Abstract
Purpose
Our recent studies found a splice region mutation in C3 accompanied by a significantly increased C3 in psoriatic peripheral blood. Mesenchymal stem cells (MSCs) are a key immunological suppression cell. We further investigate the regulation of MSCs on C3 in psoriasis.
Patients and Methods
We analyzed the C3 and its upstream S100A9, S100A8 and downstream MCP1 in psoriatic and control skin, and in normal human epidermal keratinocytes (NHEKs) co-cultured with psoriatic versus control dermal-derived mesenchymal stem cells (DMSCs) by mRNA, iTRAQ (isobaric tags for relative and absolute quantitative) and simple Western analysis.
Results
The mRNA and Simple Western analysis showed that the expression of C3, S100A8 and S100A9 are upregulated in psoriatic lesion (C3: mRNA, 9.23-fold, p = 0.0092; protein, 3.56-fold, p = 0.0244. S100A8: mRNA, 28.35-fold, p = 0.0015; protein, 4.68-fold, p = 0.0215. S100A9: mRNA, 79.45-fold, p = 0.0066; protein, 12.42-fold, p > 0.05). Moreover, the iTRAQ showed that C3 and S100A9 were significantly increased in NHEKs after co-cultured with psoriatic DMSCs compared to that of control DMSCs (C3: 3.40-fold, p = 0, FDR = 0; S100A9: 2.30-fold, p = 9.86E-241, FDR = 6.50E-239), verified by Simple Western. However, the expression of S100A8 and MCP1 was slightly different between the two groups.
Conclusion
Our results suggest that psoriatic DMSCs contribute to the increased C3 expression in psoriatic lesion via upregulating S100A9, providing the theoretical basis for the role of C3 and DMSCs in the pathogenesis of psoriasis.
Introduction
Psoriasis is a common chronic recurrent inflammatory disease.Citation1 According to the World Health Organization’s global report on psoriasis, it affects more than 2% of the world’s population,Citation2 and its incidence rate has increased from 0.123% in 1984 to 0.47% in China.Citation3 Psoriasis is mainly caused by abnormal immune response induced by excessive proliferation of keratinocytes (KCs) and the migration of immature keratinocytes to the epidermis.Citation4,Citation5 High recurrence rates seriously affect patients’ quality of life, which is a huge challenge for global public health.
Complement system is an inflammatory protein in the peripheral blood and tissue fluid. Complement C3, an important molecule in the complement pathway, plays an important role in both bypass and classical pathways. Studies showed that C3 protein is increased in serum, plasma, peripheral blood. Moreover, we found a splice region mutation of C3 and a significantly increased C3 protein in monozygotic twins discordant for psoriasis in our previous study.Citation6 The increased C3 expression may be originated from the local environment with high expression of TNF-α, IFN-γ and S100A8-S100A9.Citation7
Mesenchymal stem cells (MSCs) are a type of progenitor cells with the function of immune regulation widely existing in various tissues. MSCs play a role in promoting angiogenesis and vascular proliferation,Citation8–10 which are the most obvious pathological changes of psoriasis. Li et al found that human umbilical cord MSCs can inhibit C3a-C3aR signaling in chronic unpredictable mild stress model mice.Citation11 MSCs constitutively secrete factor H, a factor prohibiting the production of and inducing the degradation of C3.Citation12 Dermal mesenchymal stem cells (DMSCs) are a type of pluripotent stem cells isolated from skin tissue. DMSCs not only participate in the regeneration of skin tissue but also affect the skin microenvironment by secreting cytokines, growth factors and chemokines. Given that the external performance of psoriasis is skin, DMSCs have important implications for the pathophysiology of psoriasis. In the previous studies, DMSCs derived from psoriatic lesional skin, inhibit T lymphocyte proliferation,Citation13 express vascular endothelial growth factor (VEGF), promote local angiogenesis,Citation14 and induce the proliferation of KCs,Citation15,Citation16 resulting in local dermal thickening. However, the effect of DMSCs on the C3 expression of psoriatic was unknown. In this study, we investigated the impact of psoriatic DMSCs on C3 in the normal human epidermal keratinocytes (NHEKs).
Materials and Methods
Specimen Source
Eleven Chinese Han patients with quiescent psoriasis vulgaris who agreed to undergo plastic surgery at Taiyuan City Centre Hospital (6 women and 5 men, mean age 32.1 years), and eleven healthy controls (5 women and 6 men) were recorded in this study. Based on clinical and pathological characteristics, psoriasis vulgaris was diagnosed. Psoriasis area and severity index (PASI) scores were between 9.1 and 30.1 (mean 15.5) (). None of the patients had a related family history or other autoimmune diseases and had not received systematic treatment for at least 6 months before taking a skin sample. The volunteers did not develop systemic or autoimmune diseases. The experiment was approved by the medical ethics committee of Taiyuan City Centre Hospital, and the experiment is in accordance with the Declaration of Helsinki. All subjects were informed about the purpose of the study and provided written consent.
Table 1 Basic Information of Patients
Isolation and Expansion of Dermal-Derived Mesenchymal Stem Cells
Dermal mesenchymal stem cells (DMSCs) are a type of pluripotent stem cells isolated from skin tissue. The methods of isolation, culture and identification of DMSCs were consistent with previous reports.Citation17 The cell separation methods for normal skin and psoriasis lesion were referred to the above processes. The purity of the DMSCs was verified by using the flow cytometry (Beckman Coulter, Brea, CA, USA) with cell surface markers (negative for CD45, CD34, HLA-DR, but positive (>95%) for CD90, CD44, CD105, CD29, CD73, data not shown).
Co-Culture of Dermal-Derived Mesenchymal Stem Cells with Normal Human Epidermal Keratinocytes (NHEKs)
The specific experimental procedure is the same as the previous report.Citation18 In brief, the 2 × 105 NHEKs were inoculated in Transwell plate at passages 2–4 for co-culture with DMSCs of eleven psoriasis and eleven control in a 5% CO2 incubator at 37°C, respectively. In the co-culture experiment, eleven cases were performed in the P-DMSCs-Treated NHEKs group and C-DMSCs-Treated NHEKs group, respectively, in which cases were carried out in four batches. Untreated NHEKs group has four cases, and the experiment was also carried out in four batches. After 48 h, the cells were collected for further analysis. The NHEKs co-cultured with psoriatic and control DMSCs were labeled as “P-DMSC-Treated NHEKs” and “C-DMSC-Treated NHEKs”, respectively. The NHEKs in the absence of DMSC were labeled as “Untreated NHEKs”.
Quantitative Analysis of Protein Expression Using iTRAQ
The co-culture of NHEKs with five psoriatic DMSCs and five control DMSCs were used for iTRAQ. Experiment operation in accordance with our previous report.Citation19 First, we added appropriate amount of lysate and protease inhibitor, and then proteins were extracted by using an ultrasonic disruptor on the ice. Finally, the supernatant was collected after centrifugation at 4°C, and marked with iTRAQ. For the purpose of the data analysis, we established a reference database by using a list of standard peaks. The relative expression levels among samples were compared by t-test to determine the pivotal proteins of interest.
RNA Isolation and Quantitative RT-PCR Analysis
According to the operation's introduction, the tissue is placed into a mortar and is ground with liquid nitrogen. Trizol® Reagent (Ambion, USA) is designed to isolate high-quality total RNA from tissue samples, then cDNA synthesis was carried out using a PrimeScriptTM RT Master Mix Kit (Takara, Japan), and the reaction was performed at 37°C for 15 min and 85°C for 5 s. Quantitative RT-PCR was performed in a 7500 Real-Time PCR instrument (Applied Biosystems, Singapore) and PCR condition was 40 cycles of amplification (95°C, 15 s; 60°C, 30 s; and 72°C, 30 s). The relative amount of mRNA was calculated using the 2−ΔΔCt with the expression of β-actin was used as an internal reference. Primer sequences are shown in .
Table 2 Primer Sequences Used in This Experiments
Simple Western Analysis
According to the operation introduction, a Wes system is using 25-capillary cartridges of 12–230 kDa Separation Module (Proteinsimple, USA) and the Anti-Rabbit Detection Module (Proteinsimple, USA) for Simple Western analysis. In brief, combined 1 part 5× Fluorescent Master Mix with 4 parts diluted lysate of cells in a microcentrifuge tube, and heated for 5 min at 95°C. The biotinylated ladder, samples, blocking buffer, primary antibodies (Abcam, Cambridge, UK), secondary antibodies, luminol-peroxide mix substrate, and wash buffer were added to designated wells in a 25-capillary cartridge. Once the run is complete, the data are analyzed with Compass software.Citation20 The expression of β-actin was used as an internal reference.
Statistical Analysis
All data were shown as mean ± standard deviation and were analyzed using a paired Student’s t-test. Linear correlation analysis was used to analyze the correlation between protein expression and PASI score. Differences were considered statistically significant when the value of p < 0.05.
Results
The Expression of C3, S100A8 and S100A9 Increased in Psoriatic Lesion
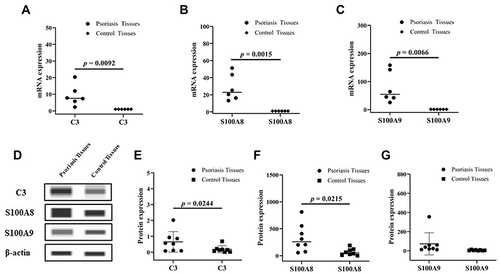
As shown in and , both the mRNA and protein expression of C3 (mRNA, 9.230 vs 1.000, p = 0.0092;protein, 0.6500 vs 0.1825, p = 0.0244) were significantly increased in psoriatic lesion compared to that of control skin. Furthermore, the expression of S100A8 and S100A9, the inducer of C3, were significantly upregulated in psoriatic lesion. The mRNA and protein expression of S100A8 were 28.35-fold (p = 0.0015) and 4.68-fold (p = 0.0215) upregulated, respectively ( and ). Consistently, the mRNA and protein expression of S100A9 were 79.45-fold (p = 0.0066) and 12.42-fold upregulated, respectively ( and ). showed Western blot images of C3, S100A8 and S100A9 (). Though the increase C3 expression was found in psoriasis, the local environment reason should be studied.
Psoriatic DMSCs Increased the C3 Expression in NHEKs Co-Cultured with Psoriatic DMSCs
MSCs, an inhibitor of C3 function, were reported to play an important role in psoriasis. To investigate the contribution of DMSCs to increase C3 expression in psoriasis, we examined the C3 expression in NHEK after co-cultured with psoriatic DMSCs. The iTRAQ results in five of eleven individuals showed that co-culture of NHEKs with psoriatic versus control DMSCs upregulated the expression levels of C3 (3.40-fold, p = 0, FDR = 0), being the significant upregulation protein (). Moreover, the Simple Western analysis results also showed that the co-culture of NHEKs with psoriatic versus compared to that of control DMSCs and untreated NHEKs significantly upregulated the expression levels of C3 (untreated NHEKs vs P-DMSCs-Treated NHEKs, 1.600 vs 2.392, p > 0.05, P-DMSCs-Treated NHEKs vs C-DMSCs-Treated NHEKs, 2.392 vs 0.857, p = 0.0011, ), being in good agreement with that of iTRAQ. The above results indicated that psoriatic DMSCs contributed to the increased C3 expression in psoriatic lesion.
Figure 2 Protein expression of iTRAQ and the Simple Western Analysis. Protein expression levels of C3 in normal human epidermal keratinocyte cells (NHEKs) co-cultured with psoriatic and control dermal mesenchymal stem cells (DMSCs) assessed by iTRAQ (A), and in NHEKs co-cultured with psoriatic and control DMSCs and untreated NHEKs assessed by Simple Western Analysis (n = 11), and the images of Western blot (B). By that analogy, protein expression levels of S100A9 by iTRAQ (C) and the Simple Western Analysis ((D), n = 11), protein expression levels of S100A8 by iTRAQ (E) and the Simple Western Analysis ((F), n = 11).
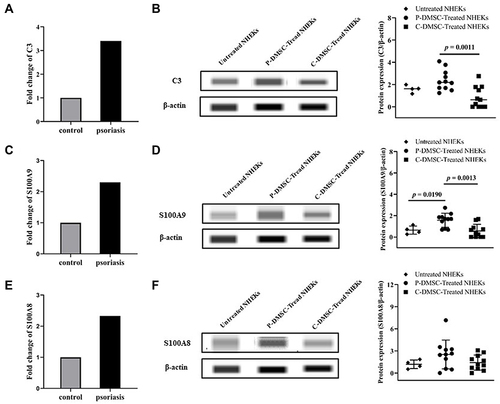
C3 Upstream-S100A8, S100A9 Expression Increased in NHEKs Co-Cultured with Psoriatic DMSCs
S100A9 and S100A8 are the upstream of C3,Citation7 adjusting the protein expression levels of C3. To investigate the induced mechanism of DMSCs on increased C3 expression, S100A9 and S100A8 were also evaluated. The iTRAQ results showed that both the expression of S100A9 (2.30-fold, p = 9.86E-241, FDR = 6.50E-239) and S100A8 (2.33-fold, p = 3.55E-34, FDR = 3.06E-33) were increased in NHEKs co-cultured with psoriatic DMSCs compared to that of control DMSCs (). In agreement with iTRAQ, the Simple Western analysis results showed that the expression of S100A9 was markedly upregulated in NHEKs co-cultured with psoriatic DMSCs compared to that of control DMSCs and untreated NHEKs (untreated NHEKs vs P-DMSCs-Treated NHEKs, 0.643 vs 1.575, p = 0.0190, P-DMSCs-Treated NHEKs vs C-DMSCs-Treated NHEKs, 1.575 vs 0.562, p = 0.0013, ). Meanwhile, the expression of S100A8 was slightly increased in NHEKs co-cultured with psoriatic DMSCs compared to that of control DMSCs and untreated NHEKs (2.516 vs 1.422), though the p value was higher than 0.05 (). However, the expression of MCP1 (the downstream of C3)Citation7 was similar in NHEKs co-cultured with psoriatic DMSCs and control DMSCs (4.855 vs 6.185, p > 0.05, ). The above results suggested that psoriatic DMSCs induce C3 expression was likely by upregulation of S100A9 in psoriatic lesion.
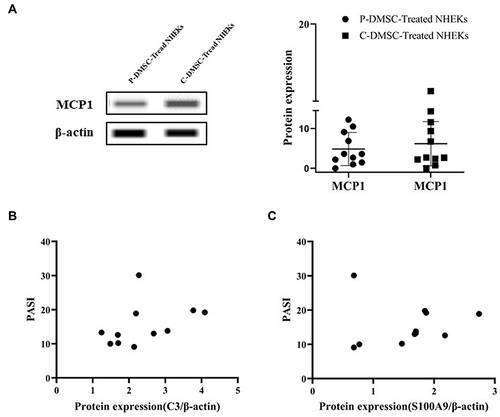
Figure 3 Protein expression of the Simple Western Analysis. Protein expression levels of MCP1 (A) in NHEKs co-cultured with psoriatic and control DMSCs assessed by Simple Western Analysis, and the images of Western blot; Linear correlation analysis for C3 (B) and S100A9 (C) levels with Psoriasis Area and Severity Index (PASI) score.
Linear Correlation Analysis Between Proteins and PASI Score
Correlation analysis is performed between the expression levels of protein and PASI score, and the statistical results show that the protein C3 with the value of p is 0.2233 (r = 0.3996), the protein S100A9 with the value of p is 0.9197 (r = 0.03453). Accordingly, the expression level of C3 and S100A9 did not correlate with PASI score in psoriatic patients ( and ). The results revealed that the C3 and S100A9 expression increased in NHEKs co-cultured with psoriatic DMSCs, did not play an obvious role in the formation of psoriasis area and severity, which may play an impact on the pathogenesis rather than the progression of psoriasis.
Discussion
Psoriasis is a complex pathophysiological disease that has been shown to be based on the interaction between immune cells and keratinocytes.Citation21 Dysregulation of KCs proliferation and differentiation is a major feature of psoriasis.Citation22
C3 acts as a potent activator for immune cells and can be produced by keratinocytes in the body.Citation23 The expression level of C3 was increased in the psoriasis lesionsCitation7 and may contribute to the inflammatory response of psoriasis.Citation24–26 Psoriatic patients had a local environment that promoted the expression of C3, such as the high level of TNF-α and IFN-γ.Citation27 Moreover, the treatment inflammatory mouse model with C3 inhibitors can significantly reduce the psoriatic skin lesions.Citation7 Additionally, the clinical treatment of psoriasis decreased the expression levels of C3. At present, PUVA is the main means of physical therapy for psoriasis, reducing the expression of IFN-γ stimulated keratinocytes producing C3 in a dose-dependent manner.Citation28 Anti-TNF-α antibody, a biological agent widely used in the treatment of psoriasis, significantly downregulated the C3 expression.Citation29 Therefore, the above mentioned research showed that C3 plays an important role in the pathogenesis of psoriasis. In this study, we found that the C3 expression in NHEKs was significantly increased after co-cultured with psoriatic DMSCs, indicating that psoriatic DMSCs participate in the pathogenesis of psoriasis by modulating the expression of C3.
S100A8-S100A9 regulate the expression of C3 by combining with the C3 promoter region. S100A8-S100A9 was thought to be a pivotal factor in psoriasis with an increased expression in psoriatic lesions. SchonthalerCitation7 et al suggested that the deletion of S100A8-S100A9 can prevent psoriasis, revealing that the upregulation of S100A8-S100A9 may be the trigger of disease. S100A8, S100A9 and MCP1 have the specific chemotactic activation, mediate intracellular inflammatory signal transduction pathways,Citation30 and play an important role in the regulatory of inflammation.Citation31 Moreover, they had extensive biological functions and participated in multiple biological processes. Hence, they are important proinflammatory cytokines. After the co-culture of NHEKs with psoriatic DMSCs, a very similar protein expression pattern was observed, including significantly upregulated expression of S100A8 and S100A9. S100A8-S100A9 participate psoriasis through its regulatory of inflammation both by direct regulation and indirect regulation via targeting C3.
In recent years, the role of skin derived MSCs in the pathogenesis of psoriasis has attracted extensive attention. The functions of MSCs include but are not limited to promoting wound healing,Citation32 inhibiting T cell proliferation,Citation13 affecting angiogenesis,Citation8 and inducing KCs proliferation.Citation16 These biological effects are mediated mainly by direct contact between cells, especially by paracrine effects. Recent studies have shown that DMSCs affect KCs connection, proliferation, differentiation, migration, and glucose metabolism through paracrine action.Citation19 We hypothesized that the increased expression of S100A9 and C3 may promote keratinocytes proliferation.
Conclusion
In this study, direct contact between NHEKs and DMSCs was avoided by co-culture. Therefore, the effect of DMSCs on NHEKs is caused by the products secreted by DMSCs. In addition, it was also found that psoriatic DMSCs show a different regulation on keratinocytes from control DMSCs. Taken together, co-culture of NHEKs with psoriatic DMSCs upregulated the expression of C3 and S100A9, providing further evidence for the pathogenesis of psoriasis induced by DMSCs. However, the pathogenesis of psoriasis induced by increased C3 expression remains to be further studied.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant [number 81773336 and 81401360]. The funding was attributed to the design of the study and the collection, analysis, and interpretation of data.
References
- Griffiths CEM, van der Walt JM, Ashcroft DM, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177(1):e4–e7. doi:10.1111/bjd.15610
- Mahler R, Jackson C, Ijacu H. The burden of psoriasis and barriers to satisfactory care: results from a Canadian patient survey. J Cutan Med Surg. 2009;13(6):283–293. doi:10.2310/7750.2009.08083
- Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–667. doi:10.1684/ejd.2012.1802
- Gamret AC, Alexandra P, Raymond MF, et al. Complementary and alternative medicine therapies for psoriasis. JAMA Dermatol. 2018;154:1330–1337. doi:10.1001/jamadermatol.2018.2972
- Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32(1):227–255. doi:10.1146/annurev-immunol-032713-120225
- Li JQ, Lin HX, Hou RX, et al. Multi-omics study in monozygotic twins confirm the contribution of de novo mutation to psoriasis. J Autoimmun. 2020;106:102349. doi:10.1016/j.jaut.2019.102349
- Schonthaler HB, Guinea-Viniegra J, Stefanie KW, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39(6):1171–1181. doi:10.1016/j.immuni.2013.11.011
- Bussche L, Gerlinde R. Peripheral blood-derived mesenchymal stromal cells promote angiogenesis via paracrine stimulation of vascular endothelial growth factor secretion in the equine model. Stem Cells Transl Med. 2014;3(12):1514–1525. doi:10.5966/sctm.2014-0138
- Hou RX, Yan H, Niu XP, et al. Gene expression profile of dermal mesenchymal stem cells from patients with psoriasis. J Eur Acad Dermatol. 2014;28(12):1782–1791. doi:10.1111/jdv.12420
- Hou RX, Yin GH, An P, et al. DNA methylation of dermal MSCs in psoriasis: identification of epigenetically dysregulated genes. J Dermatol Sci. 2013;72(2):103–109. doi:10.1016/j.jdermsci.2013.07.002
- Li J, Wang HL, Du CB, et al. hUC-MSCs ameliorated CUMS-induced depression by modulating complement C3 signaling-mediated microglial polarization during astrocyte-microglia crosstalk. Brain Res Bull. 2020;163:109–119. doi:10.1016/j.brainresbull.2020.07.004
- Tu ZD, Li Q, Bu H, et al. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19(11):1803–1809. doi:10.1089/scd.2009.0418
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi:10.1182/blood-2004-09-3696
- Chang WJ, Niu XP, Hou RX, et al. LITAF, HHEX, and DUSP1 expression in mesenchymal stem cells from patients with psoriasis. Genet Mol Res. 2015;14(4):15793–15801. doi:10.4238/2015.December.1.31
- Liu RF, Wang F, Wang Q, et al. Research note mesenchymal stem cells from skin lesions of psoriasis patients promote proliferation and inhibit apoptosis of HaCaT cells. Genet Mol Res. 2015;14(4):17758–17767. doi:10.4238/2015.December.21.49
- Santos JM, Camões SP, Filipe E, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6(1). doi:10.1186/s13287-015-0082-5
- Liu RF, Wang Y, Zhao XC, et al. Lymphocyte inhibition is compromised in mesenchymal stem cells from psoriatic skin. Eur J Dermatol. 2014;24(5):560–567. doi:10.1684/ejd.2014.2394
- Li XH, Li JQ, Lu FN, et al. Role of SPRED1 in keratinocyte proliferation in psoriasis. J Dermatol. 2020;47(7):735–742. doi:10.1111/1346-8138.15369
- Li JQ, Xing JX, Lu FN, et al. Psoriatic dermal-derived mesenchymal stem cells reduce keratinocyte junctions, and increase glycolysis. Acta DV. 2020;100(8):adv00122–7.
- Wang Y, Liang YY, Li J, et al. Expression and functional regulation of gap junction protein Connexin 43 in dermal mesenchymal stem cells from psoriasis patients. Acta Histochem. 2020;122(4):151550. doi:10.1016/j.acthis.2020.151550
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi:10.1056/NEJMra0804595
- Ekman AK, Vegfors J, Bivik EC, et al. Overexpression of psoriasin (S100A7) contributes to dysregulated differentiation in psoriasis. Acta DV. 2017;97:441–448.
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–727. doi:10.2353/ajpath.2007.070166
- Basset-Séguin PM, Dereure O, Mils V, et al. C3d, g deposits in inflammatory skin diseases: use of psoriatic skin as a model of cutaneous inflammation. J Invest Dermatol. 1993;101(6):827–831. doi:10.1111/1523-1747.ep12371702
- Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181(10):7420–7427. doi:10.4049/jimmunol.181.10.7420
- Nacken W, Roth J, Sorg C, et al. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60(6):569–580. doi:10.1002/jemt.10299
- Volanakis JE. Transcriptional regulation of complement genes. Annu Rev Immunol. 1995;13:277–305. doi:10.1146/annurev.iy.13.040195.001425
- Terui T, Funayama M, Terunuma A, et al. Ultraviolet B radiation exerts enhancing effects on the production of a complement component, C3, by interferon-gamma-stimulated cultured human epidermal keratinocytes, in contrast to photochemotherapy and ultraviolet A radiation that show suppressive effects. Br J Dermatol. 2000;142:660–668. doi:10.1046/j.1365-2133.2000.03410.x
- Chimenti MS, Perricone C, Graceffa D, et al. Complement system in psoriatic arthritis: a useful marker in response prediction and monitoring of anti-TNF treatment. Clin Exp Rheumatol. 2012;30:23–30.
- Markowitz J, Carson WE. Review of S100A9 biology and its role in cancer. Biochim Biophys Acta. 2013;1835(1):100–109. doi:10.1016/j.bbcan.2012.10.003
- Lim SY, Raftery MJ, Goyette J, et al. S-Glutathionylation regulates inflammatory activities of S100A9. J Biol Chem. 2010;285(19):14377–14388. doi:10.1074/jbc.M109.075242
- Vincent F. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi:10.1016/S0140-6736(05)67700-8