Abstract
Spontaneous regression of malignant melanoma was first reported over a century ago. Clinically, areas of blue or grey colouration in lesions may be indicative of regression. Dermoscopy is a very useful tool for diagnosing regression. An important criterion is the blue-white scar. About 10–35% of excised melanomas show features of regression histopathologically. We present a case of regressing melanoma, with clinical and dermoscopic features suggesting a collision tumour, diagnosed histopathologically. This case might improve our knowledge of the potential clinical manifestations, and the biology, of regressing melanoma.
Introduction
Spontaneous regression in malignant melanoma was first reported in 1953 by Sumner in a remarkable case.Citation1 Although the patient had several histopathologically proven tumour foci, the presumed primary was not biopsied and could only be conjectured based on history and cutaneous depigmentation.
Areas of depigmentation, blue or grey colouration may suggest regression clinically.Citation2 The blue-white scar-like structure (BWS) is considered an important dermoscopic criterion for regression in pigmented lesions.Citation3 Regression occurs in 10–35% of melanomas excised and examined histologically.Citation4 Often regressing melanomas are diagnosed histologically, ie post factum, when the process has been completed.
We present a case of regressing melanoma, showing clinical and dermoscopic features that suggest a collision tumour. Clinical and dermoscopic manifestations suggestive of collision are not normally recognised criteria for regression in melanomas. This case has the potential to offer us a window also into other characteristics that could suggest a melanoma in the process of regression to clinicians, as well as into the biology of this phenomenon.
Case Report
A 75-year-old male patient presented for a varicose ulcer on his left leg. He received a dressing for the ulcer and was instructed on how to care for it. During the instruction, the attending physician noticed a black spot on the left side of his neck. Closer examination revealed a black, approximately 1 cm × 1cm, triangular-shaped plaque, which was macular and hypopigmented on one half and nodular as well as pigmented on the other ().
Figure 1 Polymorphous plaque, with flatter depigmented area and slightly raised pigmented area on the left side of the neck, suggesting melanoma or collision lesion.

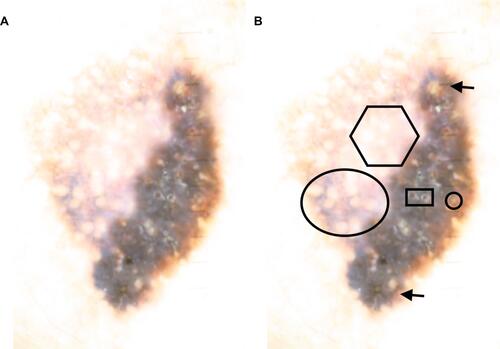
Overall, the lesion showed a multicomponent pattern. Dermoscopy showed an abrupt cut-off between two areas of differing pigment intensity. Atypical grey network irregular thick lines with incomplete rhomboids on the left side (oval shape). Irregular black blotches. Irregular streaks at 12 and 6 O’clock. White scar-like depigmented areas (hexagon), blue–gray pepper-like granules and blue-white veil. On the right side, blue-white veil, milia like cysts (circle), comedo-like openings as in seborrheic keratosis (rectangle), at 6 O’ clock thick pigmented lines around appendageal openings (arrows), which are sometimes called rhomboidal structures similar to those of lentigo maligna ().
Figure 2 (A) Dermoscopy showing abrupt cut-off between areas of stronger and milder pigmentation. Blue-whitish veil, peppering, multicomponent pattern, suggesting melanoma, with milia-like cysts and pseudofollicular openings suggesting seborrheic keratosis. (B) containing marked up photo.
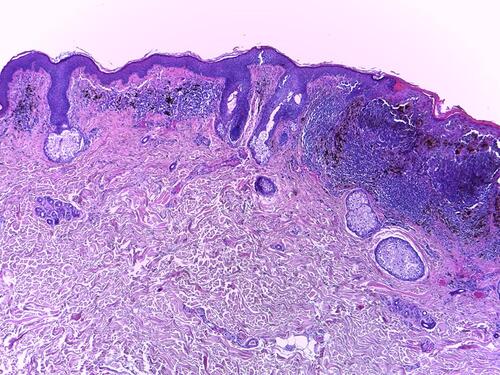
Histopathology (HE, 10x, ) shows an area of regression in the left part of the image (inflammatory infiltrate, which is predominantly lymphocytic and perivascular, encompassing also melanophages; some capillaries are newly formed; no “junctional activity” tumour cells can be identified, ie no residual tumor epidermal component is present). On the right, there are elongated cells with large hyperchromatic and atypical nuclei that are arranged in nests of varying dimension at the dermoepidermal junction, surrounded by a rich perivascular, predominantly lymphocytic infiltrate that also contains melanophages and extends along the whole of the base of the lesion. The melanoma cells involve all layers of the epidermis. The Clark level was IV and Breslow thickness 0.85 mm. No ulceration, neoplastic emboli or perineural infiltration was observed. Regression level was less than 75%.
Figure 3 Histopathology, (HE x 40) with features of regression and absence of junctional activity (left side of image) and nests of elongated tumour cells at the dermoepidermal junction (right side of image).
Surgical excision was carried out with appropriate margins, the patient recovered without complications and the excised tissue was sent for histopathology, with the results elaborated above.
The patient was subsequently referred to the oncologists.
This case report has been presented according to the CARE guidelines for case reporting. A summary of the timeline of this case is presented in .
Table 1 Timeline of Patient’s Consultation and Therapy
Written informed consent for the publication of the patient’s clinical details, including photographs and laboratory results was obtained.
Discussion
Regression is not an infrequent feature of melanocytic lesions, including melanoma.Citation4 Histopathologically, it is usually reported after the fact. Early regression is characterised by patchy disappearance of melanoma cells and their replacement by T-lymphocytes, and by fibrosis in the late phase.Citation5 CD4+ cells predominate in regressing melanoma, as well as a milieu rich in fibrogenetic cytokines, such as IL-6, platelet-derived growth factor and transforming growth factor-ß. This may explain some of the features seen in regressing primary melanomas.Citation6 Certain features have been found to correlate with regression in melanoma such as depigmentation, blue or grey colours clinically as well as BWS, multicomponent pattern and peppering dermoscopically, and these were seen in our case. Structures typical of seborrheic keratosis (milia-like cysts, comedo-like openings) are rarely found in melanoma.Citation7 Confocal microscopy promises to offer a non-invasive method of differentiating between melanomas and other skin cancers.Citation4 It may also help differentiate areas of regression from active melanoma. Round or dendritic pagetoid cells located at the dermoepidermal junction can be seen using confocal microscopy to examine melanoma tissue.Citation4 Other features that may be indicative of malignancy include epidermal disarray, pagetoid infiltration, deranged dermoepidermal junction architecture and atypical nests.Citation6 There are limited data on fibrosis studied using confocal microscopy. In classic frontal fibrosing alopecia and lichen planopilaris, confocal microscopy showed perifollicular lichenoid inflammatory infiltrate in 25% of cases, extensive perifollicular fibrosis in 75% of cases, as well as an increased number of white, ill-defined, coarse collagen fibres at the superficial dermis in 58.3% of cases and dilated blood vessels in 58.3% of cases.Citation8 The overall features, suggestive of collision clinically and dermoscopically, seen in our case, together with the histopathology suggestive of an earlier phase of regression of malignant melanoma, may offer us a window into the evolution of regressing melanomas.
The original case by SumnerCitation1 is reminiscent of the phenomenon of field cancerisation, which may be triggered in cases of immunosuppression.Citation9 Our case is a patient with diabetes, which may predispose to cancer. Further, diabetics are at high risk for cardiovascular disease. Drugs such as hydrochlorothiazides, beta-blockers and statins have been associated with increased risk of cutaneous carcinogenesis.Citation10–Citation12 This may indeed accentuate the field cancerisation. While field cancerisation is a predisposition to developing cancer, regression in melanoma is not. It may signal an immune response to the tumour, but it may also signal a poorer prognosis, including the existence of distant metastases, as it is often seen in advanced melanomas.
From a clinical perspective, when facing such patients, the first difficult task may be to accurately observe and diagnose the lesions. Indeed, one of the reasons that regressing melanoma is traditionally associated with a poor prognosis is the delayed discovery and inaccurate initial evaluation of the tumour thickness.Citation3 Such lesions evolve in two stages, first the melanocytic, usually pigmented proliferation appears; it can grow in size, darken in colour or bleed, and, left untreated, develops secondary regressive changes such as depigmentation and atrophy – flattening.Citation13 These changes occur gradually, so lesions like the one presented in this case can be observed. Frequently, the patient seeks care for other symptoms such as enlarged lymph nodes, and a diagnosis of metastatic disease is established in the absence of a visible primary tumour. Sometimes a lesion resembling an atrophic scar can be observed in the case of a completely regressed melanoma, thus differential diagnosis is all the more difficult for such patients.
The immunologic mechanism of regression is not yet fully understood. Some correlation between regressing melanoma and vitiligo-like depigmentation has been described in the literature,Citation14 and it is believed to be caused by an immune response to similar antigens shared by normal melanocytes and melanoma.Citation13 Similar discoloration has been observed in patients treated for melanoma with immunomodulators.Citation14,Citation15 This evidence may suggest that regression is based on the host immunologic response to the tumour and has led some specialists to believe that it could indicate a favourable prognosis. However, that view is not shared by all authors and the data are inconclusive. Other studies have linked regression with a poor prognosis in thin lesions, but unchanged prognosis in thicker tumours.Citation16,Citation17
The patient’s associated type II diabetes is a risk factor for cancers. Diabetes has been shown to increase the risk of developing several types of cancer, particularly liver and pancreatic cancers.Citation18 Several possible links between diabetes and carcinogenesis have been described, most important of which are hyperinsulinemia, hyperglycemia and insulin resistance, however the mechanism of carcinogenesis and its correlation with elevated glucose levels is still unclear.Citation19 The use of antidiabetic medication is also believed to influence carcinogenic potential by inducing different insulin concentrations,Citation20 thus metformin and thiazolidinediones are potentially safer than sulfonylureas and exogenous insulin,Citation21 though results have varied.Citation22
Surgical treatment in these cases can be an issue if the primary tumour has regressed completely. Our patient presented with a partially regressed melanoma and thus the pigmented part of the lesion was easier for the examining physician to notice. When the lesion is completely regressed, it is often the regional metastases that are biopsied first. Generally, in the presence of nodal dissemination, complete lymph node dissection is recommended for all patients, because it reduces local recurrence rates, it provides valuable information on prognosis and it also aids in the selection of an adjuvant treatment.Citation23 Managing metastatic melanoma almost always involves some kind of systemic therapy. Newer agents that modulate the immune response such as Ipilimumab or BRAF enzyme inhibitors such as Vemurafenib significantly prolong survival in the presence of distant metastases.Citation23,Citation24 Surgical excision of the metastases can prolong survival in some cases, but characteristics such as their number, site, resectability and also the biological aggressiveness of the disease should be carefully considered.Citation24 The 5-year survival rate for locally advanced melanoma is still only 63%, and drops to 18% in the presence of metastatic disease.Citation25 It is therefore extremely important to emphasise the role of periodic examination and self-examination, especially knowing that in the presence of regression, diagnosis can be challenging even for the experienced specialist.Citation26,Citation27 Fortunately, this patient did not suffer metastases.
Conclusion
Some features of melanomas in the process of regression have been reported, though not for the same lesion simultaneously. Our case, with features of a collision tumor, offers a rare view into potential clinical, dermoscopic and histopathologic features of a melanoma in the earlier phases of regression, thus contributing to the understanding of the biology of the phenomenon of melanoma regression.
Ethics and Consent Statements
Institutional approval was not required as this is a case report and the patient had already given written informed consent for the publication of photographs and all the test results. Written informed consent for the publication of this case was provided by the patient for the publication of photographs and all the test results.
Acknowledgments
No part of this work has been submitted or presented elsewhere.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sumner WC. Spontaneous regression of melanoma. Report of a case. Cancer. 1953;6(5):1040–1043. doi:10.1002/1097-0142(195309)6:5<1040::aid-cncr2820060525>3.0.co;2-5
- Gardner LJ, Strunck JL, Wu YP, Grossman D. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80(1):1–12. doi:10.1016/j.jaad.2018.03.053
- Ribero S, Moscarella E, Ferrara G, Piana S, Argenziano G, Longo C. Regression in cutaneous melanoma: a comprehensive review from diagnosis to prognosis. J Eur Acad Dermatol Venereol. 2016;30:2030–2037. doi:10.1111/jdv.13815
- Moscarella E, Bombonato C, Pampena R, et al. Pigmented skin lesions displaying regression features: dermoscopy and reflectance confocal microscopy criteria for diagnosis. Exp Dermatol. 2019;28(2):129–135. doi:10.1111/exd.13853
- Moretti S, Spallanzani A, Pinzi C, Prignano F, Fabbri P. Fibrosis in regressing melanoma versus nonfibrosis in halo nevus upon melanocyte disappearance: could it be related to a different cytokine microenvironment? J Cutan Pathol. 2007;34(4):301–308. doi:10.1111/j.1600-0560.2006.00616.x
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (Review). Exp Ther Med. 2022;23(1):60. doi:10.3892/etm.2021.10982
- Carrera C, Segura S, Aguilera P, et al. Dermoscopic clues for diagnosing melanomas that resemble Seborrheic Keratosis. JAMA Dermatol. 2017;153(6):544–551. doi:10.1001/jamadermatol.2017.0129
- Kurzeja M, Czuwara J, Walecka I, Olszewska M, Rudnicka L. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27(2):266–271. doi:10.1111/srt.12940
- Nwabudike LC, Tatu AL, Gambichler T, et al. Altered epigenetic pathways and cell cycle dysregulation in healthy appearing skin of patients with koebnerized squamous cell carcinomas following skin surgery. J Eur Acad Dermatol Venereol. 2019;33(1):e3–e4. doi:10.1111/jdv.15084
- Tatu AL, Ciobotaru OR, Miulescu M, et al. Hydrochlorothiazide: chemical structure, therapeutic, phototoxic and carcinogenetic effects in dermatology. Rev Chim. 2018;69(8):2110–2114. doi:10.37358/RC.18.8.6484
- Tatu AL, Elisei AM, Chioncel V, Nwabudike LC. Immunologic adverse reactions of β-blockers and the skin (review). Exp Ther Med. 2019;18:955–959. doi:10.3892/etm.2019.7504
- Nwabudike LC, Elisei AM, Buzia OD, Miulescu M, AL Tatu. A review on structural perspectives, adverse reactions and relations with non-melanoma skin cancer. Rev Chim. 2018;69(9):2557–2562. doi:10.37358/RC.18.9.6575
- Cui J, Bystryn JC. Melanoma and vitiligo are associated with antibody responses to similar antigens on pigment cells. Arch Dermatol. 1995;131:314–318. doi:10.1001/archderm.1995.01690150078015
- Scheibenbogen C, Hunstein W, Keilholz U. Vitiligo-like lesions following immunotherapy with IFN alpha and IL-2 in melanoma patients. Eur J Cancer. 1994;30:1209–1211. doi:10.1016/0959-8049(94)90493-6
- Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–3482. doi:10.1200/JCO.2001.19.15.3477
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20(4):315–322. doi:10.1111/j.1365-2559.1992.tb00988.x
- High WA, Stewart D, Wilbers CRH, Cockerell CJ, Hoang MP, Fitzpatrick JE. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53(1):89–100. doi:10.1016/j.jaad.2005.03.006
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103–1123. doi:10.1677/ERC-09-0087
- Giovannucci E, Harlan DM, Archer M. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi:10.2337/dc10-0666
- Wojciechowska J, Krajewski W, Bolanowski M, Krecicki T, Zatonski T. Diabetes and cancer: a review of current knowledge. Exp Clin Endocrinol Diabetes. 2016;124(5):263–275. doi:10.1055/s-0042-100910
- Simo R, Plana-Ripoll O, Puente D. Impact of glucose-lowering agents on the risk of cancer in type 2 diabetic patients. The Barcelona case-control study. PLoS One. 2013;8(11):e79968. doi:10.1371/journal.pone.0079968
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi:10.1007/s00125-009-1440-6
- Dzwierzynski WW. Managing malignant melanoma. Plast Reconst Surg. 2013;132(3):446e–460e. doi:10.1097/PRS.0b013e31829ad411
- Enomoto LM, Levine EA, Shen P, Votanopoulos KI. Role of surgery for metastatic melanoma. Surg Clin North Am. 2019;100:127–139.
- Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther. 2017;7(Suppl 1):5–19. doi:10.1007/s13555-016-0165-y
- Avril MF, Charpentier P, Margulis A, Guillaume JC. Regression of primary melanoma with metastases. Cancer. 1992;69(6):1377–1381. doi:10.1002/1097-0142(19920315)69:6<1377::AID-CNCR2820690613>3.0.CO;2-N
- Arpaia N, Cassano N, Vena GA. Regressing cutaneous malignant melanoma and vitiligo-like depigmentation. Int J Dermatol. 2006;45(8):952–956. doi:10.1111/j.1365-4632.2004.02468.x
