Abstract
Autoimmune and inherited bullous disorders are rare skin diseases that may have a profound negative impact on quality of life (QOL). Common symptoms include pain, pruritus, and scarring, and complications may result in the loss of the ability to perform daily tasks. Diagnosis may have a negative psychological impact, and ongoing management may require a significant allocation of time and resources by both patients and providers. To provide patient-centered care, consideration of these factors is of utmost importance for the dermatologist treating patients with bullous disorders. Herein, we present a review of the primary literature evaluating QOL in autoimmune and inherited bullous disorders, including pemphigus, pemphigoid, epidermolysis bullosa, and Hailey-Hailey disease.
Introduction
Autoimmune and inherited bullous disorders represent rare mucocutaneous diseases that may have a significant negative impact on patients’ quality of life (QOL).Citation1,Citation2 There are various physical, social, and psychiatric factors that contribute to patients’ perceived QOL. In order to provide patient-centered care, consideration of these factors is of utmost importance in the treatment of patients with bullous disorders.
In patients with bullous disorders, QOL may be assessed through general medical and dermatology-specific indices, as well as through more specific instruments as evidenced in . Which QOL assessment best represents patients’ experiences remains controversial. For example, Patsatsi et al demonstrated the Autoimmune Bullous Disease Quality of Life (ABQOL) correlated with QOL over time, while Dermatology Life Quality Index (DLQI) assessments did not.Citation3 In contrast, Ferries et al prospectively assessed the correlation between disease severity scores in pemphigus (Pemphigus Disease Area Index, PDAI), bullous pemphigoid (Bullous Pemphigoid Disease Area Index, BPDAI), and mucous membrane pemphigoid (Mucous Membrane Pemphigoid Disease Area Index, MMPDAI) to ABQOL, Treatment of Autoimmune Bullous Disease Quality of Life (TABQOL), DLQI, and Skindex-29 scores. They concluded that there may be no advantage of the ABQOL over the DLQI or Skindex-29.Citation4
Table 1 A Brief Overview of Frequently Used QOL Assessment Tools
An informed understanding of the complexities of how autoimmune and inherited bullous disorders affect QOL is critical to providing patient-centered care. This facilitates shared decision-making between patient and provider and is essential to develop a long-term therapeutic strategy for these chronic diseases. Herein, we review the primary literature evaluating QOL in autoimmune and inherited bullous disorders, including pemphigus, bullous pemphigoid (BP), epidermolysis bullosa (EB), and Hailey-Hailey disease (HHD).
Methods
A primary literature review was conducted in English using the NCBI database (PMC and PubMed filters) using the keywords “quality of life” AND “bullous,” “pemphigus,” “pemphigoid,” “epidermolysis,” “Hailey-Hailey,” “blister,” OR “blistering.” There were no limitations set on date of publication. To be included, articles must have included QOL data specific to a blistering disease. Non-primary literature, such as reviews, meta-analyses, and commentary articles lacking original evidence, were excluded. Two independent authors (JJP and RLS) evaluated titles and abstracts of the articles retrieved during the search. The full article was evaluated if titles and abstracts met the eligibility criteria, or if they did not provide enough information to enable a decision regarding eligibility to be made. Inclusion was be determined once the full text was read. After review, a total of 36 articles were identified ().
Figure 1 PRISMA flow diagram for the primary literature review detailing the database searches, the number of abstracts screened, and the full texts retrieved.
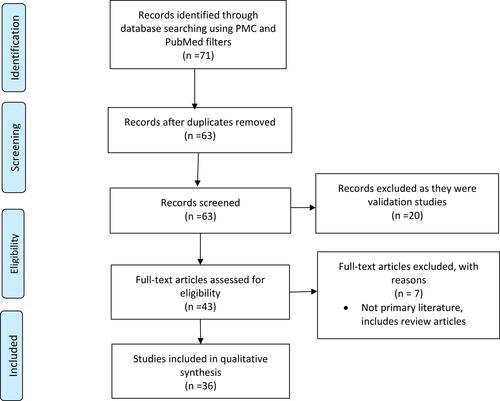
Given practical considerations among clinicians and patients with blistering diseases during the COVID-19 pandemic, a brief review of additional considerations for the clinician managing this patient population was also included.
Discussion
Autoimmune Bullous Disorders
Autoimmune bullous disorders (AIBD) are acquired diseases resulting from immunologic activity targeting constituents of the skin or mucosa. The spectrum of disease is broad and may range from isolated cutaneous erosions and vesicles to potentially deadly, diffuse sloughing of broad mucocutaneous surfaces. Numerous studies have aimed to assess the impact of AIBD on QOL as outlined in . For example, a study by Penha et al demonstrated severely impaired QOL in 84 Brazilian patients with various AIBD evidenced by a median DLQI score of 16.0.Citation2 The greatest impact was noted on symptoms/feelings and daily/leisure activities.Citation2 This suggests that the impact of AIBD on QOL may be diverse. Historically the emphasis in evaluating QOL was on disease severity but there may be a relationship to disease subtype as well. Here we review studies evaluating QOL in pemphigus, in which QOL has been most studied; BP, the most common AIBD; and other studies which evaluate patients with multiple AIBD subtypes.
Table 2 Review of Literature of QOL in AIBD, PV, and BP
Pemphigus
Pemphigus is a family of autoimmune blistering conditions related to loss of adhesion between keratinocytes. In these conditions, acantholysis, caused by autoantibodies targeting intercellular adhesion molecules, leads to intraepithelial blister formation. Pemphigus vulgaris (PV), the most common form of pemphigus, is associated with epidermolysis above the stratum basalis, resulting in flaccid blisters and erosions. In contrast, pemphigus foliaceus (PF) is associated with superficial erosions and crusting due to epidermolysis within the upper stratum spinosum or granulosum. Less common subtypes of pemphigus include pemphigus vegetans, IgA pemphigus, paraneoplastic pemphigus, and drug-induced pemphigus.
SF-36
In 2005, Terrab et al first used the SF-36 in a population of 30 pemphigus patients (PV n=14, seborrheic pemphigus n=10, PF n=4, pemphigus vegetans n=2) and found a significant decrease in all mean scores relative to healthy controls.Citation5 Most prominent score differences were noted in physical and emotional status suggesting that physical limitations and emotional frustrations may be correlated. These findings also stress the importance of assessing these domains in particular when evaluating patient’s QOL. Among a larger population of pemphigus patients (n=139, PV n=112, PF n=10, other n=4), Paradisi et al found SF-36 scores varied by demographic and disease severity.Citation6 Specifically, investigators observed worse physical and mental component scores as measured by limitations in daily activities and feelings of depression in women as compared to men. Further studies are needed to determine why this disparity exists. They also noted lower average scores in patients with 3–4 years’ disease duration and patients >50 years old suggesting that effect on QOL may have a cumulative effect and that there are specific gender- and age-related factors to consider.Citation6 A meta-analysis of 7 studies using SF-36 in pemphigus patients found the most affected dimensions of SF-36 were role-functioning physical (RP), role-emotional (RE), and vitality (VIT).Citation7 This study highlights the significant disparities in QOL findings among patients with similar characteristics using the SF-36 and Skindex-29 instruments. This disparity is likely due to lack of pemphigus-specific assessments in these instruments and these patients may benefit from the use of more specific assessment tools. Average SF-36 scores and conclusions across multiple studies are detailed in .
DLQI
A German study in 2005 examined 36 patients with a new diagnosis of PV across multiple sites and investigators determined that newly diagnosed PV patients had an elevated average DLQI score at 10 ± 6.7 as compared to other skin diseases indicating that PV had a greater impact on QOL.Citation3 Ghodsi et al found a similar mean DLQI score of 10.9 ± 6.9 in 61 newly diagnosed untreated PV patients in Tehran using the Persian DLQI.Citation9 The highest subscores were related to symptoms/feelings (2.8) and daily activities (2.2). Investigators found that DLQI score was significantly increased in patients with severe disease, mucosal involvement, positive Nikolsky sign, and itching. Disease severity and extent of symptoms likely affect ability to partake in daily activities and therefore result in lower QOL. A negative correlation between DLQI score and duration of disease was also noted suggesting increased impairment in the initial stages of the disease. This is further supported by Wysocynska et al who reported an average DLQI of 4.0 ± 5.9 in a patient population mainly composed of patients with a >5 years of disease.Citation10 Patients likely undergo an adjustment period upon initial diagnosis which affects QOL scores early on in disease course. In 2015, a meta-analysis across four studies surrounding QOL in pemphigus patients found a mean DLQI of 12.0 (95% CI 11.1–12.9) with symptoms/feelings and daily activities subscores most consistently affected.Citation7 A summary of DLQI scores reported in the literature evaluating QOL in pemphigus is noted in .
Skindex-29
In 2009, Paradisi et al performed the first large study implementing the Skindex-29 among pemphigus patients.Citation6 They assessed 112 PV patients who had mean scores of 36 in both the symptoms and emotions domains. They also noted 10 PF patients who had the highest mean scores of 52 in both the symptoms and social functioning domains.Citation6 A meta-analysis of 4 studies found similar mean scores in the symptoms and emotion domains, while slightly lower scores for social functioning.Citation7 These findings suggest that symptoms and emotional disturbance are most implicated in negative effect on QOL. In a follow-up study, Paradisi et al examined 112 pemphigus patients for treatment-related differences in QOL and found no significant difference in Skindex-29 scores.Citation11 It is unlikely that different treatment approaches have significant impact on QOL. These findings suggest that the utility of the Skindex-29, like the SF-36 and DLQI, may be limited by its lack of disease specificity or focus on mucosal involvement. Focused assessment of mucosal involvement may provide a more accurate representation of the effect of disease on QOL in conditions that significantly involve mucous membranes.
EQ-5D
The European Quality of Life Five Dimension (EQ-5D) is a tool that was developed in Europe and used to measure QOL by assessing five dimensions; mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. One study has investigated the role of the EQ-5D in assessing QOL in pemphigus patients. Tamasi et al evaluated 109 patients with either PV or PF using the EQ-5D and found that the top three dimensions affected were pain/discomfort (50%), mobility (43%), and anxiety/depression (43%).Citation12 There was no significant difference in EQ-5D scores of PF versus PV patients. EQ-5D scores significantly varied by disease severity and the number of comorbidities suggesting that these factors play a role not only in disease-related symptoms but psychosocial factors as well both of which negatively impact QOL. Compared to the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS), EQ-5D scores were better correlated with DLQI scores and average reported pain intensity.Citation12 This suggests that the EQ-5D may be a valid measure of QOL in pemphigus patients, although comparison studies to disease-specific QOL instruments should be pursued in the future.
ABQOL and TABQOL
The ABQOL and TABQOL are evaluation instruments validated for assessing QOL specifically in patients with AIBD. These tools specifically assess patient concerns for mucosal involvement such as relapse and flares, swelling associated with bullae, and the need to change clothing due to drainage from lesions. Assessment of these parameters makes these tools especially useful in assessing bullous disorders. Krain et al examined how the ABQOL, DLQI, Skindex-29, and SF-36 correlate to pemphigus-specific severity indices such as Pemphigus Disease Area Index (PDAI) and ABSIS.Citation13 After surveying 50 pemphigus patients, the change in PDAI showed a strong correlation (r=0.60–0.79) with changes in the ABQOL, Skindex-S, and Skindex-F subscales for all patients suggesting that each assessment is sensitive to symptom changes.Citation13 Therefore, any of these tools can be reliably used longitudinally to monitor patient progress. For patients with mucosal involvement (n=24), the change in PDAI showed a strong correlation with changes in the ABQOL and Skindex-S subscale suggesting that these assessments may be particularly useful in evaluating diseases with mucosal involvement.Citation13 In regards to treatment outcomes, Bax et al performed a retrospective study evaluating patients with PV and suggested that even a small amount of disease activity may have a significant impact on QOL.Citation14 This suggests that even if disease activity appears to be clinically low, patients may still experience a reduction in QOL.
Effect of Treatment on QOL
Treatment of AIBD often requires travel to treatment facilities often located in urban centers, which may be burdensome for patients living in rural regions, and treatment of the disease itself may carry significant burdens. The TABQOL is a unique tool used to assess treatment burdens on QOL, which have previously been under-addressed. In a study performed by Schultz et al, the ABQOL and TABQOL were used to examine QOL in 235 PV patients treated with rituximab versus non-rituximab modalities. Results demonstrated no difference in QOL among participants treated with rituximab versus those not.Citation15 This data suggests that ABQOL and TABQOL were either not sensitive enough to discern differences in the examined population or that there is no net benefit between these treatment modalities on QOL in PV patients.Citation15 In contrast, Joly et al found that the DLQI and Skindex-29 scores showed greater improvements in patients assigned to rituximab plus short-term prednisone as compared with those receiving prednisone alone (p=0.0411 and p=0.0137, respectively), suggesting that patients receiving rituximab had improved QOL as compared to the non-rituximab modalities.Citation16 In 2021, Werth et al compared rituximab and mycophenolate mofetil in achievement of remission rates. They noted that the estimated mean change from baseline DLQI score was −8.87 points in the rituximab group and −6.00 points in the mycophenolate mofetil group, and a post hoc analysis revealed that 62% of the patients who received rituximab had a DLQI score of 0 (suggesting no disease-related effects on QOL), while only 25% of patients receiving mycophenolate mofetil reported a DLQI score of 0.Citation17 The variations in results suggest the need for further research surrounding the effect of treatment approaches on QOL. Based on current data, we recommend an individualized patient-focused treatment approach specifically taking into account factors such as comorbidities and immunosuppression risk factors, as well as mobility issues, missed work, and commute time. A review of the literature surrounding the use of the ABQOL and TABQOL for assessing QOL is included in .
Mental Health
Several studies have assessed psychiatric comorbidities in pemphigus patients. Findings to date vary by assessments used and populations examined. One report using the GHQ-28 on 61 patients with newly diagnosed untreated PV found that more than 77% of patients experienced anxiety and depression.Citation9 Another study of 66 Korean pemphigus patients found that 47% had a positive GHQ indicating likely comorbid psychiatric conditions.Citation18 The Hamilton Rating Scale for Depression and Anxiety (HAM-D, HAM-A) is a 17 item clinician-administered questionnaire that evaluates symptoms of depression experienced over the preceding week. Using the HAM-D questionnaire, Calabria et al compared 30 patients with oropharyngeal PV (OPV) to healthy controls and showed higher HAM-A and HAM-D scores in patients with OPV.Citation19 Moreover, significant sleep impairment was observed in the OPV group, as demonstrated by elevated Pittsburgh Sleep Quality Index (PSQI) scores. Paradisi et al demonstrated that psychiatric comorbidity was associated with worse QOL in pemphigus patients based on SF-36, Skindex-29, and the GHQ-12 scores.Citation11 Segal et al demonstrated that the investigated 58 pemphigus patients had realistic illness perception and high perceived social support.Citation20 This study also concluded that patients had an improved QOL when they demonstrated understanding of the chronic nature of their condition. This suggests that mental health comorbidities and impaired QOL observed in pemphigus patients may not be as strongly related to the perception of illness or perceived level of social support. Additionally, there is likely benefit to disease education and illness expectation setting at the time of diagnosis.
Pemphigoid
Pemphigoid describes a family of autoimmune blistering diseases characterized by immunoglobulin and complement deposition within the epidermal and/or mucosal basement membrane zone, resulting in subepithelial blisters. BP and mucous membrane pemphigoid (MMP) are most commonly discussed, while pemphigoid gestationis, anti-p200 pemphigoid, and others are less common. BP presents with subepidermal blistering with rare oral involvement. In contrast, MMP more often presents with smooth-bordered mucosal erosions that result in scarring and is less likely to involve cutaneous surfaces.
Bullous Pemphigoid
Two studies have investigated QOL in BP patients. A case-control study examining QOL by DLQI, anxiety, and depression in 57 BP patients compared to healthy controls. The Hospital Anxiety Depression (HADS) is a 14-item assessment tool assessed on a Likert Scale measuring anxiety and depression with higher scores indicating more symptoms. Investigators reported a mean DLQI score of 9.45 ± 3.34, and a significant difference in the total (13.68 ± 5.66 in the BP group and 11.85 ± 3.84 in the control group) and depression subscale (7.77 ± 2.36 in BP group and 6.42 ± 2.09 in the control group) of the HADS assessment.Citation21 Kouris et al found no difference in the HADS-anxiety subscale between groups. Further studies are needed to explore the effect of BP on the severity of depression and anxiety. BP patients had a higher perceived sense of loneliness as indicated by significantly elevated Loneliness Scale-Version 3 (UCLA) scores compared to controls.Citation21 Although mixed, these results suggest impaired QOL and increased mental health comorbidities in BP patients. A second study investigated the role pruritus plays in QOL of 60 French BP patients using the 5-D Itch Scale and the ItchyQOL.Citation22 Results showed that 85% of patients experienced pruritus daily, at a mean severity of 5.2/10. Mean ItchyQOL score was 56.2/100 and 5-D Itchy scale score was 16.5/25 indicating significant QOL impairment due to pruritus which had not been specifically assessed previously. A further review of QOL assessments is outlined in .
Studies Including Multiple AIBD Subtypes
Larger reviews have evaluated multiple AIBD subtypes and their effect on QOL. Ferries et al demonstrated severely impaired QOL in 164 patients with pemphigus, BP, and MMP across multiple sites in France.Citation4 Investigators found that the ABQOL correlated with DLQI and Skindex-29 scores, and weakly correlated with changes in the PDAI, BPDAI, and MMPDAI. This suggests that the use of ABQOL, DLQI and Skindex-29 are useful in assessing QOL in patients with AIBD. The ABQOL and PDAI were more closely correlated in pemphigus and BP patients than in patients with MMP.Citation4 This was attributed to the smaller number of questions specifically focused on mucosal involvement, which may be required to better assess MMP. Heelan et al found a comparably lower mean DLQI of 6.5 ± 7.3 among 94 patients with AIBD defined as having PV, PF, IgA pemphigus, BP, MMP, epidermolysis bullosa acquisita, or lichen planus pemphigoides, suggesting a moderate effect on QOL.Citation1 They did, however, determine that patients with a higher DLQI score had greater work and overall activity impairment, providing initial evidence that AIBD may not only affect QOL but also work productivity.
In 2019, Bilgic et al examined 67 BP and PV patients for oral health-related QOL using the Oral Health Impact Profile-14 (OHIP-14). They used ABSIS scores to evaluate disease severity with a score of <17 suggestive of a moderate course and a score >17 suggestive of a significant course. They then used OHIP-14 scores to assess QOL. They found that OHIP-14 scores were higher in active patients with an average score of 42.28 ± 13.66 as compared to inactive patients with average scores of 29.08 ± 12.25.Citation22 Higher OHIP-14 scores correlated with pain scores.Citation23 This study may suggest oral health is a significant factor in the QOL of BP and PV patients, but large-scale conclusions were limited by response bias. Close monitoring of oral involvement and inclusion of dentists in the multidisciplinary approach of care should be strongly considered and may result in improved QOL outcomes.
Inherited Bullous Disorders
Epidermolysis Bullosa
Although there are many forms of inherited bullous disorders, much of the current literature focuses on epidermolysis bullosa (EB). EB is an inherited skin fragility disorder characterized by structural disruptions at the dermo-epidermal junction or in the basal epidermis. These disruptions result in increased skin fragility. Although multiple phenotypes exist, the most commonly described are epidermolysis bullosa simplex (EBS), junctional epidermolysis bullosa (JEB), and dystrophic epidermolysis bullosa (DEB). EBS is the most common type of EB, and is characterized by trauma- or friction-induced superficial skin blistering, erosions and crusting, most commonly caused by an autosomal dominant, negative missense mutation in keratinocyte proteins KRT5 and KRT14. Patients may exhibit localized (EBS-l), intermediate, or severe EBS. In contrast, DEB subtypes have a disruption in type VII collagen synthesis, classified by a mutation in COL7A1 gene.
Effect of Disease on QOL
Since the publication of the QOLEB, several studies have analyzed QOL in EBS adult patients, with mean scores ranging from 7.9 ± 5.3/51 to 13.7 ± 8.7/51.Citation24–Citation26 In children, Zigmond et al found a mean score of 15/30 in the Children’s Dermatology Life Quality Index (cDLQI).Citation27 In a subsequent study, Joen et al evaluated 16 patients using VAS, QOLEB, and total Skindex-29 scores. Mean QOLEB score was 26.62 ± 7.61 and higher scores were observed in female patients, patients with hospitalization greater than 7 days, and severe generalized dystrophic epidermolysis bullosa (RDEB-gen sev).Citation28 Although these findings were not statistically significant in this study, they suggest that a gender disparity may exist and that increased disease severity as evidenced by prolonged hospitalization likely worsens QOL outcomes. In 2017, Brun et al evaluated 57 patients in France with EBS and found that 73% of patients reported a moderate to severe impact on their QOL using the cDLQI for children and QOLEB for adults.Citation29 The mean QOLEB score was 6.6 ± 4.9/51.Citation29 They found that 87% of patients felt frustrated, 27% embarrassed, 17% depressed, 33% uncomfortable, and 40% anxious or worried by their disease.Citation29 Mean cDLQI score was 8.1 ± 5.1/30 with 76% reporting that QoL was affected by pain, 56% felt sad, and 52% had to decrease or stop any physical activity because of pain.Citation29 These findings suggest a significant psychosocial and emotional effect of disease on QOL and stress the importance of close screening for psychiatric comorbidities. In 2020, Togo et al performed a systematic review of 12 articles and concluded that women and children suffering from EB require closer monitoring than other groups, suggesting the importance of adjusting monitoring based on the demographic of the group treated.Citation30 Further studies are needed to assess the differences in QOL disturbance amongst various demographic groups. A further review of QOL assessments is outlined in .
Table 3 Review of Literature of QOL in EB, Various EB Subtypes and HHD
Effect of Chronic Wounds on QOL
Patients with EB may develop long-lasting, disfiguring wounds, which likely have a significant negative impact on QOL. Eng et al supported this by showing a correlation between larger wound size with worsening skin disease severity and worse QOL in 39 participants with RDEB.Citation31 The mean QOLEB score in their study was 20.0 ± 9 points.Citation31 Similarly, Tabolli et al found a correlation between increased EB body surface area involvement, worsened QOL, and patient-perceived severity of disease using the SF-36 and Skindex-29 assessments.Citation32 However, SF-36 mental components scores were similar in patients suffering from EB as those of the normal population.Citation32,Citation33 This suggests that although wound size has an effect on QOL, wound size does not appear to directly affect mental health comorbidities.
Although RDEB is more commonly studied, Fulchand et al performed Medical Profile and QOLEB surveys in patients with dominant DEB (DDEB) and found that self-reported severity of disease correlated with the severity of pain in the last 12 months (3.4 with mild disease vs 6.8 with severe disease on medical profile, p=0.0002) and a trend toward worse QOLEB score (33.4 vs 24.9 respectively, p=0.09) when compared with mild severity participants. The severity of self-reported disease did not correlate with the size of wounds or the number of dressing changes.Citation34 Additionally, they noted that patients with severe DDEB had more severe internal disease symptoms, such as difficulty swallowing (62.5%, p=0.01), and greater analgesic use during dressing changes (4.4% mild vs 81.3% severe, p<0.001), as compared with mild DDEB.Citation34 Increased severity of disease and greater systemic involvement correlate to worsened QOL.
Given the potential psychiatric consequences of inherited bullous disorders, it is important to assess for coexistence of mental health comorbidities, which may affect treatment and overall clinical outcomes. Margari et al used clinical interviews and standardized diagnostic protocols according to age to assess the frequency of psychiatric symptoms. They noted a high frequency of psychiatric symptoms (80%) in patients suffering from EB, but a relatively small percentage (12%) who had undergone psychopharmacological or psychotherapeutic treatments.Citation35 This disparity between psychiatric symptoms and treatment provides an area of focus that if addressed, could improve patients’ QOL substantially.
Hailey-Hailey Disease
HHD, also known as benign familial pemphigus, benign chronic pemphigus, is an autosomal dominant, intraepidermal blistering disorder that affects keratinocyte adhesion caused by a loss-of-function mutation in the ATP2C1 gene. It is characterized by painful blistering with subsequent erosions and frequent superficial infections of flexural surfaces.
A single study investigated the QOL in patients with HHD (). The study used the Skindex-29 to assess 20 patients and found that the effect on QOL was substantial, with mean Skindex-29 symptom scores at 57.1 and 60.7 with <4 and >/= 4 sites affected respectively.Citation36 This study implied that a physician’s evaluation may not correlate with a patient’s perceived handicap and effect on QOL, therefore aggressive treatments may be warranted even in patients who display seemingly low disease activity.
Considerations of Bullous Diseases During the COVID-19 Pandemic
The ongoing COVID-19 pandemic has resulted in many concerns surrounding the management of blistering disorders. The utility of telemedicine and virtual visits has become increasingly popular, especially for immunosuppressed AIBD patients, and has become a useful strategy to decrease exposure risk.Citation37–Citation39,Citation40 There remains concern over the use of immunosuppressive and immunomodulating treatment and the risk of acquiring SARS-CoV-2 infection. Joly et al noted a higher risk of COVID-19 infection in patients with AIBD among 59 patients in France.Citation39 Some providers propose postponing immunosuppressive or immunomodulatory therapies and tapering adjunctive therapies to the lowest effective dose, while other providers state that withdrawal of disease-controlling agents could result in uncontrolled disease activity.Citation41–Citation43
Mahmoudi et al found that patients receiving greater than 10 mg/day of prednisolone had a higher risk of COVID-19 and hospitalization.Citation44 Additionally, they noted that with each passing month after rituximab infusion, the patient’s risk of complication decreased.Citation44 On the contrary, Kridin et al found that the risk of developing COVID-19 (p=0.496), COVID-19-associated hospitalization (p=0.499), and COVID-19-associated mortality (p=0.789), was similar in patients with pemphigus and the age, sex, and ethnicity-matched healthy control group suggesting that the use of systemic corticosteroids and immunosuppressive adjuvant agents was not associated with worse clinical outcomes.Citation45 However, they found the risk of COVID-19-associated mortality was higher among patients with BP (p=0.023) as compared to the same matched healthy control subjects.Citation45 Although immunosuppressive and immunomodulating treatments may carry an increased risk of infection, a patient-centered approach advocates for shared decision-making, with a thorough discussion of the risks, benefits, and alternatives to treatment with each patient.Citation46
There have been rare reports of flares or new-onset AIBD triggered by the COVID-19 vaccination.Citation47–Citation49 Although reports are limited, other AIBD may carry a similar risk. This is not unique to the COVID-19 vaccine, as flares of AIBD have been reported from other vaccines.Citation50–Citation55 In addition, flares and new-onset AIBD have been reported from natural COVID-19 infections.Citation56–Citation59 Despite the small risk of disease onset or flare with vaccination, individual and public health benefits may outweigh this risk for most patients.
Conclusions
QOL is an important clinical outcome and should be monitored closely especially in patients suffering from blistering disorders such as AIBD, PV, BP, pemphigoid, EB, and HHD. A variety of validated instruments are available for the clinician to monitor QOL. Some of the primary assessment tools used include DLQI, GHQ-28, SF-36, Skindex-29, ABQOL, TABQOL, QOLEB, InTo-DermQOL. Based on the current evidence, it appears that the ABQOL and TABQOL may be more sensitive in assessing patients with AIBD, particularly those with mucocutaneous disease because there are questions specifically addressing mucosal involvement. The TABQOL is a unique assessment tool that assesses QOL burdens related to treatment. Among inherited blistering disorders, the QOLEB and InTo-DermQOL were developed specifically to assess adults and children with EB, respectively.
Although many QOL instruments have been validated in numerous languages, there are important limitations. For example, there may be a disparity between clinical disease severity and perceived QOL, which supports the regular use of both sets of instruments to accurately assess the impact of disease on patients’ lives. Frequent monitoring of QOL and ensuring appropriate supports are in place are critical to maintaining patient-centered care. Because bullous disorders affect multiple organ systems and may negatively impact mental health, a multidisciplinary approach including mental health providers, primary care physicians, and when relevant, other specialty providers, should be incorporated to improve patients’ overall QOL. In addition, providers should not forget the positive role that many patient support groups have on QOL (eg The International Pemphigus and Pemphigoid Foundation, The Rare Illness Network, and Dystrophic Epidermolysis Bullosa Research Association “DebRA” International).
Finally, while COVID-19 vaccination, like other forms of vaccination as well as natural infection, may carry a risk of disease flare, we believe that the individual and public health benefits of vaccination typically outweigh potential risks. An informed, individualized discussion should be undertaken to better assess these factors for each patient.
Disclosure
Dr David Pearson reports personal fees from Biogen Inc, outside the submitted work. The authors report no other potential conflicts of interest for this work.
Additional information
Funding
References
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19(6):546–554. doi:10.1177/1203475415582317
- Penha MÁ, Farat JG, Miot HA, Barraviera SR. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90(2):190–194. doi:10.1590/abd1806-4841.20153372
- Patsatsi A, Kokolios M, Kyriakou A, et al. Quality of life in Greek patients with autoimmune bullous diseases assessed with ABQOL and TABQOL indexes. Acta Derm Venereol. 2017;97(9):1145–1147. doi:10.2340/00015555-2737
- Ferries L, Gillibert A, Duvert-Lehembre S, et al. Sensitivity to change and correlation between the autoimmune bullous disease quality-of-life questionnaires ABQOL and TABQOL, and objective severity scores. Br J Dermatol. 2020;183(5):944–945. doi:10.1111/bjd.19173
- Terrab Z, Benchikhi H, Maaroufi A, Hassoune S, Amine M, Lakhdar H. Qualité de vie et pemphigus [Quality of life and pemphigus]. Ann Dermatol Venereol. 2005;132(4):321–328. doi:10.1016/s0151-9638(05)79276-0
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60(2):261–269. doi:10.1016/j.jaad.2008.09.014
- Rencz F, Gulácsi L, Tamási B, et al. Health-related quality of life and its determinants in pemphigus: a systematic review and meta-analysis. Br J Dermatol. 2015;173(4):1076–1080. doi:10.1111/bjd.13848
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Deutliche Einschrankung der Lebensqualitat bei Patienten mit Pemphigus vulgaris: ergebnisse der deutschen Bullous Skin Disease (BSD)-Studiengruppe [Significant decrease in quality of life in patients with pemphigus vulgaris. Results from the German Bullous Skin Disease (BSD) Study Group]. J Dtsch Dermatol Ges. 2005;3(6):431–435. doi:10.1111/j.1610-0387.2005.05722.x
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, Valikhani M, Esmaili N. Quality of life and psychological status of patients with pemphigus vulgaris using dermatology life quality index and general health questionnaires. J Dermatol. 2012;39(2):141–144. doi:10.1111/j.1346-8138.2011.01382.x
- Wysoczyńska K, Żebrowska A, Waszczykowska E. Quality of life in patients with pemphigus. Dermatology Review/Przeglad Dermatologiczny. 2013;100(3):139–145.
- Paradisi A, Cianchini G, Lupi F, et al. Quality of life in patients with pemphigus receiving adjuvant therapy. Clin Exp Dermatol. 2012;37(6):626–630. doi:10.1111/j.1365-2230.2011.04282.x
- Tamási B, Brodszky V, Péntek M, et al. Validity of the EQ-5D in patients with pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2019;180(4):802–809. doi:10.1111/bjd.16883
- Krain RL, Kushner CJ, Tarazi M, et al. Assessing the correlation between disease severity indices and quality of life measurement tools in pemphigus. Front Immunol. 2019;10:2571. doi:10.3389/fimmu.2019.02571
- Bax CE, Ravishankar A, Yan D, et al. Identifying the required degree of disease clearance to improve quality of life in pemphigus vulgaris. Br J Dermatol. 2021;184(3):573–575. doi:10.1111/bjd.19625
- Schultz B, Latour E, Fett N. Quality of life remains poor for patients with pemphigus vulgaris despite targeted therapies. Br J Dermatol. 2019;181(5):1101–1103. doi:10.1111/bjd.18167
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031–2040. doi:10.1016/S0140-6736(17)30070-3
- Werth VP, Joly P, Mimouni D, et al. Rituximab versus mycophenolate mofetil in patients with pemphigus vulgaris. N Engl J Med. 2021;384(24):2295–2305. doi:10.1056/NEJMoa2028564
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27(5):492–498. doi:10.5021/ad.2015.27.5.492
- Calabria E, Adamo D, Leuci S, et al. The health-related quality of life and psychological profile in patients with oropharyngeal Pemphigus Vulgaris in complete clinical remission: a case-control study. J Oral Pathol Med. 2021;50(5):510–519. doi:10.1111/jop.13150
- Segal O, Goldzweig G, Tako E, Barzilai A, Lyakhovitsky A, Baum S. Illness perception, perceived social support and quality of life in patients with pemphigus vulgaris: what should dermatologists know? Acta Derm Venereol. 2021;101(4):adv00441. doi:10.2340/00015555-3785
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid. A case control study. An Bras Dermatol. 2016;91(5):601–603. doi:10.1590/abd1806-4841.20164935
- Briand C, Gourier G, Poizeau F, et al. Characteristics of pruritus in bullous pemphigoid and impact on quality of life: a Prospective Cohort Study. Acta Derm Venereol. 2020;100(18):adv00320. doi:10.2340/00015555-3683
- Bilgic A, Aydin F, Sumer P, et al. Oral health related quality of life and disease severity in autoimmune bullous diseases. Niger J Clin Pract. 2020;23(2):159–164. doi:10.4103/njcp.njcp_216_19
- Frew JW, Martin LK, Nijsten T, Murrell DF. Quality of life evaluation in epidermolysis bullosa (EB) through the development of the QOLEB questionnaire: an EB-specific quality of life instrument [published correction appears in Br J Dermatol. 2010 Mar;162(3):701]. Br J Dermatol. 2009;161(6):1323–1330. doi:10.1111/j.1365-2133.2009.09347.x
- Cestari T, Prati C, Menegon DB, et al. Translation, cross-cultural adaptation and validation of the quality of life evaluation in epidermolysis bullosa instrument in Brazilian Portuguese. Int J Dermatol. 2016;55(2):e94–e99. doi:10.1111/ijd.12819
- Yuen WY, Frew JW, Veerman K, van den Heuvel ER, Murrell DF, Jonkman MF. Health-related quality of life in epidermolysis bullosa: validation of the Dutch QOLEB questionnaire and assessment in the Dutch population. Acta Derm Venereol. 2014;94(4):442–447. doi:10.2340/00015555-1758
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
- Jeon IK, On HR, Kim SC. Quality of life and economic burden in recessive dystrophic epidermolysis bullosa. Ann Dermatol. 2016;28(1):6–14. doi:10.5021/ad.2016.28.1.6
- Brun J, Chiaverini C, Devos C, et al. Pain and quality of life evaluation in patients with localized epidermolysis bullosa simplex. Orphanet J Rare Dis. 2017;12(1):119. doi:10.1186/s13023-017-0666-5
- Togo CCG, Zidorio APC, Gonçalves VSS, Hubbard L, de Carvalho KMB, Dutra ES. Quality of life in people with epidermolysis bullosa: a systematic review. Qual Life Res. 2020;29(7):1731–1745. doi:10.1007/s11136-020-02495-5
- Eng VA, Solis DC, Gorell ES, et al. Patient-reported outcomes and quality of life in recessive dystrophic epidermolysis bullosa: a global cross-sectional survey. J Am Acad Dermatol. 2021;85(5):1161–1167. doi:10.1016/j.jaad.2020.03.028
- Tabolli S, Mozzetta A, Antinone V, Alfani S, Cianchini G, Abeni D. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158(5):1029–1034. doi:10.1111/j.1365-2133.2008.08481.x
- Tabolli S, Baliva G, Lombardo GA, et al. Health related quality of life assessment in the routine clinical practice of a dermatology unit. Eur J Dermatol. 2006;16(4):409–415.
- Fulchand S, Harris N, Li S, et al. Patient-reported outcomes and quality of life in dominant dystrophic epidermolysis bullosa: a global cross-sectional survey [published online ahead of print, 2021 Sep 13]. Pediatr Dermatol. 2021. doi:10.1111/pde.14802
- Margari F, Lecce PA, Santamato W, et al. Psychiatric symptoms and quality of life in patients affected by epidermolysis bullosa. J Clin Psychol Med Settings. 2010;17(4):333–339. doi:10.1007/s10880-010-9205-4
- Gisondi P, Sampogna F, Annessi G, Girolomoni G, Abeni D. Severe impairment of quality of life in Hailey-Hailey disease. Acta Derm Venereol. 2005;85(2):132–135. doi:10.1080/00015550410025462
- De Fata Salvatores G, Villani A, Fabbrocini G, Di Guida A. Patients with bullous disorders during COVID-19 period: management and adherence to treatment. Dermatol Ther. 2020;33(6):e13697. doi:10.1111/dth.13697
- Villani A, Scalvenzi M, Fabbrocini G. Teledermatology: a useful tool to fight COVID-19. J Dermatolog Treat. 2020;31(4):325. doi:10.1080/09546634.2020.1750557
- Joly P, Gillibert A, Bohelay G, et al. Incidence and Severity of COVID-19 in patients with autoimmune blistering skin diseases: a nation-wide study [published online ahead of print, 2021 Oct 26]. J Am Acad Dermatol. 2021. doi:10.1016/j.jaad.2021.10.034
- Gupta R, Ibraheim MK, Doan HQ. Teledermatology in the wake of COVID-19: advantages and challenges to continued care in a time of disarray. J Am Acad Dermatol. 2020;83(1):168–169. doi:10.1016/j.jaad.2020.04.080
- Shakshouk H, Daneshpazhooh M, Murrell DF, Lehman JS. Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82(6):e235–e236. doi:10.1016/j.jaad.2020.04.005
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi:10.1016/S0140-6736(12)61140-4
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):882–894. doi:10.1016/S0140-6736(19)31778-7
- Mahmoudi H, Farid AS, Nili A, et al. Characteristics and outcomes of COVID-19 in patients with autoimmune bullous diseases: a retrospective cohort study. J Am Acad Dermatol. 2021;84(4):1098–1100. doi:10.1016/j.jaad.2020.12.043
- Kridin K, Schonmann Y, Weinstein O, Schmidt E, Ludwig RJ, Cohen AD. The risk of COVID-19 in patients with bullous pemphigoid and pemphigus: a population-based cohort study. J Am Acad Dermatol. 2021;85(1):79–87. doi:10.1016/j.jaad.2021.02.087
- Schultz B, Pearson DR, Mansh M. Reply to “Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84(1):e59–e60. doi:10.1016/j.jaad.2020.07.132
- Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. 2021;35(10):e645–e647. doi:10.1111/jdv.17472
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148(3):750–751. doi:10.1016/j.jaci.2021.06.026
- Kong J, Cuevas-Castillo F, Nassar M, et al. Bullous drug eruption after second dose of mRNA-1273 (Moderna) COVID-19 vaccine: case report. J Infect Public Health. 2021;14(10):1392–1394. doi:10.1016/j.jiph.2021.06.021
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review [published online ahead of print, 2021 Apr 24]. J Am Acad Dermatol. 2021. doi:10.1016/j.jaad.2021.04.061
- Toussirot É, Bereau M. Vaccination and induction of autoimmune diseases. Inflamm Allergy Drug Targets. 2015;14(2):94–98. doi:10.2174/1871528114666160105113046
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310(2):95–106. doi:10.1007/s00403-017-1790-8
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28(9):1133–1140. doi:10.1111/jdv.12366
- Guerra L, Pedicelli C, Fania L, et al. Infantile bullous pemphigoid following vaccination. Eur J Dermatol. 2018;28(5):708–710. doi:10.1684/ejd.2018.3383
- Moro F, Fania L, Sinagra JLM, Salemme A, Di Zenzo G. Bullous pemphigoid: trigger and predisposing factors. Biomolecules. 2020;10(10):1432. doi:10.3390/biom10101432
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111. doi:10.1155/2021/5575111
- Aram K, Patil A, Goldust M, Rajabi F. COVID-19 and exacerbation of dermatological diseases: a review of the available literature [published online ahead of print, 2021 Aug 27]. Dermatol Ther. 2021;e15113. doi:10.1111/dth.15113
- De Medeiros VLS, Monteiro-Neto AU, França DDT, Castelo Branco R, de Miranda Coelho ÉO, Takano DM. Pemphigus vulgaris after COVID-19: a case of induced autoimmunity [published online ahead of print, 2021 May 27]. SN Compr Clin Med. 2021;1–5. doi:10.1007/s42399-021-00971-8
- Ghalamkarpour F, Pourani MR. Aggressive course of pemphigus vulgaris following COVID-19 infection. Dermatol Ther. 2020;33(6):e14398. doi:10.1111/dth.14398
- Saleh MA, Zaraa I, Doss N, Saleh NA, Murrell DF. Assessment of the quality of life of Egyptian and Tunisian autoimmune bullous diseases’ patients using an Arabic version of the autoimmune bullous disease quality of life and the treatment of autoimmune bullous disease quality of life questionnaires. An Bras Dermatol. 2019;94(4):399–404. doi:10.1590/abd1806-4841.20197198
- Sebaratnam DF, Okawa J, Payne A, Murrell DF, Werth VP. Reliability of the autoimmune bullous disease quality of life (ABQOL) questionnaire in the USA. Qual Life Res. 2015;24(9):2257–2260. doi:10.1007/s11136-015-0965-z
- Tjokrowidjaja A, Daniel BS, Frew JW, et al. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease [published correction appears in Br J Dermatol. 2014 Feb;170(2):481-3]. Br J Dermatol. 2013;169(5):1000–1006. doi:10.1111/bjd.12623
- Yang B, Chen G, Yang Q, et al. Reliability and validity of the Chinese version of the autoimmune bullous disease quality of life (ABQOL) questionnaire. Health Qual Life Outcomes. 2017;15(1):31. doi:10.1186/s12955-017-0594-z
- Chen G, Yang B, Zhang Z, et al. Chinese version of the treatment of autoimmune bullous disease quality of life questionnaire: reliability and validity. Indian J Dermatol Venereol Leprol. 2018;84(4):431–436. doi:10.4103/ijdvl.IJDVL_538_16
- Teimourpour A, Hedayat K, Salarvand F, et al. Autoimmune Bullous Disease Quality of Life (ABQoL) questionnaire: validation of the translated Persian version in pemphigus vulgaris. Int J Womens Dermatol. 2020;6(4):306–310. doi:10.1016/j.ijwd.2020.03.043
- Kalinska-Bienias A, Jakubowska B, Kowalewski C, Murrell DF, Wozniak K. Measuring of quality of life in autoimmune blistering disorders in Poland. Validation of disease - specific Autoimmune Bullous Disease Quality of Life (ABQOL) and the Treatment Autoimmune Bullous Disease Quality of Life (TABQOL) questionnaires. Adv Med Sci. 2017;62(1):92–96. doi:10.1016/j.advms.2016.07.002
- Yazdanshenas A, Naderi E, Moravvej H, et al. The quality of life in epidermolysis bullosa (EB-QoL) questionnaire: translation, cultural adaptation, and validation into the Farsi language. Int J Womens Dermatol. 2020;6(4):301–305. doi:10.1016/j.ijwd.2020.05.012
- Chernyshov PV, Boffa MJ, Corso R, et al. Creation and pilot test results of the dermatology-specific proxy instrument: the Infants and Toddlers Dermatology Quality of Life. J Eur Acad Dermatol Venereol. 2018;32(12):2288–2294. doi:10.1111/jdv.15229
- Chernyshov PV, Sampogna F, Pustišek N, et al. Validation of the dermatology-specific proxy instrument the infants and toddlers dermatology quality of life. J Eur Acad Dermatol Venereol. 2019;33(7):1405–1411. doi:10.1111/jdv.15496
- Chernyshov PV, Marron SE, Tomas-Aragones L, et al. Initial validation of the epidermolysis bullosa-specific module of the infants and toddlers dermatology quality of life questionnaire. Dermatol Ther. 2020;33(6):e14128. doi:10.1111/dth.14128
- Beissert S, Mimouni D, Kanwar AJ, Solomons N, Kalia V, Anhalt GJ. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130(8):2041–2048. doi:10.1038/jid.2010.91
- Horn HM, Tidman MJ. Quality of life in epidermolysis bullosa. Clin Exp Dermatol. 2002;27(8):707–710. doi:10.1046/j.1365-2230.2002.01121.x
- Kýrová J, Bučková H. Kvalita života pacientů s epidermolysis bullosa. Czecho-Slovak Dermatology/Cesko-Slovenska Dermatologie. 2013;88:3.
- Eismann EA, Lucky AW, Cornwall R. Hand function and quality of life in children with epidermolysis bullosa. Pediatr Dermatol. 2014;31(2):176–182. doi:10.1111/pde.12262F
- Dănescu S, Sălăvăstru C, Sendrea A, et al. Correlation between disease severity and quality of life in patients with epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2019;33(5):e217–e219. doi:10.1111/jdv.15371
- Angelis A, Kanavos P, López-Bastida J, et al. Social/economic costs and health-related quality of life in patients with epidermolysis bullosa in Europe. Eur J Health Econ. 2016;17 Suppl 1(Suppl1):31–42. doi:10.1007/s10198-016-0783-4
