Abstract
Background
Botulinum toxin type A treatment has been used for over 20 years to enhance the appearance of the face. There are several commercially available botulinum toxin type A products used in aesthetic clinical practice. The aim of this retrospective analysis was to compare the clinical efficacy of the most commonly used botulinum toxin type A preparations in daily practice.
Methods
Physicians from 21 centers in Germany completed questionnaires based on an inspection of subject files for subjects 18 years of age or over who had received at least two, but not more than three, consecutive treatments with incobotulinumtoxinA, onabotulinumtoxinA, or abobotulinumtoxinA within a 12-month period in the previous 2 years. Data on subject and physician satisfaction, treatment intervals, dosages, and safety were collected from 1256 subjects.
Results
There were no statistically significant differences between incobotulinumtoxinA and onabotulinumtoxinA with respect to physician and subject satisfaction, dosages, and adverse effects experienced. Both botulinum toxin type A preparations were well tolerated and effective in the treatment of upper facial lines. Due to low treatment numbers, abobotulinumtoxinA was not included in the statistical analysis.
Conclusion
The results of this retrospective analysis confirm the results of prospective clinical trials by demonstrating that, in daily practice, incobotulinumtoxinA and onabotulinumtoxinA are used at a 1:1 dose ratio and display comparable efficacy and safety.
Introduction
Botulinum toxins have been used for over 20 years for aesthetic procedures to improve the appearance of the face.Citation1,Citation2 Botulinum toxin type A injections are the most common nonsurgical procedures performed in the US with almost 2.5 million procedures carried out in 2010.Citation3
There are several commercially available botulinum toxin type A products. OnabotulinumtoxinA (Botox®/Vistabel®; Allergan Inc, Irvine, CA) is indicated for the treatment of glabellar frown lines and is commonly used for the treatment of facial wrinkles.Citation4 The terms “incobotulinumtoxinA”, “NT 201”, “Xeomin®”, and “Bocouture®” (Merz Pharmaceuticals GmbH, Frankfurt, Germany) all refer to the same botulinum toxin type A (150 kDa) that, unlike onabotulinumtoxinA, is free from complexing proteins. IncobotulinumtoxinA is currently licensed widely across Europe, the US, and parts of South America and Asia for aesthetic indications. IncobotulinumtoxinA has demonstrated clinical efficacy in aesthetic indications in a number of clinical trials.Citation5–Citation7
In comparative, head-to-head trials in healthy volunteers, and in the therapeutic indications of blepharospasm and cervical dystonia, incobotulinumtoxinA had an identical time course of action (eg, time to onset, duration of effect, and time to waning of effect) as onabotulinumtoxinA.Citation8 Furthermore, in a large head-to-head study comparing incobotulinumtoxinA with onabotulinumtoxinA for the treatment of glabellar frown lines, the percentage of responders 4 and 12 weeks after injection of the same number of units (U) of either preparation were similar, and demonstrated that both treatments were highly effective according to the assessment of independent raters, investigators, and subjects.Citation7 No statistically significant difference in efficacy was observed in a proof-of-concept study in the treatment of crow’s feetCitation6 and in the treatment of forehead linesCitation9 using a 1:1 dose ratio of the two products.
The aim of this retrospective analysis was to investigate the clinical efficacy of incobotulinumtoxinA compared with onabotulinumtoxinA or abobotulinumtoxinA (Dysport®/Azzalure®; Ipsen Ltd, Berkshire, UK) when used in daily practice by physicians to treat wrinkles of the upper face. IncobotulinumtoxinA was launched in Germany in 2005, making it the best suited location for this study by ensuring sufficient data could be obtained for this analysis including in off-label indications.
Parameters relating to subject and physician satisfaction, the time interval between doses administered (duration between treatment cycles), dosages used, and adverse effects experienced were investigated. Any differences in these parameters may indicate that the product with lower subject and physician satisfaction, shorter interval between treatments, or higher dosages was less clinically effective.
Materials and methods
The parameters used as indicators of clinical efficacy in daily practice were subject and physician satisfaction, the time interval between injections, dosage, and adverse effects. In order to collect the relevant data to address these questions, physicians known to use incobotulinumtoxinA at 41 sites in Germany were contacted and asked to complete questionnaires () based on an inspection of files for those subjects who had received at least two consecutive botulinum toxin type A injections, but not more than three, in the upper face within 12 months during the last 2 years (ie, to treat glabellar frown lines, lateral periorbital wrinkles, and/or horizontal forehead lines, which includes common on- and off-label usage in clinical practice). A different questionnaire was filled in for each indication, giving a maximum of three completed questionnaires per subject. The selected subjects had therefore been treated according to the treatment flows shown in . For the analysis, subjects were divided into two groups: subjects who did not change product and subjects who changed product (irrespective of whether the change occurred at visit 2 or visit 3 or both).
Figure 1 Diagram showing treatment flow.
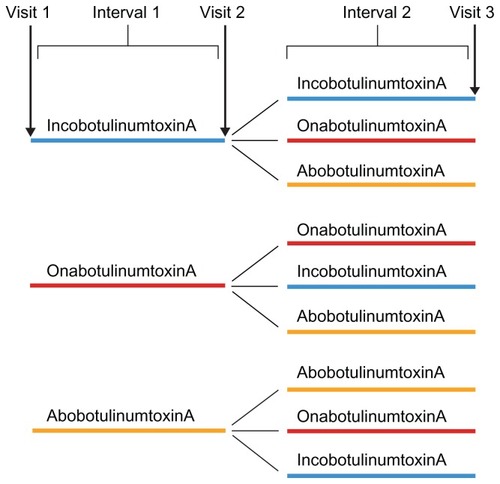
Table 1 Components of questionnaire completed by physicians
The documentation period was from March 2011 to June 2011 and data that would allow a subject to be identified were not collected. Male or female subjects aged 18 years and over who had received treatment with botulinum toxin type A were eligible for inclusion in this study. Initial treatments and touch-up treatments were reasons to exclude a subject from the study. No ethics committee approval was required for this retrospective study without invasive measures.
In daily practice, it is very common to treat “aesthetic units”, for example the “upper face”, rather than single isolated areas, such as forehead lines, the glabella or crow’s feet. Consequently, analysis was performed on all treatments of the upper face rather than single isolated areas. Differences between treatment groups were assessed using appropriate statistical analyses. Any differences in subject and physician satisfaction and adverse effects were analyzed using Fisher’s Exact test, while analysis of doses at each visit and treatment intervals used the Wilcoxon–Mann–Whitney test. The Student’s t-test was used to analyze the total mean dose across all visits. Data were collected from a sufficient number of subjects (n = 1256) to enable statistical analyses to be carried out.
Results
Of the 41 sites contacted, 21 sites returned completed questionnaires. In total, 2316 questionnaires were returned and 46 were excluded. Forty-five questionnaires were excluded because they only recorded one visit (rather than the required minimum of two) and one was excluded because it was a duplicate, leaving 2270 evaluable questionnaires. In total, data from 1256 subjects were included. Demographic and baseline characteristics are shown in . Subject numbers in the incobotulinumtoxinA and onabotulinumtoxinA treatment groups were sufficient to ensure that robust statistical data could be obtained, but the number of abobotulinumtoxinA injections was so low (1.6%, which might reflect daily practice in Germany), that no statistical evidence could be conveyed. Hence, these were removed from the analysis. Most subjects received incobotulinumtoxinA injections and the majority of subjects did not change product within the time limits of this retrospective analysis (). The most common reason for product change given was that the usual product was unavailable at the time of treatment.
Table 2 Demographic and baseline characteristics
Subject and physician satisfaction
The vast majority of subjects were satisfied with their treatment (96.4% for incobotulinumtoxinA and 95.8% for onabotulinumtoxinA). There was no statistically significant difference in subject satisfaction between the two products (P = 1.000). Similarly, the rates of physician satisfaction were also very high for both products: 96.3% and 95.3% were satisfied with incobotulinumtoxinA and onabotulinumtoxinA, respectively (P = 0.825).
Interval between two treatments
Any difference in the mean treatment interval (ie, the time between the first and second injections [interval 1] or the second and third injections [interval 2]; ) was assessed separately in subjects who did not change product in order to give an indication of the duration of the effect. There was no statistically significant difference between the treatment intervals in subjects who did not change product (). The mean length of interval 1 was 25.25 weeks and 24.90 weeks for subjects treated with incobotulinumtoxinA and onabotulinumtoxinA, respectively (P = 0.9646). For interval 2, the mean length was 22.43 weeks and 21.95 weeks, respectively (P = 0.8696).
Table 3 Average interval between treatments in the upper face for subjects without product change
Because the group of subjects who did change product included subjects who changed product at visit 2 or visit 3 or both, no conclusions could be drawn from any differences between the lengths of the treatment intervals in this group of subjects.
Dosage
In order to analyze the dosages administered, subjects were again divided into two groups: those who did not change product and those who did. Within these two groups, the mean dosage of each product was calculated at each visit. For the subjects who did not change product, the mean dosages for incobotulinumtoxinA and onabotulinumtoxinA were 18.92 U and 18.79 U at visit 1 (P = 0.4335), 18.12 U and 18.44 U at visit 2 (P = 0.4262), and 18.20 U and 18.94 U at visit 3 (P = 0.6900), respectively. In the group of subjects who did change product, the mean dosages for incobotulinumtoxinA and onabotulinumtoxinA at each visit were 17.48 U and 16.99 U (visit 1; P = 0.9138), 17.13 U and 18.63 U (visit 2; P = 0.3168), and 17.97 U and 18.11 U (visit 3; P = 0.7007), respectively. The mean total treatment dose for the upper face at each treatment visit did not differ significantly between incobotulinumtoxinA and onabotulinumtoxinA for subjects who did and those who did not change product (). Furthermore, there was no statistically significant difference between the mean total dose of incobotulinumtoxinA and onabotulinumtoxinA across all visits (P = 0.35).
Table 4 Mean total treatment doses in the upper face at each treatment visit for subjects with and without product change
Safety
Adverse effects were analyzed in subjects who did not change product in order to assess any differences in adverse effects experienced by subjects receiving either product. In these subjects, all the adverse effects were mild or moderate in intensity. There were no severe side effects reported for either product. Of the subjects treated only with incobotulinumtoxinA, 6.7% experienced adverse effects compared with 9.5% of those treated with onabotulinumtoxinA, though this was not a statistically significant difference (P = 0.178). Localized pain was reported in 2.2% of subjects receiving incobotulinumtoxinA and 3.4% of subjects receiving onabotulinumtoxinA while local hematoma was reported in 3.0% and 2.7% of subjects, respectively. Headache was reported by 1.7% of incobotulinumtoxinA-treated subjects and 2.0% of onabotulinumtoxinA-treated subjects.
Discussion
This retrospective study, comparing use of botulinum toxin type A preparations in daily clinical practice, analyzed subject and physician satisfaction, the time interval between doses administered (duration between treatment cycles), dosages used, and adverse effects experienced, and found no differences between incobotulinumtoxinA and onabotulinumtoxinA. The low number of abobotulinumtoxinA injections meant that they were excluded from the analysis. This low number presumably reflects the habits of the physicians who responded to the questionnaire. Amongst these physicians, abobotulinumtoxinA is the least commonly used of the three products included in this study. More subjects were treated with incobotulinumtoxinA compared with onabotulinumtoxinA due to the fact that mainly incobotulinumtoxinA injectors were approached. However, all physicians were equally proficient and experienced in administering all products and both the subject and physician satisfaction were similar for incobotulinumtoxinA and onabotulinumtoxinA.
Subject and physician satisfaction levels were similarly high for both products, and product change was rare and most often simply because the usual product was unavailable at the time, reflecting physician and subject confidence and satisfaction with both products.
The treatment intervals in subjects who did not change product were of a similar length. However, it should be noted that the length of time between two treatments is influenced by several factors, including financial and time constraints on the subject.
In this study no questions were asked relating to the reasons why subjects returned for subsequent treatments when they did, so the impact of the factors that may affect treatment interval could not be assessed here. In addition, the intention had been to analyze the different treatment areas separately, but this was not possible due to the confounding factor that allocation of individual injection points to a single isolated area was not necessarily possible (for example, some glabellar injection points also affect forehead lines and vice versa). Thus, the data were pooled and analyzed as “upper face”.
Similar clinical efficacy between incobotulinumtoxinA and onabotulinumtoxinA with regard to the other parameters tested suggests that these two products have similar clinical efficacy when used at the same dosage. In addition, the total dosages for both products were similar and low, as expected for cosmetic procedures in the face. Therefore, the results from this large retrospective study (n = 1256) are in line with published results of smaller prospective clinical studies which have shown similar efficacy of incobotulinumtoxinA compared with onabotulinumtoxinA in the treatment of glabellar frown lines in 381 subjects,Citation7 crow’s feet in 21 subjects,Citation6 and forehead lines in 12 subjectsCitation9 in aesthetic indications using identical dosages, as well as in therapeutic indications with similar dosages.Citation10,Citation11
The results from the large number of subjects presented in this retrospective analysis of daily clinical practice support those observed in prospective, randomized, controlled trials. Therefore, evidence from both routine clinical practice and the clinical trial setting suggests that incobotulinumtoxinA and onabotulinumtoxinA have similar clinical efficacy in therapeutic and aesthetic indications. In addition, the results of this retrospective analysis demonstrated that the identical dosage (20 U) of both products for the treatment of glabellar frown lines is appropriate.
Conclusion
In daily aesthetic practice, similar clinical efficacy between incobotulinumtoxinA and onabotulinumtoxinA in terms of subject and physician satisfaction, dosage given, and safety were observed. These data support comparable and similar therapeutic efficacy of these two products and clinicians may alternate between incobotulinumtoxinA and onabotulinumtoxinA as product availabilities dictate.
Acknowledgment
This retrospective analysis and publication were supported by Merz Pharmaceuticals GmbH.
Disclosure
WP has served as a consultant and lecturer for Allergan Inc, Merz Pharmaceuticals GmbH and Galderma Pharma SA. MI has served as a consultant, speaker, and investigator in clinical trials for Merz Pharmaceuticals GmbH. UK received honoraria as an investigator in clinical trials and speaker for Merz Pharmaceuticals GmbH. L-PK has spoken at workshops and training sessions for Merz Pharmaceuticals GmbH. WGP-D has acted as a consultant and lecturer for Allergan Inc, Merz Pharmaceuticals GmbH, and Galderma Pharma SA. TP has served as a consultant and speaker at workshops and meetings for Merz Pharmaceuticals GmbH and Galderma Pharma SA. She has received honoraria as an investigator in clinical trials for Merz Pharmaceuticals GmbH, Galderma Pharma SA, and Ipsen Pharma. Editorial assistance was provided by Ogilvy 4D and funded by Merz Pharmaceuticals GmbH.
References
- CarruthersAKieneKCarruthersJBotulinum A exotoxin use in clinical dermatologyJ Am Acad Dermatol1996345 Pt 17887978632076
- KleinAWCarruthersAFagienSLoweNJComparisons among botulinum toxins: an evidence-based reviewPlast Reconstr Surg20081216413e422e
- American Society for Aesthetic Plastic SurgeryThe American Society for Aesthetic Plastic Surgery Statistics Available from: http://www.surgery.org/media/statisticsAccessed November 9, 2011
- FlynnTCAdvances in the use of botulinum neurotoxins in facial estheticsJ Cosmet Dermatol2012111425022360334
- ImhofMKühneUA Phase III study of incobotulinumtoxinA in the treatment of glabellar frown linesJ Clin Aesthet Dermatol2011410283422010053
- PragerWWissmüllerEKollhorstBWilliamsSZschockeIComparison of two botulinum toxin type A preparations for treating crow’s feet: a split-face, double-blind, proof-of-concept studyDermatol Surg201036Suppl 42155216021134046
- SattlerGCallanderMGrablowitzDNoninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown linesDermatol Surg201036S42146215421134045
- JostWHBlumelJGrafeSBotulinum neurotoxin type A free of complexing proteins (Xeomin) in focal dystoniaDrugs200767566968317385940
- Oliveira de MoraisOMatos Reis-FilhoEVilela PereiraLMartins GomesCAlvesGComparison of four botulinum neurotoxin type A preparations in the treatment of hyperdynamic forehead lines in men: a pilot studyJ Drugs Dermatol201211221621922270205
- BeneckeRJostWHKanovskyPRuzickaEComesGGrafeSA new botulinum toxin type A free of complexing proteins for treatment of cervical dystoniaNeurology200564111949195115955951
- RoggenkämperPJostWHBihariKComesGGrafeSEfficacy and safety of a new Botulinum toxin type A free of complexing proteins in the treatment of blepharospasmJ Neural Transm2006113330331215959841