Abstract
Background
Oronine H® ointment, which contains chlorhexidine gluconate as its active component, is a well known disinfectant, and has been widely used for treatment of acne in Japan. In this study, we investigated the inhibitory effect of this ointment on the formation of comedones induced by application of 50% oleic acid on the orifices of the external auditory canals of rabbits.
Methods
The application sites were observed with a dermatoscope, and the area of the hair pores was measured using an Image analysis software program.
Results
The chlorhexidine gluconate ointment inhibited comedone formation significantly more effectively than the liquid paraffin used as a control (P < 0.001). We also investigated the therapeutic effect of this ointment on comedones. After starting application of chlorhexidine gluconate ointment or liquid paraffin on the comedone area, the hair pore size was gradually decreased in the group treated with chlorhexidine gluconate ointment compared with the hair pore size at baseline.
Conclusion
These results suggest that chlorhexidine gluconate ointment is effective for inhibiting comedone formation as well as for treating already formed comedones. Chlorhexidine gluconate ointment is a useful topical medicine for the treatment of early-stage acne and for preventing acne.
Introduction
Chlorhexidine gluconate ointment (Oronine H®) is an over-the-counter drug widely used for the treatment of acne in Japan. Although many commercial preparations of various agents have been used, this ointment is still one of the most popular treatments for acne. However, an objective assessment of the therapeutic effect of this ointment on acne has not been performed. In the present study, we studied the effects of this ointment on comedone formation and also examined its therapeutic effect on already formed comedones in an experimental rabbit model of comedones induced by topical application of 50% oleic acid on the orifices of the external auditory canal.
Materials and methods
Reagents and test substance
Chlorhexidine gluconate ointment (Oronine H®, Otsuka Pharmaceutical Factory Inc, Tokushima, Japan) consists of 0.2% chlorhexidine gluconate, lauromacrogol, polysorbate 80, aluminum potassium sulfate, macrogol, glycerol, olive oil, stearyl alcohol, white beeswax, Vaseline®, self-emulsifying glycerol stearate, perfume, and purified water. Oleic acid (Nacalai Tesque Inc, Kyoto, Japan) was diluted with liquid paraffin (Nacalai Tesque Inc) to prepare 50% (v/v) oleic acid, which was used to induce comedone formation. All other reagents were of analytical grade.
Animal model and comedone formation
Japanese white rabbits were purchased from Sankyo Labo Service Corporation and used in this study. The rabbits were maintained under specific pathogen-free conditions at the Institute for Animal Experiments of the University of Toyama. All experiments using rabbits were conducted according to the guidelines set down by the University of Toyama Institutional Animal Care and approved by Toyama University Institutional Animal Care and Use Committee.
Study design
To induce comedone formation, 50% oleic acid was applied to the orifices of the external auditory canals of the rabbit ears daily, for one, two, or three weeks as described previously.Citation1–Citation3 We first examined the inhibitory effects of chlorhexidine gluconate ointment on experimentally induced comedones. Chlorhexidine gluconate ointment and 50% oleic acid were applied to the orifices of the external auditory canals once daily for 21 days in the actively treated rabbits, and liquid paraffin and 50% oleic acid was applied to the orifices of the external auditory canals of the controls (). Next, we examined the therapeutic effect of chlorhexidine gluconate ointment on pre-existing comedones. To induce early-stage comedones, 50% oleic acid was applied once a day for seven days to the orifices of the external auditory canals of the rabbits (days 1–7). Chlorhexidine gluconate ointment was then applied to the comedones for 14 days in the treatment group (days 8–21). In the control group, liquid paraffin was applied for 14 days instead of chlorhexidine gluconate ointment (). The auditory orifices of both ears on three rabbits were used as the experimental sites (six sites in total) in each of the experiments.
Figure 1 Study design. (A) Inhibitory effects of chlorhexidine gluconate ointment on comedone formation. Once-daily application of 50% oleic acid, followed by application of the test substance (chlorhexidine gluconate ointment or liquid paraffin), was carried out for 21 consecutive days (n = 3 in each group). (B) Therapeutic effect of the ointment on preformed comedones.
Dermatoscopic observation and assessment
The inner surfaces of the auditory canals were observed using a dermatoscope (Delta20®, Heine, Germany) at the start of application of the experimental substances (chlorhexidine gluconate ointment or liquid paraffin) and 7, 14, and 21 days thereafter. The area of each group of 120 comedones (20 comedones on one ear, and six sites in each ear) was measured using the Image J analysis software program, and the efficacy of this treatment was assessed by comparing the differences between areas measured before and after application of the test substance.
Histological examination
Skin tissues were excised on day 21 after treatment with the test substance, and the specimens were fixed in 10% formaldehyde. Tissue sections (5 μm) were stained with hematoxylin and eosin, and observed using a microscope.
Statistical analysis
Values are expressed as the mean ± standard deviation of the respective test or control group. Statistically significant differences between the control and test groups were sought using the Student’s t-test. Values of P < 0.05 were considered to be statistically significant.
Results
Inhibitory effect on comedone formation
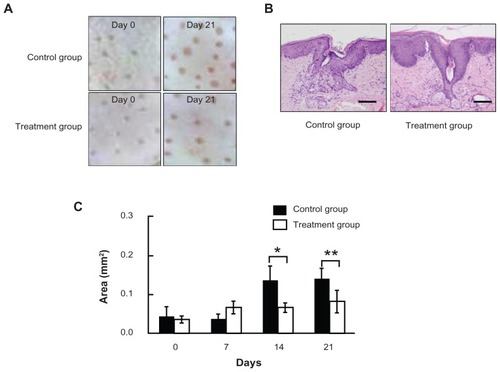
We first investigated whether chlorhexidine gluconate ointment could inhibit comedone formation. In the control group, enlarged hair pores were observed in the rabbit auditory canals after 21 days of application 50% oleic acid and liquid paraffin in comparison with hair pores on day 0 by dermatoscopic observation. In contrast, hair pores in the chlorhexidine gluconate ointment-treated group were markedly smaller than those in the liquid paraffin-treated control group on day 21, although on day 21 the hair pores were slightly larger than those on day 0 (). Histological examination of the hair pores showed markedly dilated hair follicles and hypertrophy of the sebaceous glands on day 21 in the control group, and these findings are characteristic of comedone formation. In contrast, chlorhexidine gluconate ointment significantly inhibited these histological changes, as shown in . Furthermore, the area of the hair pores was calculated quantitatively using the Image J analysis software program, and it was found that application of chlorhexidine gluconate ointment had significant inhibitory effects on comedone formation as compared with the control group on days 14 and 21 (). According to these results, chlorhexidine gluconate ointment was considered to be effective for inhibiting formation of comedones.
Figure 2 Inhibitory effects of chlorhexidine gluconate ointment on comedone formation. (A) Dermatoscopic findings on days 0 and 21 for rabbit ear skin treated with 50% oleic acid and liquid paraffin (control group) or with 50% oleic acid and chlorhexidine gluconate ointment (treatment group). (B) Histological findings of hair pores treated with 50% oleic acid and liquid paraffin (control group), and with 50% oleic acid and chlorhexidine gluconate ointment (treatment group) on day 21. Bar = 200 μm. (C) Quantitative assessment of the inhibitory effects of treatment on comedone formation.
Therapeutic effect on pre-existing comedones
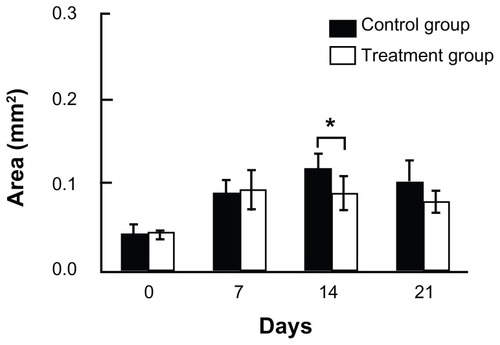
We next investigated the therapeutic effect of chlorhexidine gluconate ointment on comedones. Seven days of 50% oleic acid application caused a remarkable increase in hair pore area, suggesting that comedones were formed by day 7 (). These hair pores were then treated with chlorhexidine gluconate ointment (treatment group) or with liquid paraffin (control group) for 14 days. The hair pore size continued to increase in the control group in spite of 50% oleic acid application having been stopped on day 7. In contrast, mean hair pore size in the treatment group had gradually decreased by days 14 and 21 compared with baseline on day 7. Further, comparison of the two groups on day 14 showed that hair pore size became smaller in the treatment group than in the control group on day 14 (P < 0.005, ). This result suggests that chlorhexidine gluconate ointment might also be an effective treatment for pre-existing comedones.
Figure 3 Therapeutic effect on comedones.
Discussion
Acne is a common skin disorder of the pilosebaceous glands that occurs predominantly in the skin of the face, back, and trunk. It is thought to be caused by abnormal sebum production. Affected areas are characterized by formation of comedones, papules, pustules, cysts, and nodules.Citation4,Citation5 Bacteria, particularly Propionibacterium acnes and Staphylococcus epidermis, are usually involved in the development of acne.Citation6 P. acnes proliferates in sebum and is involved in comedone formation.Citation7 Comedones are noninflammatory eruptions appearing at the early stage of acne, and result from abnormal production and differentiation of ductal keratinocytes. Triglycerides, a component of sebum, are decomposed by the action of bacterial lipase to form free fatty acids, which stimulate the development of abnormal keratinization in the infundibular hair follicles, leading to retention of sebum, and keratinous debris dilates the follicles and leads to formation of comedones.Citation8–Citation10 Therefore, removal of free fatty acids, as well as suppression of P. acne growth, is an important strategy for inhibiting comedone formation.
Chlorhexidine gluconate ointment is a hydrophilic product containing macrogol and Vaseline® as the base. It has been used as a therapeutic drug for acne, but its efficacy and mechanism of action is not fully understood. This ointment is thought to exert its effect in the early stages of acne, especially on comedone formation. In this study, the efficacy of this ointment was evaluated in a rabbit model of experimentally induced comedones. We found that chlorhexidine gluconate ointment significantly inhibited comedone formation. We also studied the therapeutic effect of this ointment, and observed that it decreased comedone size, indicating that the ointment is useful not only for prevention but also for the treatment of acne.
The therapeutic effects of this ointment on acne were previously considered to be most likely due to the antibacterial activity of chlorhexidine gluconate, a frequently used antiseptic, against P. acnes. However, there was no bacterial involvement in this experimental model. Thus, it is speculated that the inhibitory effect of this ointment is probably due to a mechanism other than its antibacterial activity. Although macrogol, which is used as the ointment base, has high affinity and compatibility with free fatty acids such as oleic acid, it is a water-soluble substance, and thus inhibits transdermal absorption of any substances dissolved in it.Citation11 Therefore, it is suggested that macrogol may have inhibited comedone formation by dissolving and removing free fatty acids, such as oleic acid, thereby preventing them from stimulating the follicular endothelium, leading to suppression of abnormal keratinization. Accordingly, when chlorhexidine gluconate ointment was used at the early stage of acne, it presumably inhibited comedone formation through multiple processes, ie, the dissolving action of macrogol on free fatty acids, as well as the antibacterial action of chlorhexidine gluconate, preventing comedones from progressing to papules or pustules.
In conclusion, chlorhexidine gluconate ointment has inhibitory effects on the formation of comedones and can be used for the treatment of existing comedones. Therefore, it is a useful topical medicine for the treatment of early-stage acne and for preventing acne. We consider that further work will be necessary to obtain a better understanding of the actions of this ointment, and we intend to perform a detailed study to analyze the key mechanisms by which it inhibits comedone formation.
Disclosure
TS has received industry consulting and research grants from Otsuka Pharma and YH is an employee of Otsuka Pharmaceutical Factory Inc.
References
- ChoiEHAhnSKLeeSHThe changes of stratum corneum interstices and calcium distribution of follicular epithelium of experimentally induced comedones (EIC) by oleic acidExp Dermatol1997629359067704
- MaedaTAn electron microscopic study of experimentally-induced comedo and effects of vitamin A acid on comedo formationJ Dermatol1991183974071724252
- OhCWMyungKBAn ultrastructural study of the retention hyperkeratosis of experimentally induced comedones in rabbits: the effect of three comedolyticsJ Dermatol1996231691808935627
- FeldmanSCarecciaREBarhamKLHancoxJDiagnosis and treatment of acneAm Fam Physician2004692123213015152959
- KumarABabootaSAgarwalSPTreatment of acne with special emphasis on herbal remediesExpert Rev Dermatol20083111122
- JeremyAHHollandDBRobertsSGThomsonKFCunliffeWJInflammatory events are involved in acne lesion initiationJ Invest Dermatol2003121202712839559
- JarrousseVCastex-RizziNKhammariAModulation of integrins and filaggrin expression by Propionibacterium acnes extracts on keratinocytesArch Dermatol Res200729944144717684752
- CunliffeWJHollandDBJeremyAComedone formation: etiology, clinical presentation, and treatmentClin Dermatol20042236737415556720
- HolmesRLWilliamsMCunliffeWJPilosebaceous duct obstruction and acneBr J Dermatol1972873273324263289
- KurokawaIDanbyFWJuQNew developments in our understanding of acne pathogenesis and treatmentExp Dermatol20091882183219555434
- NodaYWatanabeKSanagawaAPhysicochemical properties of macrogol ointment and emulsion ointment blend developed for regulation of water absorptionInt J Pharm201141913113621820500