Abstract
Introduction
Squamous cell carcinoma (SCC) is a non-melanoma skin cancer, with a rising worldwide incidence. Wide excision with an intraoperative frozen section decreases its recurrence rate and metastases.
Case
We reported an SCC case in a 50-year-old woman with clinical manifestations of a 4 × 6 × 0.3 cm solitary ulcer that easily bled. Dermoscopy and histopathological examination support the diagnosis of SCC. Due to its size, a wide excision was initiated, followed by a frozen section being carried out to determine the cancer cell-free margin. We performed an additional 1 cm margin excision as residual tumor still remained in the margin on the first excision. The forehead interpolation flap reconstruction was performed right after the excision to cover the extensive defects on the cheek, followed by a full-thickness skin graft (FTSG) for the forehead. The patient recovered well without complication within eight weeks post-procedure.
Discussion
SCC with a diameter larger than 2 cm is considered as high-risk, and a wide excision is the standard treatment in this condition. However, this may risk incomplete excision, leaving residual tumor and increased recurrence rate. Intraoperative frozen section aids the surgeon to determine tumor margin, thus improving the success rate of therapy by up to 95%. A skin graft on the cheek was avoided due to concerns of wound contraction, which may lead to lower tissue survival rates with poor color and texture matching. Therefore, we preferred a skin flap to increase tissue survival and preserve facial contour as well as skin color. Forehead interpolation flap was carried out as it could cover the large size of the skin defect. The forehead skin as donor was later closed by a FTSG.
Conclusion
Wide excision surgery with frozen sections is the best option for managing large SCC while a skin flap is preferred to close defects on the cheek.
Introduction
Squamous cell carcinoma (SCC) is a non-melanoma skin cancer characterized by malignant proliferation of epidermal keratinocytes and is the second most common skin cancer after basal cell carcinoma (BCC).Citation1,Citation2 Based on epidemiological data, the incidence of SCC has increased in the last thirty years. Data from The Rochester Epidemiology Project show a 263% increase in SCC incidence globally between 1976–1984 and 2000–2010.Citation3 SCC is more common in the elderly,Citation4 especially those aged 60 years or older,Citation1,Citation3 and white women.Citation1 The risk factors for SCC involve genotypic, phenotypic, and environmental factors.Citation1 A genetic predisposition, such as light skin complexion and variations in the melanocortin-1 receptor, are risk factors for SCC.Citation1,Citation2 Environmental factors such as cumulative ultraviolet (UV) exposure and chronic inflammation of the skin might contribute to SCC development and progression.Citation1,Citation5 UV exposure is thought to be the most important environmental factor that leads to SCC development. This is indicated by the localization of SCC on sun-exposed areas along with increasing age and high cumulative UV irradiation.Citation1,Citation6
Clinically, SCC appears as hyperkeratotic and well-defined papules or plaques, often accompanied by central ulceration.Citation4,Citation7 SCC lesions are generally firm and easily bleed on palpation.Citation7,Citation8 Dermoscopy benefits in making SCC diagnosis without any invasive procedure.Citation1–Citation3 Vascular patterns, such as glomerular-like vessels, dotted vessels,Citation3 or irregular vessels with keratin might also be found.Citation2 Diagnosis of SCC is generally established by histopathological examination. There are two main histological classifications of SCC: the well-differentiated and the poorly-differentiated one. In well-differentiated SCC, the nuclei are less pleomorphic and the keratinization is strongly evident as seen in parakeratosis, individual cell dyskeratosis, formation of horn pearls,Citation1,Citation3,Citation7 and intercellular bridges or desmosomes. In the contrary, pleomorphism with a high degree of atypia, irregular cell mitosis activities, and little keratinization area are seen in poorly-differentiated SCC.Citation1,Citation3
A surgical procedure mainly aims to eradicate the tumor cells. However, this should be done with effort to preserve the function and aesthetic of the face.Citation7 A wide excision technique is the primary option for SCC management due to its excellent outcome,Citation1 but an inaccurate surgical margin may increase the recurrence rate.Citation9,Citation10 To prevent improper determination of surgical margin, intraoperative frozen section has been used to establish the tumor-free margin.Citation11
Reconstruction for postoperative defects by flaps and/or skin grafts in SCC might be challenging due to its functional and aesthetical reasons.Citation11,Citation12 A skin flap is performed to close surgical wounds that interfere with the patient’s functioning and aesthetical aspects, while skin grafts can be performed for defects that cannot be appropriately closed by a skin flap.Citation13 This case reports aims to elaborate an SCC management on the cheek by wide excision and intraoperative frozen section, while closure of the defect was done using a forehead interpolation flap reconstruction combined with a skin graft.
Case
A 50-year-old woman came with the chief complaint of an intermittently painful ulcer on her left cheek. The lesion started five years ago as a pruritic, erythematous papule with scabs on her left maxillae. Three years later, the skin lesions enlarged and developed to an erythematous plaque covered in brownish crust. It continued to enlarge, reaching the size of a baby’s palm, while becoming painful, wet, and easily bled with light pressure. There was no history of a similar lesion before. The patient denied recent weight loss. She had a tremendous amount of sun exposure in the past by having daily sunbaths for approximately 30 minutes and working outdoors, yet she has never used any sunscreen or any protective apparels.
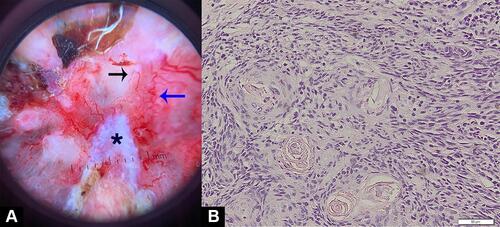
On the physical examination, the lesion appeared large, with a 4 × 6 × 0.3 cm dimension, solitary, irregular border, and covered by crust. Upon crust removal, we revealed a deep, irregular ulcer (). On dermoscopic examination, there were serpentine and hairpin vessels, erosions, and white structureless areas (). No regional lymph node enlargement was found. Blood examination results were unremarkable. Histopathological examination revealed a tumor mass consisting of condensed, hyperplastic round, oval-to-polygonal tumor cells with partially eosinophilic cytoplasm. The nuclei appear polymorphic, hyperchromatic, and vesicular with clear daughter nuclei (). A computed tomography scan (CT-Scan) of the head showed no bone or other organ involvement.
Figure 1 The lesion covered with a thick crust (A and B) and ulcer revealed within by removing the crust (C).
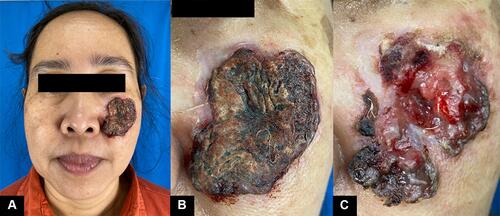
Figure 2 Dermoscopy examination (A) and histopathology examination (B). The dermoscopy, there are a hairpin vessel (black arrow), serpentine vessels (blue arrow), and white structureless area (asterisk).
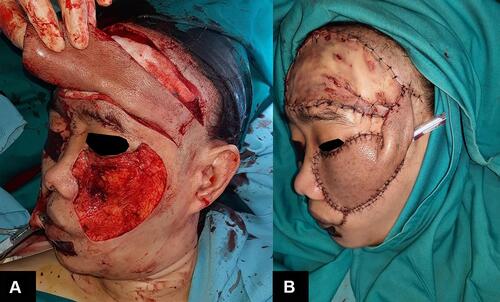
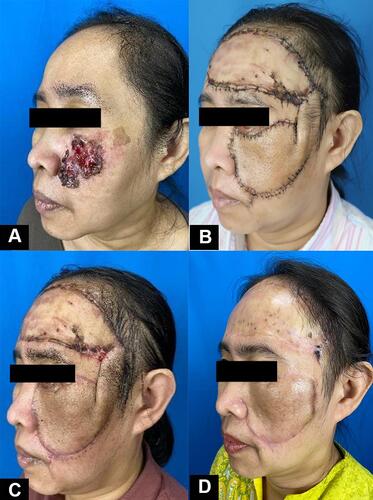
The patient was diagnosed with SCC and consulted to the Department of Oncology Surgery. We planned for a wide excision with 1 cm margin with intraoperative frozen section. Due to the potentially large defect, the closure was planned using a forehead interpolation flap, followed by a skin graft to the forehead. At the time of the procedure, the residual tumor was found by frozen section on the margin 0–1 to 1–3, thus additional excision was done with a 1 cm margin. After the second excision, we confirmed the incision margins 1–2, 3–4, 4–5, and 5–0 were free of malignant tumor cells. The final defect on the cheek after excision was measured as 12 × 14 cm. Forehead skin was opted as a donor to accommodate the size of the defect. Interpolation skin flaps technique was done by pivoting the forehead skin to the left cheek with a left temporal skin pedicle as its axis. The left temporal pedicle was meant to be removed after the survival of the skin flap was assured (planned for at least within four weeks). A full-thickness skin graft (FTSG) was carried out for the forehead skin defect and the tissue donor was taken from the abdomen (). The patient recovered in three days without complication and was discharged afterwards. The temporal pedicle was removed eight weeks after procedure. The comparison of before procedure, two and six weeks after procedure, and after pedicle removal can be seen in . At the end of observation, there were a visible unwanted result such as ectropion of the left lower eyelid, and dissimilarity of the skin graft with surrounding skin. Nevertheless, the patient was generally satisfied with the result.
Discussion
The ideal management of SCC is predicated on local tumor control along with maximum preservation of function and cosmetics.Citation1,Citation7 Excision of the tumor with a predetermined margin of normal skin should ensure that the lesion is fully excised.Citation14 The initial excision margin depends on SCC risk classification. A high-risk SCC is considered when the diameter of the tumor is larger than 2 cm as tumors of this size double the risk of SCC recurrence and triple the rate of metastasis compared to the smaller lesion.Citation3,Citation14 Therefore, a larger margin for excision is required on the high-risk SCC. Study by Bordland et alCitation15 demonstrated that at least 6-mm excision margin for high-risk SCC is required,Citation15 compared to the smaller 4–6 mm margin for the low-risk SCC,Citation1,Citation15 to achieve an acceptable cure rate. However, determining the resected area is completely cancer-free is challenging without additional reassurance procedure.Citation10 Inaccurate margin may result in incomplete excision and high recurrence rates.Citation9,Citation10 Residual tumor skin should never be left under the flap or a skin graft as recurrence may be hidden from later visual examination, and lead to a delay in re-excision.Citation16 A study by Bogdanov-Berezovsky et alCitation10 showed that determining the border of carcinoma lesions using frozen sections could increase the success rate of excision at initial surgery by 70%. Research by WintherCitation17 demonstrated that histopathological diagnostic tests using the frozen section method could achieve 95.1% accuracy. Especially in this case report, it is particularly important to ensure the free-tumor margin for better success rate, as SCC lesions larger than 2 cm tend to metastasize.Citation18
Reconstruction after facial tumor surgery is essential as tumors might cause facial asymmetry.Citation19 As the lesion is on the cheek, the surgeon may face a reconstructive challenge due to the extremely visible site, as well as limited local tissue supply. In addition, the cheek may perform several important functions such as face expression because it harbors several facial muscles underneath.Citation20 As mentioned by Millard,Citation21 the cheek may be divided into three overlapping units: (1) suborbital; (2) preauricular; and (3) buccomandibular. As in this case report, the lesion is located on the zone 1, which is the suborbital zone. This particular zone extends along the lateral border of the nose to the nasolabial fold, across the cheek below the gingival sulcus toward the sideburn, up the anterior sideburn to the lateral crow’s-foot line, and then along the lower eyelid cheek junction.Citation21 This location might not be amenable to primary or skin graft closure.Citation20 The highly visible site of the cheek and the size of the defect is the main reason that skin graft is unattainable. To obtain the optimal aesthetic result, the most suitable skin graft technique is FTSG as it preserves similar thickness, texture, and color to surrounding skin. However, the increased thickness of FTSG results in an increased metabolic demand and a higher rate of graft failure. Due to its high metabolic demand, FTSG should not be used for closure of lesions larger than 5 cm.Citation22 The patient in this case report had an excised wound for 12 × 14 cm and this is one of the reasons we did not opt for FTSG as wound closure on the cheek.
Local skin flap is still the preferred method for closure on the cheek. It may provide favorable contours, texture, and functionality.Citation12 Skin flaps also have a 96% success rate for closing skin cancer defects such as SCC and BCC, without complications such as hematoma or infection.Citation19 Some authors recommend rhomboid, circular, bilobed, or cheek advancement flaps for wound closure on zone 1.Citation20,Citation23,Citation24 However, as for this case, we considered a different approach as those aforementioned techniques might only work on a smaller defect. Therefore, the forehead interpolation flap was chosen because it accommodates the need of a large size skin donor. This was done by two steps: (1) the flap was designed and its pedicle was set on the left temporal region to accommodate the movement of donor skin to defect location and to maintain blood flow of the donor skin through superficial temporal artery; and (2) the dividing of the temporal pedicle within eight weeks after the procedure. Flap donors taken from the forehead skin are suitable for reconstructing defects in the cheek, oral cavity, maxillary antrum, and mouth corners due to their similarity in texture and color, along with appropriate vascularization on the recipient tissue.Citation11,Citation19
As for the donor site on the forehead, the wound was extensive so that primary closure would not be appropriate. Therefore, FTSG for wound closure on the forehead was performed. Choosing a skin donor and grafting technique depend on the vascularity, contour, texture, and recipient area.Citation11,Citation25 The use of the abdominal skin as a donor has its benefit as it may provide a large coverage, tension-free results at the site of the defect, and cosmetically symmetrical result, therefore it is preferred by patients.Citation25
Overall, the patient in this case report responded very well upon observation. There was no major complication following the procedure, and the skin flap and graft were also able to survive. However, we found an unwanted result, which was an ectropion on the left eye caused by the skin flap. This was expected because the lower eyelid was unable to sustain the weight of the skin flap. A similar result was also reported by Mahadevan et alCitation26 and became one of the reasons for the patient’s dissatisfaction towards the procedure. The other unwanted result was the visible dissimilarity of the affected skin especially on the forehead which underwent FTSG. This was inevitable as no matter that the donor skin was carefully chosen and treated, the abdominal skin may never be equal to the skin of the forehead. Nevertheless, these conditions were considered mild as cancers of the head and neck must be optimally removed, and concerns for eventual aesthetic should not hinder the optimal removal of the malignant tumor.
In general, SCC has a high risk of local invasion and metastasis.Citation8 In one study, 65% of SCC cases were at high risk of recurrence in the first year after diagnosis.Citation8 The size of the lesion is directly related to the risk of metastasis, hence the prognosis. The patient in this case report, although there was no apparent local lymph node involvement, still needs to be under surveillance due to her large lesion. Hopefully, eliminating the tumor by wide excision combined with the intraoperative frozen section may improve the prognosis and life expectancy of the patient.
Conclusion
Wide excision surgery with frozen sections are the standard for the management of SCC. Cheek reconstruction may be done with forehead interpolation flaps considering the extensive size of defect, while FTSG from abdominal skin is preferable to close the defect on the forehead.
Ethical Statement
Patient’s consent and institutional approval had been obtained for the purpose of image publication.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Acknowledgments
The authors would like to thank the Department of Surgery and Department of Dermatology and Venereology for providing meeting, editorial, and general administrative support.
Disclosure
The authors have no conflicts of interest to declare in this work.
Additional information
Funding
References
- Lonsdorf A, Hadaschik E. Squamous cell carcinoma and keratoachantoma. In: Kang S, Amagai M, Bruckner A, Enk A, Margolis D, McMichael A, editors. Fitzpatrick’s Dermatology. 9 ed. New York: McGraw-Hill; 2019:1901–1919.
- Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 2. Treatment. Eur J Cancer. 2020;128:83–102. doi:10.1016/j.ejca.2020.01.008
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247.
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17(5):491–508.
- Maru GB, Gandhi K, Ramchandani A, Kumar G. The role of inflammation in skin cancer. Inflammation Cancer. 2014;1:437–469.
- Green AC, Olsen C. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373–381.
- Firnhaber JM. Diagnosis and treatment of basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86(2):161–168.
- Gupta G, Madan V, Lear J. Squamous cell carcinoma and its precursor. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s Textbook of Dermatology. New Jersey: John Wiley & Sons; 2016:p.142.1 -.40.
- Badash I, Shauly O, Lui CG, Gould DJ, Patel KM. Nonmelanoma facial skin cancer: a review of diagnostic strategies, surgical treatment, and reconstructive techniques. Clin Med Insights Ear, Nose Throat. 2019;12:1179550619865278.
- Bogdanov-Berezovsky A, Rosenberg L, Cagniano E, Silberstein E. The role of frozen section histological analysis in the treatment of head and neck skin basal and squamous cell carcinomas. Isr Med Assoc J. 2008;10(5):344.
- Agbara R, Fomete B, Obiadazie AC, Omeje KU, Amole OI. The forehead flap: a valuable option in resource depleted environment. Plast Aesthetic Res. 2016;3(4):115–120. doi:10.20517/2347-9264.2015.123
- Hidayatullah O, Asif M, Tahir M, Aslam M. Aesthetic outcome of sliding island flap for reconstruction of cheek after tumour ablation. J Ayub Med Coll Abbottabad. 2005;17(3):15–18.
- Sheehan J, Kingsley M, Rohrer T. Excisional surgery and repair, flaps, and grafts. In: Wolff K, Goldsmith L, Katz S, Gilchres T, Paller A, Leffell D, editors. Fitzpatrick’s Dermatology in General Medicine. 8 ed. New York: McGraw-Hill; 2012:2930–2944.
- Samarasinghe V, Madan V, Lear JT. Management of high-risk squamous cell carcinoma of the skin. Expert Rev Anticancer Ther. 2011;11(5):763–769. doi:10.1586/era.11.36
- Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27(2):241–248. doi:10.1016/0190-9622(92)70178-I
- Manstein ME, Manstein CH, Smith R. How accurate is frozen section for skin cancers? Ann Plast Surg. 2003;50(6):607–609. doi:10.1097/01.SAP.0000069073.38391.91
- Winther C, Graem N. Accuracy of frozen section diagnosis: a retrospective analysis of 4785 cases. APMIS. 2011;119(4–5):259–262. doi:10.1111/j.1600-0463.2011.02725.x
- Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg. 2002;28(3):268–273. doi:10.1046/j.1524-4725.2002.01169.x
- Souza CD. Reconstrução de grandes defeitos de couro cabeludo e fronte em oncologia: tática pessoal e experiência - análise de 25 casos. Revista Brasileira de Cirurgia Plástica. 2012;27(2):227–237. doi:10.1590/S1983-51752012000200011
- Heller L, Cole P, Kaufman Y, editors. Cheek Reconstruction: Current Concepts in Managing Facial Soft Tissue Loss. Semin Plast Surg. © Thieme Medical Publishers; 2008.
- Millard D. Principlization of Plastic Surgery. Boston, MA: Little, Brown; 1986.
- Acosta AEA, Sumaira Z, MacNeal R, Messingham M, Arpey C. Skin Grafting. In: Robinson JK, Hanke C, Siegel D, Fratila A, editors. Surgery of the Skin. New York: Elsevier Saunders; 2015:306–323.
- Haimovic A, Sheehan JM, Rohrer TE. Excisional surgery and repair, flaps, and grafts. In: Kang S, Amagai M, Bruckner A, Enk A, Margolis D, McMichael A, editors. Fitzpatrick’s Dermatology. 9 ed. New York: McGraw-Hill; 2019:3726–3760.
- Cook JL, Goldman GD, Holmes TE. Random pattern cutaneous flaps. In: Robinson JK, Hanke C, Siegel D, Fratila A, editors. Surgery of the Skin. New York: Elsevier Saunders; 2015:253–285.
- Foulke EJ, Clegg D, Peters D. Lower Abdomen as a Donor Site for Large Full-Thickness Skin Grafts. Am Surg. 2020;1:0003134820951454.
- Mahadevan K, Sruthi S, Sridevi S, Vivek R. Fourth dimension in reconstruction of defects following excision of basal cell carcinoma of head and neck! J Cutaneous Aesthetic Surg. 2018;11(3):110. doi:10.4103/JCAS.JCAS_100_17