Abstract
Halobetasol propionate and tazarotene lotion 0.01%/0.045% (HP/TAZ) is a topical medication approved for the treatment of plaque psoriasis in adults. As a treatment modality, HP/TAZ has a combinatory therapeutic effect because it contains both a corticosteroid (HP) and a retinoid (TAZ) component. Here, we review the important clinical efficacy and safety data derived from pivotal clinical trials for HP/TAZ in the treatment of plaque psoriasis. We also discuss the mechanism of action, dosage guidelines, pharmacokinetics/pharmacodynamics, and clinical considerations for HP/TAZ, including why HP/TAZ should be avoided in pregnant patients.
Introduction
Psoriasis is a chronic, immune-mediated condition that affects an estimated 3.2% of adults in the United States.Citation1 Plaque-type psoriasis, the most common clinical variant, is responsible for approximately 90% of psoriasis cases.Citation2 It is characterized by the recurrent development of thick, scaly, well-circumscribed, erythematous plaques often associated with pruritus and pain. In addition to its dermatologic symptoms, which can incur significant impairment in quality of life, psoriasis is also associated with several comorbidities such as obesity,Citation3 depression,Citation4 psoriatic arthritis,Citation5 cardiovascular disease,Citation6,Citation7 and diabetes.Citation8
Topical therapies have long been utilized as first-line therapeutic options for the treatment of plaque psoriasis. Both providers and patients are typically comfortable with the use of topical therapies for the treatment of mild psoriasis, which accounts for an estimated 70% to 80% of cases.Citation9 While biologics are increasingly prescribed as mainstay treatments for moderate-to-severe psoriasis, topical therapies, such as corticosteroids, are still routinely used as adjuncts for the treatment of psoriasis flares or plaques refractory to biologic therapy.
Halobetasol propionate and tazarotene lotion 0.01%/0.045% (HP/TAZ; Duobrii™, Ortho Dermatologics, Bridgewater, NJ, USA) is a topical medication first approved in April 2019 by the Food and Drug Administration (FDA) for the treatment of plaque psoriasis in adults. The combination of HP and TAZ allows for dual mechanistic action in treating psoriasis; HP, a topical corticosteroid, provides a primarily anti-inflammatory effect and TAZ, a vitamin A derivative, impairs keratinocyte proliferation. Although HP and TAZ have both demonstrated success in the treatment of plaque psoriasis when used independently,Citation10–Citation13 each have limitations in their clinical utility, particularly due to their side-effect profiles. As such, potential benefits of combination therapy with both HP and TAZ have been evaluated in various clinical trials. The results of these trials and important implications for clinical use of HP/TAZ are discussed in this review. Important considerations for the use of HP/TAZ are summarized in .
Table 1 HP/TAZ Summary Table
Methods
A literature search of the PubMed, Embase, and US NLM (National Library of Medicine) clinicaltrials.gov databases was conducted for the terms “psoriasis” and “halobetasol propionate” or “tazarotene” or “Duobrii” or “IDP-118.” Searches were limited to English-language articles published prior to or on March 23, 2020. Results of any relevant articles were manually identified by the authors for review. Duplicate articles were excluded.
Mechanism of Action
Halobetasol Propionate
Halobetasol propionate (HP) is an ultra-high potency topical steroid (class I) that exerts its therapeutic effect by inhibiting aberrant inflammation and mitotic activity. As with other corticosteroids, HP binds to glucocorticoid receptor proteins to modulate inflammatory protein synthesis, decrease prostaglandin synthesis, and suppress mRNA required for interleukin-1 (IL-1) formation.Citation14,Citation15 Despite its efficacy, continuous use of HP is typically limited to two weeks due to local side-effects such as skin atrophy, striae, telangiectasias, purpura, and possible systemic adverse effects, including hypothalamic–pituitary–adrenal (HPA) axis suppression.Citation16
Tazarotene
TAZ is a vitamin A derivative useful in treating psoriasis due to its anti-inflammatory properties and the ability to mediate keratinocyte differentiation and proliferation. As a retinoid, TAZ binds to retinoic acid receptors (RARs) following conversion to its active form, tazarotenic acid, which has a particular affinity for RAR-β and RAR-γ.Citation17,Citation18 In doing so, TAZ alters transcription of several genes to improve cellular adhesion, inhibit excess keratinocyte proliferation, and decrease the expression of inflammatory cytokines.Citation19–Citation21 Commonly reported side-effects of TAZ include erythema, skin irritation/burning sensation, xeroderma, and desquamation.
The clinical utility of HP/TAZ is in providing a synergistic therapeutic effect and an improved side-effect profile by combining a topical corticosteroid and retinoid. TAZ has demonstrated efficacy for the treatment of psoriasis when used alone, as well as when combined with corticosteroids of various strengths.Citation21 HP/TAZ presents an opportunity to optimize the therapeutic effect of HP and TAZ while minimizing limiting factors. The dosage, efficacy, and safety of HP/TAZ are discussed in detail below.
Dosage
HP/TAZ is formulated as a 0.01%/0.045% lotion. Each gram of HP/TAZ contains 0.1 mg HP and 0.45 mg TAZ.Citation22 Due to concerns for HPA axis suppression, the total weekly dosage of HP/TAZ should not exceed 50 g.Citation22 HP/TAZ should be applied onto dry skin affected by psoriasis and is not indicated for the treatment of face, groin, or axillary psoriasis.
Pharmacodynamics and Pharmacokinetics
Pharmacodynamics
Based on data derived from vasoconstrictor assays (human skin blanching assays) in healthy subjects, HP/TAZ is classified as a high to super-high range topical corticosteroid.Citation22 As such, an open-label, randomized, pharmacodynamics/pharmacokinetics (PD/PK) study was conducted to evaluate the potential for HPA axis suppression. Adult subjects with at least 20% body surface area (BSA) involvement of plaque psoriasis applied a median dose of 8.2 g of HP/TAZ once daily for 28 days.Citation22 HPA axis suppression (defined as a serum cortisol level of less than or equal to 18 µg per deciliter 30 mins after stimulation with cosyntropin (adrenocorticotropic hormone)) was evaluated at week four and week eight.Citation22 HPA axis suppression was found in three out of 20 (15%) of subjects at week four and none at week eight.Citation22 Of note, current dosage guidelines limit HP/TAZ to 50 g per week, which is lower than the median dose used by subjects in this PK/PD study. Additionally, subjects in this study were evaluated following 28 days of consecutive use, but the recommended maximum consecutive use is 14 days. Thus, when used according to current dosage guidelines, HP/TAZ is unlikely to cause HPA axis suppression in patients.
Pharmacokinetics
The PK of HP/TAZ has been evaluated in 22 adult subjects following daily application for 28 days. On day 28, systemic concentrations of HP (lower limit of quantification (LLOQ) = 50pg/mL) and TAZ (LLOQ = 5pg/mL) were quantifiable in 59% and 82% of subjects, respectively.Citation22 Tazarotenic acid (LLOQ = 5 pg/mL), the active moiety of TAZ, was quantifiable in 100% of subjects on day 28.Citation22 The mean concentration maximum (Cmax; pg/mL) of HP, TAZ, and tazarotenic acid on day 28 was 101.9 (standard deviation (SD) = 135.4), 24.6 (SD = 27.3), and 523.4 (SD = 523.3), respectively.Citation22
Efficacy and Safety
Phase III Clinical Trials
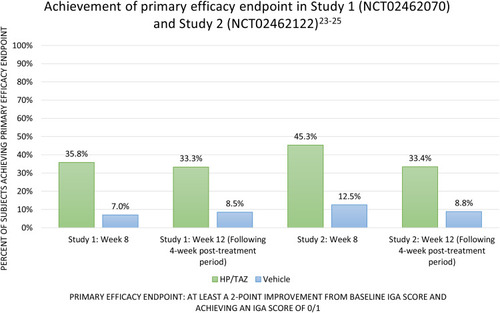
Two multicenter, randomized, double-blinded, vehicle-controlled, phase III clinical trials (n=418) have been conducted using HP/TAZ (NCT02462070; Study 1 and NCT02462122; Study 2).Citation23,Citation24 Both studies recruited adult subjects with moderate-to-severe psoriasis, defined as an Investigator Global Assessment (IGA) score of 3 or 4 and body surface (BSA) involvement between 3% and 12%. Study subjects were randomized 2:1 to receive either HP/TAZ lotion or vehicle applied to psoriasis plaques once daily for 8 weeks. The maximum allowable dose was 50 g per week. The primary efficacy endpoint was the percentage of subjects who achieved treatment success at week 8, with treatment success defined as at least a 2-point improvement in IGA score from baseline and achieving an IGA score of clear or almost clear (IGA 0/1).Citation25 When compared to vehicle, HP/TAZ was significantly more effective by week two in Study 2 and week four in Study 1.Citation25 At week eight, 35.8% (Study 1) and 45.3% (Study 2) of those receiving HP/TAZ lotion had achieved the primary efficacy outcome (vs 7.0% (Study 1) and 12.5% (Study 2) of those receiving vehicle) (both p<.001).Citation25 At week 12, following a four-week post-treatment period, subjects who had received HP/TAZ lotion demonstrated sustained therapeutic success when compared to those who had received vehicle (33.3% vs 8.5% (Study 1); 33.4% vs 8.8% (Study 2); (both p<.001)).Citation25 The results of these trials are summarized in .
Figure 1 Achievement of primary efficacy endpoint in study 1 (NCT02462070) and study 2 (NCT02462122).Citation23–Citation25
Safety evaluations in both studies were conducted through week 12. The most common treatment-related adverse events were contact dermatitis (6.3%), pruritus (2.2%), and application site pain (2.6%).Citation25 No treatment-related serious adverse events occurred in patients treated with HP/TAZ.Citation25
Blauvelt et al conducted a post hoc analysis of Study 1 and Study 2 discussed above using the product of IGA and BSA involvement (IGA x BSA) to assess treatment response, as this takes into account both disease extent and plaque qualities.Citation26 In this study, a clinically meaningful outcome was defined as an achievement of greater than or equal to 75% reduction in IGA x BSA score from baseline (IGA x BSA-75). At the week 8 evaluation, IGA x BSA scores improved from baseline by 51.9% and 9.21% (p < 0.001) for HP/TAZ and vehicle, respectively.Citation26 41.7% of patients treated with HP/TAZ and 9.9% of patients treated with vehicle (p<0.001) achieved IGA x BSA-75 by week 8.Citation26 At week 12, following a four-week post-treatment period, mean percent changes in IGA x BSA scores from baseline were 46.6% and 7.92% (p<0.001), respectively.Citation26 This improvement was consistent with the IGA x BSA-75 evaluation at week 12, with 41.4% of HP/TAZ lotion patients achieving IGA x BSA-75 compared with 10.7% of vehicle-treated patients (p<0.001).Citation26 The rate of improvement in baseline IGA x BSA scores was similar even when patients were stratified by baseline disease severity.
Lebwohl et al conducted a separate post hoc analysis of Study 1 and Study 2 to specifically examine patients with severe localized psoriasis (n=62), defined as those enrolled with an IGA of 4.Citation27 As with the Blauvelt et al post hoc analysis discussed previously, this post hoc analysis also defined the achievement of a clinically meaningful treatment response as an achievement of IGA x BSA-75. At week eight, 34.8% of these patients treated with HP/TAZ were considered treatment successes compared with 0.0% on the vehicle (p=0.004).Citation27 The investigators also noted that when evaluating individual psoriasis signs and symptoms (erythema, plaque elevation, and scaling) on a 4-point scale, HP/TAZ was significantly superior to the vehicle. At week eight, HP/TAZ patients achieved at least a 2-point improvement in the following: 47.4% (erythema), 66.4% (plaque elevation), and 65.4% (scaling), compared with 14.0% (p=0.016), 14.8% (p<0.001) and 14.7% (p<0.001), respectively, with patients receiving vehicle.Citation27 Patients treated with HP/TAZ lotion achieved a 32.8% reduction in baseline mean BSA, compared with a 39.6% increase seen with vehicle (p=0.013).Citation27 This post hoc analysis also reiterated the findings from Study 1 and Study 2 that HP/TAZ has the potential to provide demonstrable improvement early on following initiation of treatment. HP/TAZ lotion achieved a statistically significant reduction in mean IGA x BSA compared to the vehicle by week 2 (p<0.001 vs vehicle).Citation27 By week 8, almost half of HP/TAZ-treated patients achieved a clinically meaningful response (IGA x BSA-75) and 52.9% reduction in mean IGA x BSA scores, compared with a 17.5% increase in IGA x BSA scores in vehicle-treated (p<0.001).Citation27 The most frequently reported treatment-related adverse events in the HP/TAZ patients were application site pain (7.9%), contact dermatitis (5.3%), and pruritus (5.3%).Citation27
Long-term efficacy and safety results (up to 1 year) of HP/TAZ have been reported by Lebwohl et al in an open-label, phase III study (n=555) in adults with moderate-to-severe plaque psoriasis, defined as having a baseline IGA of 3 or 4.Citation28 In this study, 4.7% and 20.9% of subjects discontinued treatment with HP/TAZ by weeks 12 and 24, respectively, due to inefficacy.Citation28 The most commonly reported treatment-related adverse events were application site reactions resulting in dermatitis, pruritus, and pain, with 7.5% of subjects discontinuing treatment with HP/TAZ due to these side effects.Citation28 Overall, the incidence of adverse events peaked around day 60 of the trial and remained stable from day 90 until the end of the study.Citation28 When compared to the previously discussed phase III clinical trials, no new safety concerns for HP/TAZ were identified in this study.
Phase II Clinical Trials
Bhatia et al conducted a multicenter, randomized, double-blinded, parallel-group, vehicle-controlled, phase II clinical trial (n=154) in which adult subjects were randomized 2:2:1 to receive either HP/TAZ lotion, HP cream, or vehicle applied topically once daily for 2 weeks.Citation29 As with the phase III studies, this study included patients with IGA scores of 3 or 4 and BSA involvement of 3 to 12%. The primary efficacy endpoint was achieving at least a 2-point improvement from baseline IGA score and also achieving an IGA score of 0/1 (clear or almost clear). At week two, HP/TAZ was more effective than the vehicle and comparable to HP in achieving these aims (32.79% in HP/TAZ vs 33.97% and 3.33%, respectively; p=0.002 vs vehicle).Citation29 There was also a 25% reduction in mean baseline BSA with HP/TAZ as compared to 24.8% in HP patients and 5.0% in patients treated with vehicle (p<0.001) at week 2.Citation29 Investigators also identified a target lesion in each subject to evaluate improvement. Each target lesion was evaluated on a 4-point scale for erythema, plaque elevation, and scaling and improvement was assessed across all three cohorts. At week two, at least a 2-point improvement from baseline was achieved by 34.43% (erythema, p=0.08 vs vehicle), 54.10% (plaque elevation, p=0.003 vs vehicle), and 60.66% (scaling, p=0.003 vs vehicle) of HP/TAZ-treated patients.Citation29 In comparison, the HP-treated cohort achieved 43.49%, 50.79%, and 50.79% of HP-treated patients (all nonsignificant versus HP/TAZ) and 16.67%, 20.67%, and 27.33% of vehicle-treated patients, respectively.Citation29 Although HP/TAZ was comparable in efficacy to HP alone, the investigators noted greater improvements in plaque elevation and scaling with HP/TAZ, attributing this to the keratolytic action of TAZ.Citation29 In regard to safety, treatment-related adverse events were rare, with the most common being application site pain and application site atrophy. Application site pain was reported in 1.6% of HP/TAZ patients, 4.8% of HP patients, and 0% of vehicle patients.Citation29 Application site atrophy was reported in 1.6% of HP/TAZ patients, 0% of HP patients, and 3.4% of vehicle patients.Citation29
Sugarman et al conducted a larger, multicenter, randomized, double-blinded, phase II clinical trial (n=212) in which subjects were randomized 2:2:2:1 to receive either HP/TAZ lotion, HP lotion, TAZ lotion, or vehicle lotion once daily for 8 weeks.Citation30 As with the other reported studies, enrolled subjects had baseline IGA scores of 3 or 4 and a BSA of 3% to 12%. Efficacy was assessed by the achievement of at least a 2-point improvement from baseline IGA score and an IGA of 0/1 by week eight. Improvement in erythema, plaque elevation, and scaling of a specific target lesion were also evaluated in each patient. At week 8, 52.5% of HP/TAZ subjects achieved the primary efficacy endpoint, compared to 33.3%, 18.6%, and 9.7% of HP (p=0.033), TAZ (p<0.001), and vehicle (p<0.001) subjects, respectively.Citation30 At week eight, HP/TAZ lotion was superior to HP alone, TAZ alone, and vehicle in reducing erythema, plaque elevation, and scaling of the target lesion.Citation30 Two-point improvement in IGA was achieved by 54.2% of subjects for erythema, 67.8% for plaque elevation, and 64.4% for scaling in the HP/TAZ group.Citation30 At week 12, following a four-week post-treatment period, 67.7% of the HP/TAZ-treated participants who achieved treatment success maintained their improvement compared with 61.9% of those in the HP group and 54.5% of those in the TAZ group.Citation30 TAZ patients had a 79.7% adherence rate compared with 94.9% in the HP/TAZ group.Citation30 The most frequently reported treatment-emergent adverse events were application site reactions. Side effects, such as application site pain, erythema, and pruritus occurred most often in the TAZ group (22.4%) vs the HP/TAZ group (10.6%).Citation30
Multiple post hoc analyses have been subsequently conducted on the Sugarman et al study.Citation31,Citation32 Kircik et al aimed to evaluate to what extent, if any, HP/TAZ provided a synergistic therapeutic effect as compared to HP and TAZ alone at week eight and at week 12 following the 4-week post-treatment period.Citation31 Synergy was established when the benefit of combination HP/TAZ lotion was greater than the sum of benefit of HP alone plus TAZ alone, with a ratio >1.0 (HP/TAZ divided by HP+TAZ). At week eight, treatment success with HP/TAZ lotion relative to the vehicle was 42.8% compared with 32.5% for HP plus TAZ and percent change from baseline in IGA x BSA score relative to the vehicle was 51.6% compared with 40.6% for HP plus TAZ.Citation31 At week 12, treatment success with HP/TAZ lotion relative to the vehicle was 31.3% compared with 20.0% for HP plus TAZ.Citation31 Percent change from baseline in IGA x BSA score relative to the vehicle was 47.3% compared with 34.2% for HP plus TAZ.Citation31 HP/TAZ lotion provided synergistic benefits in terms of achieving a clinically meaningful outcome, with a ratio of 1.3 and 2.0 at weeks eight and 12.Citation31
In a separate post hoc analysis, Gold et al evaluated efficacy from the Sugarman et al study by using a similar approach to Blauvelt et al’s post hoc analysis of the phase III HP/TAZ trials. Gold et al examined improvements in IGA x BSA at week eight and following the post-treatment period at week 12 from the Sugarman et al cohort.Citation32 By week 8, HP/TAZ achieved a 63.5% reduction in mean IGA x BSA composite score (p<0.001 vs TAZ and vehicle) that was sustained 4-weeks post-treatment (p<0.001 vs TAZ and vehicle and p=0.003 vs HP).Citation32 A 25% and 50% improvement in IGA x BSA was achieved within 1.9 and 4.6 weeks, respectively, and 47.5% of patients achieved IGA x BSA-75 by week eight in the HP/TAZ cohort.Citation32
In another analysis, results revealed that when compared to both vehicle and TAZ alone, HP/TAZ demonstrated statistically significant superiority for treatment success (p=0.047 and p = 0.029, respectively) in as early as 2 weeks.Citation33 By week 2, 47.5% of patients were “mild”, “almost clear”, or “clear” compared with 33.3%, 16.9%, and 12.9% of patients treated with HP, TAZ, or vehicle, respectively.Citation33 Plaque elevation and scaling were significantly improved compared with HP, TAZ, or vehicle, and erythema was significantly improved compared with TAZ. Improvements in baseline itching (45.6%), dryness (42.2%), burning/stinging (55.9%) with HP/TAZ lotion at 2 weeks were similar to those seen with HP, and greater than those achieved with TAZ (30.8% (p=0.099), 35.4%, and 13.3%, respectively).Citation33
Another analysis of the Sugarman et al study found that at the end of the 4-week post-treatment period, 38.2% of patients who had been treated with HP/TAZ were treatment successes; compared with 21.0%, 12.8% and 6.9% of patients who had been treated with HP (p=0.042), TAZ (p=0.004), or vehicle (p=0.002).Citation34 HP/TAZ lotion was superior in maintaining reductions in erythema, plaque elevation, and scaling of the target lesion. At week 12, following the 4-week posttreatment period, 49.1%, 54.5%, and 54.5% of patients, respectively, were treatment successes compared with 38.7% (p=0.26), 48.4% (p=0.51), and 48.4% (p=0.51) of patients who had been treated with HP; 29.8% (p=0.049), 31.9% (p=0.022), and 23.4% (p=0.001) who had been treated with TAZ; and 13.8% (p=0.002), 20.7% (p=0.003), and 20.7% (p=0.003) who had been treated with vehicle.Citation34 Side effects were minimal and tended to resolve during the posttreatment period.Citation34
Pregnancy
Due to concerns regarding embryofetal risk, HP/TAZ is contraindicated in pregnancy. Animal studies have identified the teratogenic effects of TAZ,Citation35 although the level of exposure required to induce teratogenic effects in humans is unknown.Citation22 It is recommended that providers obtain a pregnancy test from patients of reproductive potential within 2 weeks prior to initiating treatment with HP/TAZ. Additionally, HP/TAZ should be initiated during a menstrual period, if possible, and patients of reproductive potential on HP/TAZ who are sexually active should use an effective and reliable method of contraception. Because the level of absorption of HP/TAZ in human breastmilk is unknown, breastfeeding women should not apply HP/TAZ to the nipple and/or areola.Citation22 Additionally, both providers and patients should weigh the potential benefits and risks of starting HP/TAZ in breastfeeding women.
Photosensitivity
HP/TAZ, primarily due to the photosensitizing properties of TAZ, can increase susceptibility to developing sunburns. As such, providers should encourage patients to avoid exposure to sunlight as much as possible. Additionally, providers should educate patients on appropriate sun protection techniques, including the correct application of sunscreen and the importance of stopping HP/TAZ if a sunburn occurs until it heals. Prior to initiating HP/TAZ, providers should also consider whether patients are taking other photosensitizing medications such as thiazides, tetracyclines, fluoroquinolones, and sulfonamides.Citation22
Conclusion
Topical therapeutics remain mainstays for treating mild psoriasis and are often utilized as adjuncts for treating moderate-to-severe psoriasis. Despite their efficacy, topical medications are often limited due to side effects. HP/TAZ, a combination of a topical corticosteroid and a retinoid, can be used as a topical medication in adults with plaque psoriasis. Following the review of the available clinical trials, HP/TAZ appears to be clinically useful for the treatment of plaque psoriasis, particularly for patients with localized disease. Additionally, there are data to suggest that as a combination, HP/TAZ provides a synergistic effect compared to TAZ or HP alone, although additional research is necessary to further validate this conclusion. The available long-term safety data have not revealed any new concerning side-effects beyond the usual side-effects noted in both the short-term trials and trials evaluating HP and TAZ individually. However, as with all topicals, there may be a concern for poor adherence due to side-effects, with topical corticosteroids potentially resulting in skin thinning or atrophy and topical retinoids causing burning or irritation. Although only one study thus far has reported on long-term data, there is some evidence to suggest that about one-fifth of subjects treated with HP/TAZ discontinue treatment due to inefficacy by week 24. Additionally, as with other combination-based topicals, the cost of medication can often be prohibitive for some patients. Providers must consider potential barriers prior to deciding the best therapeutic option for an individual patient to minimize non-adherence. Future research on HP/TAZ should focus on its utility as an adjunctive therapy for patients with psoriasis who are managed with biologics and/or phototherapy. Currently, ongoing Phase IV clinical trials are evaluating the pharmacokinetics and safety of HP/TAZ in pediatric patients as well as in combination with biologics in adult patients with plaque psoriasis.Citation36,Citation37
Abbreviations
HP/TAZ, halobetasol propionate and tazarotene lotion, 0.01%/0.045%; FDA, Food and Drug Administration; NLM, National Library of Medicine; HP, halobetasol propionate; TAZ, tazarotene; RAR, retinoic acid receptor; IL, interleukin; HPA axis, hypothalamic–pituitary–adrenal axis; IGA, Investigator Global Assessment; BSA, body surface area.
Disclosure
Vidhatha Reddy, Eric Yang, and Bridget Myers have no disclosures or conflicts of interest to report. Tina Bhutani is currently an investigator for Celgene, Galderma, Janssen, and Regeneron. She has served as an advisor for Abbvie, Lilly, and Pfizer.
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi:10.1016/j.jaad.2013.11.01324388724
- Boehncke W-H, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-726025581
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54–e54. doi:10.1038/nutd.2012.2623208415
- Olivier C, Robert PD, Daihung D, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi:10.1001/archdermatol.2010.18620713823
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi:10.1016/j.jaad.2016.07.06428212759
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi:10.1001/jama.296.14.173517032986
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31(3):433–443. doi:10.1097/HJH.0b013e32835bcce123249828
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi:10.1001/2013.jamadermatol.40623407990
- Schön MP, Boehncke W-H. Psoriasis. N Engl J Med. 2005;352(18):1899–1912. doi:10.1056/NEJMra04132015872205
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1 betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25(6, Part 2):1153–1156. doi:10.1016/0190-9622(91)70315-S1757607
- Goldberg B, Hartdegen R, Presbury D, Harvey Smith E, Yawalkar S. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25(6, Part 2):1145–1148. doi:10.1016/0190-9622(91)70313-Q1757605
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25(6, Part 2):1149–1152. doi:10.1016/0190-9622(91)70314-R1757606
- Weinstein GD, Koo JYM, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48(5):760–767. doi:10.1067/mjd.2003.10312734506
- Awad N, Preuss CV. Halobetasol Cream In: StatPearls. Treasure Island (FL): StatPearls Publishing 2020 Available from: http://www.ncbi.nlm.nih.gov/books/NBK544234/.Accessed 317, 2020.
- Kragballe K. Topical corticosteroids: mechanisms of action. Acta Derm Venereol Suppl (Stockh). 1989;151:7–10.2533778
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15. doi:10.1016/j.jaad.2005.01.01016384751
- Chandraratna R. Tazarotene—first of a new generation of receptor-selective retinoids. Br J Dermatol. 1996;135(s49):18–25. doi:10.1111/j.1365-2133.1996.tb15662.x
- Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Available from: https://www-ncbi-nlm-nih-gov.ucsf.idm.oclc.org/pmc/articles/PMC2699641/. Accessed 317, 2020.
- Heath MS, Sahni DR, Curry ZA, Feldman SR. Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol. 2018;14(9):919–927. doi:10.1080/17425255.2018.151519830134735
- Duvic M, Nagpal S, Asano AT, Chandraratna RAS. Molecular mechanisms of tazarotene action in psoriasis. J Am Acad Dermatol. 1997;37(2, Part 3):S18–S24. doi:10.1016/S0190-9622(97)80396-99270552
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4, Supplement):S139–S143. doi:10.1016/S0190-9622(98)70311-19777792
- DUOBRII™ Lotion [package insert]. Bridgewater, NJ: bausch Health US, LLC. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209354s000lbl.pdf. Accessed 3 11, 2020.
- Safety and efficacy of IDP-118 in the treatment of plaque psoriasis - full text view - clinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02462070. Accessed 311, 2020..
- Safety and efficacy of IDP-118 in the treatment of plaque psoriasis - full text view - clinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02462122. Accessed 311, 2020.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 Phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79(2):287–293. doi:10.1016/j.jaad.2018.03.04029614243
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18(3):297–299.30909352
- Lebwohl MG, Sugarman JL, Gold LS, et al. Efficacy, safety, and tolerability of a halobetasol 0.01%/tazarotene 0.045% fixed combination in the treatment of severe localized plaque psoriasis: post hoc analysis of two phase III randomized controlled trials. J Drugs Dermatol. 2019;18(10):1012–1018.31584780
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80(1):282–285. doi:10.1016/j.jaad.2018.09.00230227193
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthetic Dermatol. 2018;11(11):15–19.
- Sugarman JL, Gold LS, Lebwohl MG, Pariser DM, Alexander BJ, Pillai R. A Phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16(3):197–204.28301614
- Kircik LH, Papp KA, Gold LS, Harris S, Pharm TL, Pillai R. Assessing the synergistic effect of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18(3):279–284.30909333
- Gold LS, Bagel J, Lebwohl M, Lin T, Martin G, Pillai R. Halobetasol and tazarotene: further defining the role of a unique fixed combination topical lotion in moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2018;17(12):1290–1296.30586261
- Gold LS, Kircik LH, Pariser D, et al. Rapid onset of action in patients with moderate-to-severe plaque psoriasis with halobetasol 0.01%/tazarotene 0.045% fixed combination. J Drugs Dermatol. 2018;17(8):863–868.30124725
- Pariser DM, Green LJ, Gold LS, Sugarman JL, Lin T, Pillai R. Halobetasol 0.01%/tazarotene 0.045% lotion in the treatment of moderate-to-severe plaque psoriasis: maintenance of therapeutic effect after cessation of therapy. J Drugs Dermatol. 2018;17(7):723–726.30005093
- PubChem. Tazarotene. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5381. Accessed 323, 2020.
- A safety and pharmacokinetics study of IDP-118 lotion in pediatric participants with plaque psoriasis - full text view - clinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT03988439. Accessed 325, 2020.
- Duobrii in Combination with Biologics – Full Text View – ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04119102. Accessed 325, 2020.
