Abstract
Purpose
Acne is a skin condition of the pilosebaceous unit that affects mainly the face, chest and trunk. Approximately 50% of subjects with facial acne also have acne of the trunk. This study investigated the clinical benefit of a cleansing gel containing salicylic acid 2%, zinc gluconate 0.2% and lipohydroxy acid 0.05% in truncal acne after 84 days of daily use.
Materials and Methods
A single center, open label, non-randomized study with 51 subjects with mild to moderate truncal acne was conducted. Thirty-five (35) subjects completed the study; mean age was 23 years. Inflammatory and non-inflammatory lesions, Transepidermal water loss (TEWL) and local tolerance were assessed at baseline, Day 42 and Day 84 and total lesion count was calculated.
Results
The total lesion count was significantly reduced (p<0.05) after 42 days (−21.5%) and 84 days (−56.3%). Non-inflammatory lesions were significantly decreased after 84 days (−64.0%) only, while inflammatory lesions were decreased at Day 42 (−29.2%), and Day 84 (−48.2%). A statistically significant skin barrier improvement was observed at Day 84 (−21.26%). No adverse events or relevant local intolerance were reported.
Conclusion
The use of the cleansing gel studied was effective in improving mild to moderate truncal acne and contributed to the skin barrier improvement. The product was well tolerated.
Clintrial Data Base Identifier
NCT05584150.
Introduction
Acne vulgaris is a chronic inflammatory disease, mainly of the face, but also of the trunk.Citation1 Acne may be caused by internal and external factors.Citation2,Citation3 Only a very small amount of data exists concerning truncal acne.Citation4,Citation5 The condition affects about 9% of the population worldwide, with 50% of subjects with facial acne also presenting with truncal acne.Citation6,Citation7
It is commonly thought that truncal and facial acne have a common pathogenesis.Citation8,Citation9 While increased sebum secretion, follicular epidermal hyperproliferation, skin microbiome with mainly Cutibacterium acnes (C. acnes) strains and inflammation are commonly thought to trigger acne, research conducted by Kim et al showed that hyperseborrhoea may not play a main role in truncal acne.Citation10,Citation11
Until recently, clinicians frequently followed the same therapeutic approach as for facial acne, with treatment adherence remaining an issue.Citation6,Citation12,Citation13
This study investigated the clinical benefit of a cleansing gel containing salicylic acid 2%, zinc gluconate 0.2% and lipohydroxy acid (LHA 0.05% (Effaclar® cleansing gel, La Roche-Posay Laboratoire Dermatologique, France), hereafter DC) in truncal acne after 84 days of daily use Salicylic acid decreases sebocyte lipogenesis, zinc gluconate is indicated in the treatment of acne in reducing inflammation, while LHA has skin renewing, exfoliating, and acne treating properties.Citation14–17
Patients and Methods
For this single center, open label, non-randomized exploratory study, 51 subjects with mild to moderate truncal acne were recruited. The study received ethics committee approval (PRÓ-CARDÍACO Hospital, CAAE: 20641819.7.0000.5533), conformed to all local legal requirements for the conduct of a clinical study and complied with the Principles of the Declaration of Helsinki. All subjects provided written informed consent prior to inclusion. The investigator counted the number of inflammatory, non-inflammatory and total lesions, the sum of both inflammatory and non-inflammatory acne lesions at Baseline, Day 42 and 84. Skin barrier function was appraised via the assessment of transepidermal water loss (TEWL) using a Tewameter® (Courage + Khazaka Electronic GmbH, Germany) on a predefined area of the upper back of the subjects. Safety was assessed by considering the evaluation of clinical signs and reporting of symptoms, and local adverse reactions. Subjects assessed efficacy at Day 42 and 84 and product perception at Day 84.
Subjects were asked to gently massage the wet skin of the trunk with the product in the morning and evening for at least 30 seconds, and then thoroughly rinse with tempered water and pat dry.
For each parameter, the evolution over time was calculated using either the Student’s t-test for paired data or the Wilcox on Signed Rank Test. A 5% significance level was applied to show significance.
Results
In total, 35 subjects with a mean age of 23±4 years completed the study. Slightly more females (57%) were included. A majority of subjects had phototype III (60%); 66% were Caucasians. The mean inflammatory lesion count was 42.1 ±43.7, the mean non-inflammatory lesion count was 44.8 ±56.9 and the total lesion count was 86.9 ±87.4; details are given in .
Table 1 Demographic and Baseline Characteristics
After 42 days, inflammatory lesions had significantly (p<0.005) decreased by 29.2% and by 48.2% after 84 days, while non-inflammatory lesions significantly (p<0.005) decreased only after 84 days (−64.0%). The total number of lesions had significantly (p<0.05) decreased by 21.5% at Day 42, sustaining until Day 84 (−56.3%). So did the transepidermal water loss at Day 84 (12.7±2.0, −21.3%, p<0.005), compared to baseline (16.1±2.1).
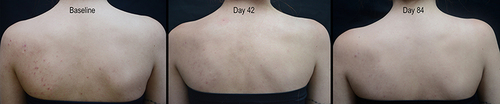
provides visual support of truncal acne on the back of a female acne at baseline and after 42 and 84 days of care.
Moreover, we evaluated the gender impact of the gel cleanser with regards to the improvement of truncal acne. At baseline, women reported fewer total lesions (78.4±89.4) than men (93.5±87.2). After 84 days, females confirmed a higher percentage of improvement of their lesions (inflammatory lesions: −68.6%), non-inflammatory lesions: −68.5%, total lesions: −68.6%) compared to males (inflammatory lesions: −37.7%, non-inflammatory lesions: −58.1%, total lesions: −49.4%). More than 90% of all subjects considered that the cleansing gel was easy to apply, to spread and to rinse, and considered that the product was gentle on the skin and suitable for daily use.
Local tolerance was good.
Discussion
The present results demonstrate that the use of a cleansing gel containing salicylic acid 2%, LHA 0.05% and zinc gluconate 0.2% significantly (p<0.05) improves truncal acne as early as after 42 days.
The gender analysis showed that acne improved more in females than in males. We do not have any explanation for that. However, this finding may be due to the difference in total lesion count at baseline.
Acne vulgaris is associated with intrinsic abnormalities of epidermal barrier functions. The follicular epithelial barrier disruption is directly involved with changes that occur both in comedogenesis, in inflammatory phases of acne, especially with the rupture of the follicle.Citation18 The degree of stratum corneum permeability barrier impairment is correlated with the severity of the condition. TEWL is an indicator of the disturbed skin barrier. TEWL assessments from this study showed an increase of water loss within 42 days which than was reduced after 84 days confirming that the DC improves the damaged skin barrier. Subjects highly appreciated the tested DC.
In conclusion, the use of a deep cleansing gel containing salicylic acid 2%, zinc gluconate 0.2% and LHA 0.05% is beneficial in the management of mild to moderate truncal acne. The deep cleansing gel significantly reduces the number of truncal acne lesions and improves the skin barrier by reducing TEWL. It is well tolerated and highly accepted.
Data Sharing Statement
Priscila Correia, the corresponding author will share upon reasonable request for one year after publication of this manuscript the study protocol and all data collected and statistically analysed and in relationship with this study, except deidentified participant data.
Ethical Statement
The present study received ethic committee approval from PRÓ-CARDÍACO Hospital ethics committee, CAAE: 20641819.7.0000.5533, on October 2, 2019. The study complied with the Principles of the Declaration of Helsinki.
Disclosure
Priscila Correia, Mariana Fajgenbaum Feiges, José Euzébio Gonçalves Junior, Beatriz Sant’Anna, Delphine Kerob and Caroline Le Floc’h are employees of L’Oréal Group. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors acknowledge the writing support of Karl Patrick Göritz, SMWS, France.
Additional information
Funding
References
- Nast A, Dreno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1):1–29. doi:10.1111/j.1468-3083.2011.04374.x
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):5754. doi:10.1038/s41598-020-62715-3
- Dréno B, Bettoli V, Araviiskaia E, Sanchez Viera M, Bouloc A. The influence of exposome on acne. J Eur Acad Dermatol Venereol. 2018;32(5):812–819. doi:10.1111/jdv.14820
- Tan JK, Tang J, Fung K, et al. Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol. 2008;7(6):551–556.
- Tan J, Thiboutot D, Popp G, et al. Randomized Phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–1699. doi:10.1016/j.jaad.2019.02.044
- Eichenfield DZ, Sprague J, Eichenfield LF. Management of acne vulgaris: a review. JAMA. 2021;326(20):2055–2067. doi:10.1001/jama.2021.17633
- Poli F, Auffret N, Leccia MT, Claudel JP, Dréno B. Truncal acne, what do we know? J Eur Acad Dermatol Venereol. 2020;34(10):2241–2246. doi:10.1111/jdv.16634
- Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77(5):285–289.
- Gollnick HP. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol. 2015;29(Suppl 5):1–7.
- Kim BR, Chun MY, Kim SA, Youn SW. Sebum secretion of the trunk and the development of truncal acne in women: do truncal acne and sebum affect each other? Dermatology. 2015;231(1):87–93. doi:10.1159/000382125
- Dagnelie MA, Poinas A, Dréno B. What is new in adult acne for the last 2 years: focus on acne pathophysiology and treatments. Int J Dermatol. 2022;61(10):1205–1212. doi:10.1111/ijd.16220
- Bell KA, Brumfiel CM, Haidari W, Boger L. Trifarotene for the treatment of facial and truncal acne. Ann Pharmacother. 2021;55(1):111–116. doi:10.1177/1060028020934892
- Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456. doi:10.1111/j.1365-4632.2010.04416.x
- Brocard A, Dreno B. Innate immunity: a crucial target for zinc in the treatment of inflammatory dermatosis. J Eur Acad Dermatol Venereol. 2011;25(10):1146–1152. doi:10.1111/j.1468-3083.2010.03934.x
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 Suppl 1):s11–6.
- Zeichner JA. The use of lipohydroxy acid in skin care and acne treatment. J Clin Aesthet Dermatol. 2016;9(11):40–43.
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786–794. doi:10.1111/exd.13934
- Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? Do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6(2):18–24.