Abstract
Background
Autoimmune subepidermal bullous dermatoses have similar clinical features to those of a spectrum of immune reactants at the dermoepidermal junction (DEJ). It is difficult to obtain a precise diagnosis without an immunofluorescence assay because of their similar clinical presentations. The aim of this study was to describe the cellular cutaneous infiltration among autoimmune subepidermal bullous dermatoses.
Materials and methods
This retrospective analysis was conducted at a hospital in Riyadh, Saudi Arabia using biopsy-based data collected from 65 patients.
Results
Spongiotic changes, neutrophils, and lymphocyte infiltrations in the epidermis differed among the subepidermal bullous diseases. The DEJ showed a difference in the extent of neutrophil infiltration. The dermis showed differences in perivascular lymphocytic infiltration, neutrophilic infiltration, eosinophilic infiltration, and dermal edema.
Conclusion
The dermal and DEJ showed most of the histopathologic changes in subepidermal autoimmune bullous dermatoses.
Introduction
Autoimmune subepidermal bullous diseases are a heterogeneous group of disorders. They encompass many specific disorders, including the bullous pemphigoid (BP), pemphigoid gestationis (PG), linear IgA bullous dermatosis (LAD), dermatitis herpetiformis (DH), epidermolysis bullosa acquisita (EBA), bullous lupus erythematosus (BLE), lichen planus pemphigoides (LPP), and bullous vasculitis (BV).
Immunofluorescence techniques, direct and indirect, and enzyme-linked immunosorbent assay have a crucial role in diagnosis, but determining the types of inflammatory cell infiltrate is still of great value for differentiating between these disorders.Citation1 Numerous inflammatory cells (neutrophils, eosinophils, lymphocytes, and plasma cells) have been identified in the upper dermis of patients with these disorders.Citation2–Citation5 We performed a detailed histopathologic study of a series of patients with these diseases to identify certain histopathologic features that might help to describe them.
Materials and methods
We conducted a retrospective study using biopsy-based data collected at a hospital in Riyadh, Saudi Arabia. This study was approved by the Institutional Ethical Review Board, College of Medicine, King Saud University. Patients’ consent to review their medical records was not required by the Institutional Review Board due to being retrospective in nature and related to histopathology. We assured the confidentiality of all patients.
We utilized histopathologic laboratory reports to identify all patients diagnosed with subepidermal bulbous dermatosis. We examined all biopsies collected between 1997 and 2016 (65 cases). All cases were examined by the dermatology clinic, and histopathologic findings and direct immunofluorescence assays were performed. All the original pathology reports were reviewed and evaluated by a dermatopathologist.
A histopathologic analysis of the biopsies was carried out based on the epidermal, subepidermal, and dermal changes. The presence or absence of acanthosis, para-keratosis, spongiosis, and dermal edema was noted. We also identified the presence of inflammatory cells, including lymphocytes, plasma cells, histiocytes, neutrophils, eosinophils, fibrin, and mast cells. Data were collected and stored in a spreadsheet using Microsoft Excel 2010®. Data management and coding were also performed in Excel. The data were analyzed using SPSS® version 21.0 (IBM Corporation, Armonk, NY, USA). A descriptive analysis was performed using categorical variables presented in the form of frequencies and percentages.
Results
A total of 65 cases of autoimmune subepidermal bullous diseases were diagnosed based on the histopathologic features and direct immunofluorescence assay on skin biopsies. The most common cause was BP (38%), followed by PG (34%), LAD (6%), BLE (6%), EBA (6%), DH (5%), LPP (3%), and BV (2%). The histopathologic features of the different diseases are enumerated and shown in – and –, respectively.
Figure 1 Bullous pemphigoid.
Abbreviation: H/E, Haemotoxylin and Eosin.
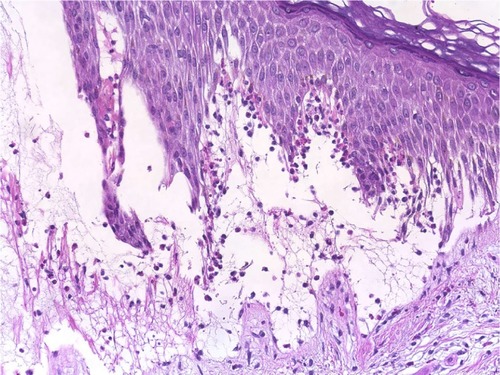
Figure 2 Linear IgA bullous dermatosis.
Abbreviation: H/E, Haemotoxylin and Eosin.
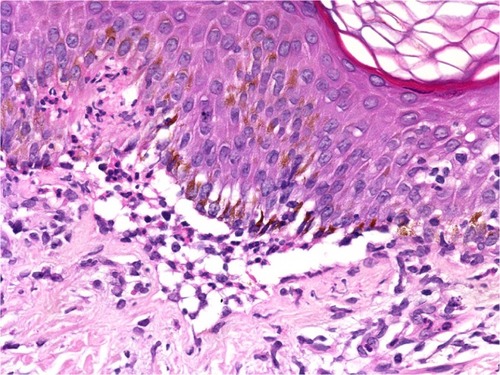
Figure 3 Bullous vasculitis.
Abbreviation: H/E, Haemotoxylin and Eosin.
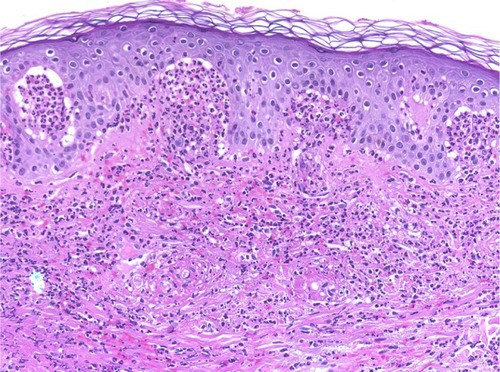
Figure 4 Dermatitis herpetiformis.
Abbreviation: H/E, Haemotoxylin and Eosin.
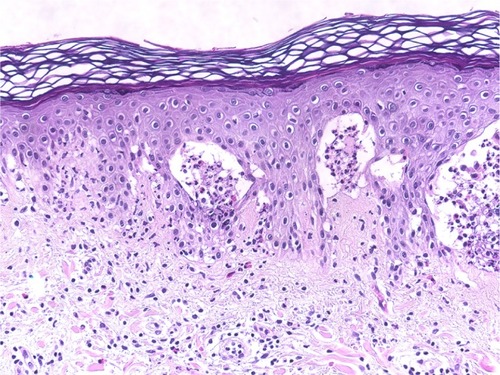
Figure 5 Bullous lupus erythematosus.
Abbreviation: H/E, Haemotoxylin and Eosin.
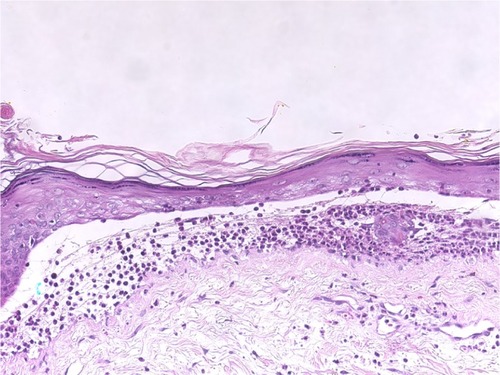
Figure 6 Epidermolysis bullosa acquisita.
Abbreviation: H/E, Haemotoxylin and Eosin.
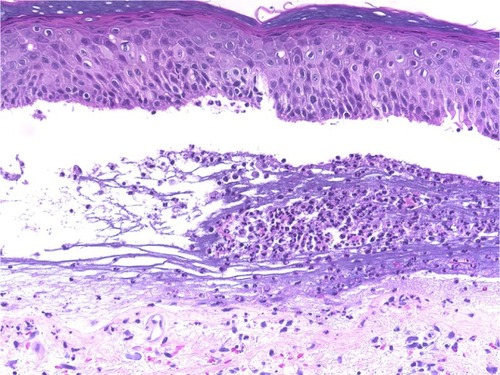
Figure 7 Pemphigoid gestationis.
Abbreviation: H/E, Haemotoxylin and Eosin.
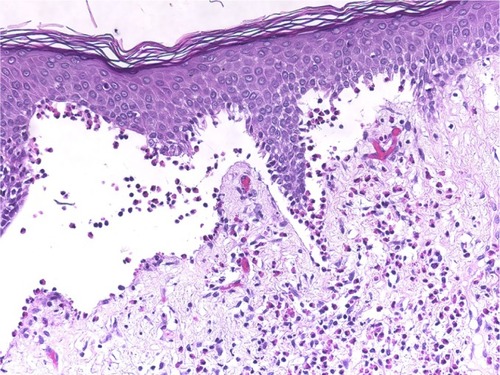
Table 1 Epidermal changes observed for the different diagnoses
Table 3 A comparison of the dermal changes among the different diagnoses
The frequencies of each histopathologic finding were investigated for all types of autoimmune subepidermal bullous diseases. Overall, the most significant histopathologic changes were found in the dermal and dermoepidermal junction (DEJ).
lists the epidermal changes. Acanthosis was more evident in BP and EBA (12.0% and 25.0% of cases, respectively), and parakeratosis was observed in BP, PG, and DH (8.0% 22.7%, and 33.3%, respectively). Epidermal spongiosis was significantly present in both PG and DH (72.0% and 66.7%, respectively). No significant epidermal inflammatory cell infiltration apart from eosinophilic infiltration (eosinophilic spongiosis) in BP and PG (8.0% and 22.7%, respectively) and neutrophil and lymphocyte infiltration in BLE (25.0%) was noted.
lists the changes at the DEJ. The LAD, EBA, and BV contained significantly higher levels of neutrophil infiltration of nearly 100%, and 50.0% of BLE had neutrophils tagging the DEJ. Only 16.0% of BP had neutrophil infiltration along the DEJ. On the other hand, eosinophils significantly tagged the DEJ in BP, PG, LAD, and LPP (40.0%, 54.5%, 50.0%, and 50.0%, respectively). These results support the theory that these cells play a major role in the pathogenesis of bulla formation. No significant lymphocytic infiltrations along the DEJ were noted compared with other inflammatory cells. Fibrin was deposited in bullous dermatosis, which has the tendency to form scar-like BLE or EBA.
Table 2 Dermo-epidermal junction alterations observed for the different diagnoses
lists the dermal changes. Perivascular lymphocytic infiltrates were significantly found in PG (100%), LPP (100%), EBA (75%), DH (66.7%), LAD (50.0%), BP (32%), and BLE (25%). A significant number of neutrophils were positively correlated with LAD (100%), BLE (100%), EBA (75%), DH (100%), and BV (100%). Eosinophils were highly infiltrated in BP (80%), PG (95.5%), EBA (75%), DH (66.7%), and LPP (100%), but they were present in only 50% of the LAD cases. However, they were less frequently observed in BLE or not found in BV. Dermal edema correlated significantly with PG (100%), LAD (100%), and BLE (50%); only 28% of BP had dermal edema. Plasma cells, histiocytes, and mast cells did not exhibit any significant differences.
Discussion
The diagnosis of subepidermal autoimmune bullous dermatoses is complex, and there is considerable clinical and histologic overlap between these diseases that necessitates more complicated assays to reach a definite diagnosis.Citation6–Citation8 At present, the diagnosis of autoimmune bullous disorders is based on immunologic and molecular findings rather than a clinical- or histopathologic-based diagnosis alone. Indirect immunofluorescence, immunoblotting, and immune electron microscopy may have great value in such diagnoses, but these technologies are available only in advanced research laboratories.Citation8,Citation9
In this study, 72% of the patients were in the pemphigoid group, which was consistent with the findings of international studies.Citation10 Although it is difficult to give a precise estimate of the disease incidence of other subepidermal autoimmune bullous dermatoses because of their rarity, an annual incidence rate ranging from 0.26 to 0.67 cases per million people per year has been reported for LAD, BLE, and EBA.Citation11–Citation14 The incidence of DH ranges from 11.5 to 39.2 cases per 100,000 people.Citation15,Citation16 Bullous lesions are very rare in vasculitis, and they reflect the severity of inflammation.Citation17,Citation18 In Henoch–Schönlein purpura, the incidence of bullous lesions among patients was roughly 19%.Citation19 As in other studies, our data indicated how rare these diseases are; their prevalence in our study ranged between 2% and 6%.
All subepidermal autoimmune bullous dermatoses exhibit nonspecific histopathologic changes in the epidermis. Epidermal changes in PG are not rare. A recent study showed increased Toll-like receptor 4 expression in the basal and suprabasal layers of BP lesions,Citation20 and thickening of the epidermis and hyperkeratosis have been described in a BP model.Citation21 The epidermis displays edema and focal eosinophilic spongiosis in PG.Citation22 In our study, we found statistically significant eosinophilic spongiosis changes in 72.7% of the cases of PG; such changes were observed in only 20.0% of cases of BP (). Epidermal changes in LAD were rare and not statistically significant, although mild acanthosis has been reported in some cases.Citation23 In our patients, we did not observe any significant epidermal changes (). Other studies have reported epidermal atrophy in BLE and extensive necrosis of the epidermis.Citation24,Citation25 We found epidermal spongiosis in 25.0% of our patients (). The histopathology of EBA depends on whether the presentation is an inflammatory or noninflammatory variant. Eosinophilic spongiosis has been reported in the inflammatory type.Citation26 Although the epidermis of DH sometimes exhibits neutrophilic accumulation,Citation27 the primary histopathologic changes are in the papillary dermis. LPP is characterized by the development of classical lichen planus lesions as well as tense bullae over lichenoid lesions and the de novo formation of these features in the uninvolved skin.Citation28,Citation29 The histopathologic features of LPP typically depend on the site of biopsy (from the bullous lesion on the top of lichenoid lesions or from the de novo bullous lesions). The epidermal changes in a bullous lesion on the top of lichenoid lesions will show features that are indistinguishable from classical lichen planus, and a de novo lesion may show eosinophilic spongiosis without features associated with lichen planus.Citation29–Citation33 The epidermal features of vasculitis appear to depend on the underlying disease, type, and severity of inflammatory cell infiltrations, and they may reveal variations in the extent of spongiosis, neutrophils, or eosinophils.Citation34–Citation36
The changes in the DEJ are the primary pathology in autoimmune subepidermal bullous diseases. The distribution and density of the inflammatory cells are very helpful for differentiating between these bullous eruptions. The separation of the DEJ in the pemphigoid groups depends on the accumulation of inflammatory cells, especially eosinophils, neutrophils, and their mediators.Citation37,Citation38 A linear arrangement of eosinophils and neutrophils along the DEJ is not uncommon in the pemphigoid group, especially in PG.Citation39 We have noted a moderate-to-rich eosinophilic cell infiltration and tagging of eosinophils along the basal layer, which was observed in 54.5% and 40.0% of PG and BP patients, respectively (; and ). Infiltration of neutrophils into the DEJ was noted in 16% of BP patients, 8% of whom had lymphocytes (). On the other hand, LAD is associated with a linear arrangement of neutrophils along the basal layer.Citation39,Citation40 We observed neutrophils at the DEJ in 100% of our patients, and these neutrophils were mixed with eosinophils in 50.0% of cases (; ). However, the presence of eosinophils may lead to a diagnostic confusion between LAD and BP.Citation41 Primary histopathologic changes in BLE occurred in the DEJ and dermis, which included acute inflammation, interface change, intense leukocytoclastic hydropic basal degeneration, mucin deposition, and neutrophilic microabscesses at DEJ.Citation24,Citation42,Citation43 Neutrophils were the dominant cellular infiltrates in the DEJ in our patients. However, lymphocytic and eosinophilic infiltrates were also present in 25% of cases (; ). The initial changes observed in EBA are around the DEJ and it is associated with neutropilic infiltration.Citation44 One hundred percent of the specimens in our study exhibited neutrophilic infiltration around the DEJ, which was associated with fibrin deposition in 25% of these cases (; ).
The primary histopathologic changes of DH are in the papillary dermis. Piérard microabscesses are the characteristic histopathology changes in the papillary dermis, and this condition is characterized by neutrophilic accumulation. Occasionally, eosinophils occur in the early stage of DH. Furthermore, inflammatory perivascular infiltrates commonly occur, especially in the superficial and medial dermis. Late stages may show fibrin deposition, neutrophil fragments, and edema.Citation45–Citation49 In our study, only 33.3% of specimens exhibited neutrophils at the DEJ and the absence of eosinophils (). The changes in the DEJ in LPP may reflect the clinical types. The primary changes in a bullous lesion on the top of a lichenoid lesion are liquefactive degeneration of basal keratinocytes with a few civatte bodies. On the other hand, similar changes in the classical cases of BP typically occur in the de novo lesion.Citation29–Citation33 In our cases, biopsies were taken from de novo bullous lesions and showed almost normal epidermis and scattered basal cell necrotic keratinocytes ().
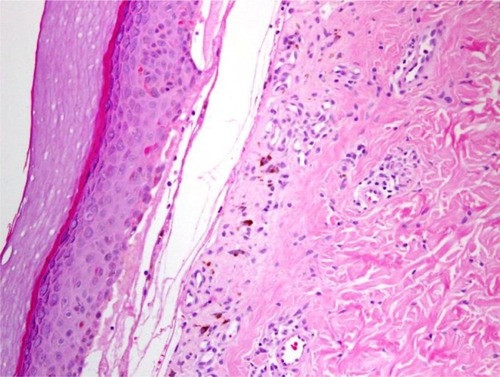
Figure 8 Lichen planus pemphigoides.
Abbreviation: H/E, Haemotoxylin and Eosin.
Cellular dermal infiltrations typically depend on the stage and severity of the lesions. Early prevesicular lesions of BP often display a significant number of eosinophils and dermal edema of the upper and middle dermis with both superficial and deep perivascular lymphohistiocytic infiltration. On the other hand, inflammatory cells, such as neutrophils, lymphocytes, and mast cells, may be present in the upper dermis of lesions.Citation50–Citation54 We characterized general dermal infiltration patterns in the pemphigoid group, and 80%–95% of the biopsies consistently revealed that eosinophils were the predominant dermal inflammatory infiltrate. Furthermore, nearly all of the specimens consistently exhibited perivascular lymphohistiocytic infiltration. The other inflammatory cells that were noted in the histologic specimens included neutrophils (12%–27%), plasma cells (5%–8%), and mast cells (13%, only in PG). Papillary edema was a constant feature in PG, and it occurred in 100% of the cases. In contrast, the occurrence rate was only 28% in BP cases. Cellular dermal infiltrations of LAD were mostly superficial and involved dermal infiltration by the neutrophils with papillary neutrophilic microabscesses and leukocytoclasia.Citation40,Citation55 Histopathology is useful for differentiating between LAD and IgA pemphigoid.Citation56 Our patients exhibited mild neutrophilic and eosinophilic infiltration in the upper dermis, although neutrophilic infiltration was a constant feature in our patients at the DEJ. We also noted a mild perivascular lymphohistiocytic infiltration ().
Cellular dermal infiltration in BLE mostly involved constant neutrophilic infiltration, papillary edema, and papillary microabscesses. However, lymphocytic infiltration, mild dermal fibrosis, and mucin have also been reported.Citation24–Citation27 Our results revealed significant neutrophilic infiltrations in 100% of our patients and eosinophilic infiltrations in 25% of our patients, as well as mild dermal edema in 50% of cases ().
Cellular dermal infiltration in EBA can exhibit little inflammatory infiltration within the dermis, especially around the vessels, follicles, and in the interstitium in classical EBA. On the other end of the spectrum, rich neutrophilic infiltrations can be mixed with variable numbers of eosinophils and mononuclear cells in other types of EBA associated with variable fibrosis.Citation44,Citation57,Citation58 Most of our patients had mixed inflammatory cell infiltrations, including neutrophils, eosinophils, incident histiocytes, and perivascular lymphocytic infiltrations (). The analysis of the cellular dermal infiltration of DH showed that 100% of our patients displayed the presence of neutrophils, 66.7% had eosinophils, and 66.7% had perivascular lymphocytes (; ).
Cellular dermal infiltrations in LPP correlated with the type of lesion from which the biopsy was obtained. The primary histopathologic features in a bullous lesion on the top of a lichenoid lesion were band-like lymphocytes beneath the DEJ, perivascular lymphocytic infiltrations, and scattered eosinophils. In contrast, dermal changes in de novo bullous lesions mostly exhibited dominant eosinophilic infiltrations with few perivascular lymphocytic infiltrations.Citation29–Citation33 All of our patients showed eosinophilic infiltrations with perivascular lymphocytic infiltrations and fibrin around the cleft (). Neutrophilic or eosinophilic infiltrations are the most common inflammatory cells associated with perivascular infiltrations, extravasated red blood cells, nuclear dust, and fibrinoid necrosis.Citation34–Citation36 Our patients exhibited dense neutrophilic infiltrations in the upper dermis associated with perivascular neutrophils, red blood cell extravasations, and fibrinoid necrosis.
Conclusion
The most significant histopathologic changes in subepidermal autoimmune bullous dermatoses were found in the dermal and DEJ. Eosinophil-rich infiltrates are characteristic of the pemphigoid group, and neutrophil-rich infiltrates are observed in LAD, DH, and BLE. EBA typically exhibits a cell-poor subepidermal bulla, and the inflammatory type of EBA reveals mixed neutrophilic and eosinophilic infiltrates.
Disclosure
The authors report no conflicts of interest in this work.
References
- SaxeNKahnLBSubepidermal bullous disease. A correlated clinicopathologic study of 51 casesJ Cutan Pathol1976328894791977
- VerdoliniRCerioRAutoimmune subepidermal bullous skin diseases: The impact of recent findings for the dermatopathologistVirchows Arch2003443218419312802582
- KolankoEBickleKKeehnCGlassLFSubepidermal blistering disorders: a clinical and histopathologic reviewSemin Cutan Med Surg2004231101815095911
- HusseinMRAliFMOmarAEImmunohistological analysis of immune cells in blistering skin lesionsJ Clin Pathol2007601627117213348
- BarnadasMAGonzalezMJPlanagumaMClinical, histopathologic, and therapeutic aspects of subepidermal autoimmune bullous diseases with IgG on the floor of salt-split skinInt J Dermatol200140426827211454083
- WojnarowskaFVenningVAImmunobullous diseasesBurnsTBreathnachSCoxNGriffithsCRook’s Textbook of Dermatology8th edOxfordBlackwell2010162
- ArundhathiSRagunathaSMahadevaKCA cross-sectional study of clinical, histopathological and direct immunofluorescence spectrum of vesiculobullous disordersJ Clin Diagn Res20137122788279224551638
- SchmidtEZillikensDModern diagnosis of autoimmune blistering skin diseasesAutoimmun Rev2010102848920713186
- MinzRWChhabraSSinghSRadotraBDKumarBDirect immunofluorescence of skin biopsy: perspective of an immunopathologistIndian J Dermatol Venereol Leprol201076215015720228544
- JainSBasavarajVVimalaMGUtility of direct immunofluorescence studies in subclassification of autoimmune subepidermal bullous diseases: A 2-year study in a tertiary care hospitalTurk Patoloji Derg2016322919827136107
- LingsKBygumALinear IgA bullous dermatosis: a retrospective study of 23 patients in DenmarkActa Derm Venereol201595446647125350667
- GammonWRBriggamanRABullous eruption of systemic lupus erythematosusWojnarowskaFBriggamanRAManagement of Blistering DiseasesLondonChapman and Hall Ltd1990263275
- IranzoPHerrero-GonzálezJEMascaró-GalyJMSuárez-FernándezREspañaAEpidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in SpainBr J Dermatol201417151022103024890437
- WoodleyDTBurgesonRELunstrumGBruckner-TudermanLReeseMJBriggamanRAThe epidermolysis bullosa acquisita antigen is the globular carboxyl terminus of type VII procollagenJ Clin Invest19888136836873278005
- MobackenHKastrupWNilssonLAIncidence and prevalence of dermatitis herpetiformis in SwedenArch Derm Venereol1984642400404
- GawkrodgerDJBlackwellJNGilmourHMRifkindEAHeadingRCBarnetsonRSDermatitis herpetiformis diagnosis, diet, and demographyGut19842521511576693042
- IshiiYTakizawaTArakawaHHemorrhagic bullous lesions in Henoch-Schönlein purpuraPediatr Int200547169469716354228
- KobayashiTSakuraokaKIwatmotoMKuriharaSA case of anaphylactoid purpura with multiple blister formation: possible pathophysiological role of gelatinase (MMP-9)Dermatology1998197162649693190
- PoteruchaTJWetterDAGibsonLECamilleriMJLohseCMHistopathology and correlates of systemic disease in adult Henoch-Schönlein purpura: a retrospective study of microscopic and clinical findings in 68 patients at Mayo ClinicJ Am Acad Dermatol201368342042422959233
- SunXKChenJFShenHImmunohistochemical study of toll-like receptors 2, 4, and 9 expressions in pemphigus and bullous pemphigoid lesionsArch Dermatol Res2016308642943627221282
- HiroseMReckeABeckmannTRepetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in miceJ Immunol201118731176118321705619
- ShimanovichIBröckerEBZillikensDPemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic toolsBJOG2002109997097612269691
- JosephTISathyanPGoma KumarKULinear IgA dermatosis adult variant with oral manifestation: a rare case reportJ Oral Maxillofac Pathol2015191838726097313
- Merklen-DjafriCBessisDFrancesCBlisters and loss of epidermis in patients with lupus erythematosus: a clinicopathological study of 22 patientsMedicine (Baltimore)20159446e210226579826
- TingWStoneMSRacilaDScofieldRHSontheimerRDToxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic panepidermolysis (ASAP): a case report, concept review, and proposal for new classification of lupus erythematosus vesiculobullous skin lesionsLupus2004131294195015645750
- Barreiro-CapurroAMascaró-GalyJMIranzoPEstudio retrospectivo de las características clínicas, histológicas e inmunológicas en una serie de 9 pacientes con epidermólisis ampollosa adquirida [Retrospective study of the clinical, histologic, and immunologic features of epidermolysis bullosa acquisita in 9 patients]Actas Dermosifiliogr201310410904914 Spanish23895729
- FaureMDermatitis herpetiformisSemin Dermatol1988711231293153433
- ZaraaIMahfoudhASellamiMKLichen planus pemphigoides: four new cases and a review of the literatureInt J Dermatol201352440641223331194
- DavisALBhogalBSWhiteheadPLichen planus pemphigoides: Its relationship to bullous pemphigoidBr J Dermatol199112532632711911320
- WillesteedEBhogalBSDasAKWojnarowskaFBlackMMMcKeePHLichen planus pemphigoides: a clinicopathological study of nine casesHistopathology19911921471541757068
- GhoshAVoraVNDaveJNShahSVCardoscoBJLichen planus pemphigoidesIndian J Dermatol Venereol Leprol199763168
- GawkrodgerDJStavropoulosPGMcLarenKMBuxtonPKBullous lichen planus and lichen planus pemphigoides-Clinicopathological comparisonsClin Exp Dermatol19891421501532598489
- MoraRGNesbittLTJrBrantleyJBLichen planus pemphigoides: Clinical and immunofluorescent findings in four casesJ Am Acad Dermatol1983833313366339569
- PoteruchaTJWetterDAGibsonLECamilleriMJLohseCMHistopathology and correlates of systemic disease in adult Henoch-Schönlein purpura: a retrospective study of microscopic and clinical findings in 68 patients at Mayo ClinicJ Am Acad Dermatol201368342042422959233
- GulatiGSivJWareAEBullous skin lesions in an adult male: a diagnostic dilemmaAm J Case Rep20151621521925858335
- IshibashiMKudoSYamamotoKShimaiNChenKRChurg-Strauss syndrome with coexistence of eosinophilic vasculitis, granulomatous phlebitis and granulomatous dermatitis in bullous pemphigoid-like blistersJ Cutan Pathol201138329029420132420
- BorregoLMaynardBPetersonEADeposition of eosinophil granule proteins precedes blister formation in bullous pemphigoid. Comparison with neutrophil and mast cell granule proteinsAm J Pathol199614838979098774144
- StanleyJRCell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesionAdv Immunol1993532913268512037
- AndrachukLGhazarianDSiddhaSAl HabeebALinear arrangement of neutrophils along the basal layer in bullous pemphigoid: a unique histological findingAm J Dermatopathol201234219219322441369
- SmithSBHarristTJMurphyGFLinear IgA bullous dermatosis v dermatitis herpetiformis. Quantitative measurements of dermoepidermal alterationsArch Dermatol198412033243286367664
- TsaiICChuCYChenHJWangLFChiuHCLinear IgA bullous dermatosis: a clinical study of 16 cases at National Taiwan University HospitalDermatol Sinica20102812126
- PennysNSWileyHE3rdHerpetiform blisters in systemic lupus erythematosusArch Dermatol19791551214271428
- OlanskyAJBriggamanRAGammonWRKellyTFSamsWMJrBullous systemic lupus erythematosusJ Am Acad Dermatol1982715115206754773
- GuptaRWoodleyDTChenMEpidermolysis bullosa acquisitaClin Dermatol2012301606922137228
- FaureMDermatitis herpetiformisSemin Dermatol198871231293153433
- SmithJBTaylorTBZoneJJThe site of blister formation in dermatitis herpetiformis is within the lamina lucidaJ Am Acad Dermatol1992272 Pt 12092131430358
- ClarindoMVPossebonATSoligoEMUyedaHRuaroRTEmpinottiJCDermatitis herpetiformis: pathophysiology, clinical presentation, diagnosis and treatmentAn Bras Dermatol201489686587525387490
- ImberMJKibbiAGMihmMCJrDermatitis herpetiformis: Histopathologic findingsClin Dermatol1991932892931806216
- CaproniMAntigaEMelaniLFabbriPItalian Group for Cutaneous ImmunopathologyGuidelines for the diagnosis and treatment of dermatitis herpetiformisJ Eur Acad Dermatol Venereol200923663363819470076
- NishiokaKHashimotoKKatayamaISarashiCKuboTSanoSEosinophilic spongiosis in bullous pemphigoidArch Dermatol19841209116611686383222
- NatioKMoriokaSIkedaSOgawaHExperimental bullous pemphigoid in guinea pigs: The role of pemphigoid antibodies, complement, and migrating cellsJ Invest Dermatol19848232272306366073
- WintroubBUMihmMCJrGoetzlEJSoterNAAustenKFMorphologic and functional evidence for release of mast-cell products in bullous pemphigoidN Engl J Med19782988417421340950
- DvorakAMMihmMCJrOsageJEKwanTHAustenKFWintroubBUBullous pemphigoid, and ultrastructural study of the inflammatory response: Eosinophil, basophil and mast cell granule changes in multiple biopsies from one patientJ Invest Dermatol1982782911017035575
- YaoitaHGullinoMKatzSIHerpes gestationis. Ultrastructure and ultrastructural localization of in vivo-bound complementJ Invest Dermatol197666638338858951
- BlenkinsoppWKHaffendenGPFryLLeonardJNHistology of linear IgA disease, dermatitis herpetiformis, and bullous pemphigoidAm J Dermatopathol1983565475546364874
- HorváthBNiedermeierAPodstawaEIgA autoantibodies in the pemphigoids and linear IgA bullous dermatosisExp Dermatol201019764865320500772
- De JongMCBruinsSHeeresKBullous pemphigoid and epidermolysis bullosa acquisita: differentiation by fluorescence overlay antigen mappingArch Dermatol199613221511578629822
- KazamaTYamamotoYHashimotoTKomaiAItoMApplication of confocal laser scanning microscopy to differential diagnosis of bullous pemphigoid and epidermolysis bullosa acquisitaBr J Dermatol199813845936019640362
