Abstract
Purpose
This prospective, single-center study aims to evaluate the safety and effectiveness of NEAUVIA Intense, a PEG cross-linked polymeric hydrogel, in correcting moderate-to-severe nasolabial folds (NLF) in a routine clinical setting. The study investigates the aesthetic outcomes, patient satisfaction, and adverse events associated with the injectable filler.
Patients and Methods
Seventy patients were initially enrolled, with 60 meeting study parameters. The post-market study involved a single session treatment, employing NEAUVIA Intense on each side of the NLF. Assessments utilized the Modified Fitzpatrick Wrinkle Scale (MFWS), Global Aesthetic Improvement Scale (GAIS), and Visual Analogical Scale (VAS).
Results
The study demonstrated a statistically significant improvement in tissue depression immediately post-injection (p < 0.001), with sustained effects up to 6 months. MFWS assessments revealed that responder patients were 96.6% immediately after treatment, 76.6% one month, 48.3% after 3 months, and 28.3% at 6 months (p < 0.001). Additionally, there was a significant change in the frequency distribution of MFWS scores post-treatment (p < 0.001), with the majority of patients experiencing improvement in tissue depression. Maximum improvement was observed at 30- and 90-days post-treatment based on GAIS assessments. Patient and physician satisfaction, measured by VAS, remained stable over time, with fluctuations at 4 and 24 weeks after treatment (p < 0.001, Anova; p < 0.05, Wilcoxon). Throughout the entire follow-up duration of the patients enrolled in the study, no adverse effects related to the use of the product were observed.
Conclusion
NEAUVIA Intense proved to be an effective solution for correcting NLF, providing significant and lasting improvements in tissue depression and aesthetic outcomes. The study underscores the necessity for continuous assessment in aesthetic medicine to align outcomes with evolving patient expectations and optimize long-term results. The findings contribute to the understanding of this specific hydrogel filler and highlight the broader context of injectable fillers in comprehensive facial aesthetic strategies.
Introduction
A novel branch of aesthetic medicine has emerged, offering a minimally invasive alternative to plastic surgery for addressing facial ageing. This specialized field focuses on techniques like intra-dermal or subcutaneous filler injections, particularly hyaluronic acid (HA) fillers, known for their remarkable aesthetic improvements with minimal downtime.Citation1–3 Dermal fillers, classified as class III medical devices, effectively restore facial volume and treat aesthetic defects such as wrinkles and scars. In particular, the nasolabial folds (NLF) are deep wrinkles located from the side of the nose to the corner of the mouth, commonly known as “laugh lines”, and they change with age due to the loss of the integrity of the underlying tissues and of the volume itself, due to the decrease in HA and the alteration of collagen and tissue elasticity.Citation4–8 These “smile lines” can already be seen in younger adulthood, but this occurs mostly in the 40–50 age group. Injectable HA gels can be used to treat NLF defects by restoring volume and improving the natural three-dimensional contour of the area.Citation8 This treatment offers several advantages, including the reestablishment of a more appropriate psychosocial function.Citation9,Citation10 Common complications of fillers include minor infections, allergic reactions, and swelling. Although rare, autoimmune reactions, visual disturbances, and strokes have been reported.Citation11 Therefore, doctors need to receive proper training in the use of dermal fillers to be knowledgeable about potential complications and able to manage them immediately.Citation12,Citation13 Doctors must have experience in selecting the right injectable product for patients based on their characteristics and facial anatomy. To prevent adverse events, safe preparation and injection techniques must be used. Hyaluronidase is a necessary antidote to protect against unexpected complications, especially for HA-based fillers.Citation14,Citation15 Injectable fillers have various applications in specific pathologies, such as the recovery of subcutaneous fat atrophy, a peculiarity of HIV-infected patients.Citation16,Citation17 They are also used for correcting facial defects and traumatic facial injuries of all types, which can cause significant psychological discomfort and negative effects on the psyche and social life of the patient. Facial asymmetries, scars, and other deformities have an unfavorable impact on the emotionality of the traumatized subject, especially among young people involved in road accidents, sporting accidents, violent actions or tumbles.Citation18 Less invasive treatments are being developed to restore facial volume and improve the appearance of scars. Dermal fillers, autologous fat grafting, laser, and dermabrasion are effective methods that can complement major surgery or be used as less invasive substitutes. They positively impact facial volume and the appearance of scars, making them better accepted by traumatized individuals.Citation18–20 HA fillers are widely used for facial rejuvenation and are the most common dermal filler treatment.Citation1 HA is a biopolymer naturally found in the body and has many beneficial properties.Citation1 It is non-toxic, biodegradable, non-immunogenic, and has various biological functions.
Naturally occurring HA has a short life of around 1–2 days and is eliminated through the liver and lymphatic system. To maintain its properties and prevent degradation, cross-linking is necessary. This process helps to prolong its residence time in the dermis, and conjugation is also important.Citation21 Hyaluronic acid (HA) is used in bioengineering, orthopedics, and ophthalmology for creating biomedical devices. It is available in forms like nanoparticles, nanocomplexes, matrices and hydrogels.Citation22 HA-based hydrogels are used in various medical applications, including dermal fillers. To make them safer for use in the body, cross-linking agents are used to reduce the biodegradation rate and balance the viscoelastic properties.Citation23,Citation24 Research aims to find maximum safety and quality of hydrogels. Cross-linking principles are fundamental to knowing the hydrogel’s rheological and swelling peculiarities, which are essential for the definition and clinical application.Citation25 NEAUVIA Intense is a PEG cross-linked polymer hydrogel. It is composed of stabilized sodium hyaluronate 28 mg/mL, glycine and L-proline in pyrogen-free buffered water. It is used to transiently modify the defects already present or acquired from the soft fabrics of the face through injection in the dermis, and its deterioration takes place precisely after the introduction, focusing on the polyethylengicolydilidiletere (PEGDE) reticulation method, which ensures biocompatibility, biointegration and rheological characteristics of quality.Citation26–29 The cross-linking agent polyethylene glycol (PEG) has been a source of in-depth study regarding its characteristics for implementation in the biotechnological and pharmaceutical fields. PEG is safer and less toxic than other cross-linking compounds used in the recent past. Furthermore, its specificity leads to a reduction of immunogenicity and antigenicity.Citation30–32
In the literature, currently, there are no studies regarding the safety and effectiveness of PEG cross-linked HA hydrogel (NEAUVIA Intense) regarding the treatment of facial soft tissue deficits, especially regarding the remodeling of problems with moderate or severe NLF. Therefore, our work aims to evaluate the actual effectiveness of NEAUVIA Intense in correcting facial soft tissue defects in the ways previously reported.
Materials and Methods
This work was a post-market, non-profit, prospective, single-center, single-arm, real-life, open-label study. Our research’s characteristics lead to evaluating the subjects treated in a broad context of routine clinical practice. Each procedure, treatment or use of resources was chosen at attending physician’s discretion within the therapeutic procedures recommended by the Scientific Community.
Our work aims to resolve the efficacy and safety of the injectable implant based on cross-linked PEG hydrogel HA (NEAUVIA Intense – Matex Lab) for the correction of moderate-to-severe NLF to obtain results in this regard to a risk/benefit assessment in a routine clinical setting. In this first targeted study, our goal is to highlight the effectiveness of NEAUVIA Intense dermal fillers, specifically. This evaluation of merit will be carried out through the photographs produced by the investigator, comparing the images before the treatment with those after the execution of the same. We used two assessment scales to achieve our goal: the Modified Fitzpatrick Wrinkle Scale (MFWS)Citation33 and the Global Aesthetic Improvement Scale (GAIS).Citation34 Finally, the evaluation status was completed through the satisfaction of the aesthetic result by both the researcher and the subject treated with the NEAUVIA Intense dermal filler, using the Visual Analogical Scale (VAS).Citation35,Citation36
The study was approved by the local ethical committee of Pavia (number of protocol P-20200010552) in accordance with the International Conference on Harmonization (ICH) and Good Clinical Practice (GCP) and the Declaration of Helsinki. In addition, the study was conducted in compliance with all applicable local and international laws and regulatory requirements relevant to the use of medical devices. The study was conducted at the Centro Medico Polispecialistico of Pavia (Italy).
Product Description and Treatment
NEAUVIA Intense consists of highly purified sodium hyaluronate of non-animal origin derived from bacterial fermentation, which is non-pathogenic for humans. The hydrogel is gelatinous, transparent and colorless. The device is supplied in pre-filled 1.0 mL disposable syringes with a Luer-lock connection. The package contains the device, instructions for use, needle and product identification labels.
Product delivery was performed under aseptic conditions by bolus or linear retrograde injection employing a 27G needle (G) or a 22G or 25G cannula. The tissue correction took place on the subcutaneous or supraperiosteal anatomical level. After implantation, participants remained in the medical room for approximately 60 minutes to be monitored and evaluated for possible adverse events. No ice, topical anaesthetics or nerve blocks were used prior to treatment.
Selection of Study Population
Seventy patients were enrolled, but only 60 fell within the total parameters of the study protocol. Fifty-eight were females (96.6%) and 2 were males (3.4%).
Patient inclusion criteria were:
to be men or women, at least 18 but not more than 70 years of age at enrolment.
to have congenital or acquired soft tissue deficits of the NLF with a score of at least 1.5 according to the MFWS;Citation33
to have a reasonable potential for benefit from correction;
to be able to understand and comply with the requirements of this study;
to be willing and able to provide medical history and Informed Consent prior to any study-related procedures being performed;
to be willing to comply with all aspects of the treatment and follow-up schedule and procedures.
Patient exclusion criteria were:
to be pregnant, lactating, or trying to become pregnant;
to be children or teenagers;
to have had prior therapy (eg, other permanent or biodegradable injectable fillers or surgical correction) within 3 months prior to the HA injection;
to have had previous tissue augmentation with permanent implants (eg, silicone) in the area to be treated;
to have any active inflammation, infection (acne, herpes, dermatitis, etc.) or unhealed wound of the face;
to have varices in the area of the implant;
to have auto-immune disorders affecting the skin;
to undergo radiation or ultrasound therapy in the area of the implant;
to have a known hypersensitivity to the test product (HA or its ingredients);
to tend to develop hypertrophic scarring;
to suffer from untreated epilepsy;
to have a history of anaphylaxis or history of severe allergies;
to use simultaneously laser treatment, deep chemical peels or dermabrasion;
to use aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) within 2 weeks prior to the treatment or to take concomitant anticoagulant therapy, anti-platelet therapy, or to have a history of bleeding disorders;
to use photosensitizing drugs (antidepressants, retinoids) within 2 weeks prior to the treatment.
A subject was to be withdrawn from the study for any of the following reasons:
Lost to follow-up;
Withdrawal of consent;
The Principal Investigator believed that for safety reasons (eg, AE, concurrent illness), it was in the best interest of the subject to be withdrawn from study participation;
The subject’s attending physician requested that the subject be withdrawn from the study;
Lack of compliance with study procedures or poor visit attendance;
A significant protocol deviation or violation.
Assessment
All patients were assessed at 1 month, 3 months and 6 months post-injection by the Principal Investigator using the MFWS,Citation33 a validated 7-point rating scale used to quantify the results obtained from the treatment, and GAIS,Citation34 a 5-point scale rating global aesthetic improvement in appearance. Moreover, for the clinical assessments, patients were asked to complete a satisfaction score module for the tested product to judge their change in appearance after the treatment. This module was submitted to patients immediately after the treatment, 1 month after the treatment, 3 months after the treatment and 6 months after the treatment. The satisfaction score was based on VAS;Citation35,Citation36 the score was determined by measuring the distance on the 10-cm line between the “no satisfaction” and “high satisfaction”. The patients, and later the PI or CIT as well, scored themselves, providing a range that included a value between 0 and 10.
Data Collection
Baseline information, including standardized facial photographs, was collected prior to treatment. Details of the treatment session were recorded: these details included needle/cannula size, technique, and volume of product used. Details of the session were recorded, with safety outcomes and treatment of adverse events retrieved from patient medical records. All patients were asked for details of any post-treatment adverse events they might have experienced.
To quantify the results obtained from the treatment, the MFWS,Citation33 a validated 7-point rating scale was used, incorporating 5 categories, as follows: 0 = no wrinkle; 0.5 = very shallow yet visible wrinkle; 1 = fine wrinkle; 1.5 = visible wrinkle and slight indentation, <1 mm wrinkle depth; 2 = moderate wrinkle: clear visible wrinkle 1–2 mm wrinkle; 2.5 = prominent and visible wrinkle: more than 2 mm and less than 3 mm wrinkle depth; 3 = deep wrinkle more than 3 mm wrinkle depth.
To grade of appearance, GAISCitation34 was used, incorporating 5 categories, as follows: 1 = very much improved; 2 = much improved; 3 = improved; 4 = no change; 5 = worse.
Additionally, for clinical evaluations, patients were asked to complete a satisfaction score form with the tested product, to rate their change in appearance after treatment. This form was sent to patients immediately after treatment, 1 month after treatment, 3 months after treatment, and 6 months after treatment.
The satisfaction score was based on the VAS;Citation35,Citation36 the score was determined by measuring the distance on the 10 cm line between “no satisfaction” and “high satisfaction” and the patient’s sign, giving a range of scores from 0 to 10. A higher score indicated greater satisfaction. The patients obviously completed the self-assessment in total autonomy. The same form was then drawn up by the PI the CIT, to assess the investigator’s satisfaction with the treatment.
Statistical Analysis
All statistical analyses were performed using Jamovi software version 2.2.5.Citation37,Citation38 The software used to perform the statistical analysis, as well as the data management activities, is fully validated. The analysis was mainly descriptive, with quantitative variables expressed as means and standard deviations and qualitative variables expressed as frequencies and percentages. Quantitative variables are compared using the T Test for paired data. Frequencies and percentages are analyzed by Chi-square test, while the comparison of non-parametric scales measured before and after the NEAUVIA Intense injection was performed by ANOVA for repeated measurements (Friedman Chi-square) and also by the Wilcoxon test. A p-value of <0.05 was considered statistically significant for all analyses.
Results
Initially, 70 patients were enrolled in this study, but 10 (14.3%) did not complete the study due to non-compliance with the survey rules. Therefore, they were excluded from the protocol. Patients received NEAUVIA Intense injectable subdermal filler treatment in one session on each side of the NLF for correction. The mean total dose injected into each side of the NLF was 1 mL. Therefore, as described above, a total of 60 patients were prospectively evaluated, of which 58 females (96.7%) and 2 males (3.3%), with a mean age of 56.3 years (range: 36–70 years).
Effectiveness
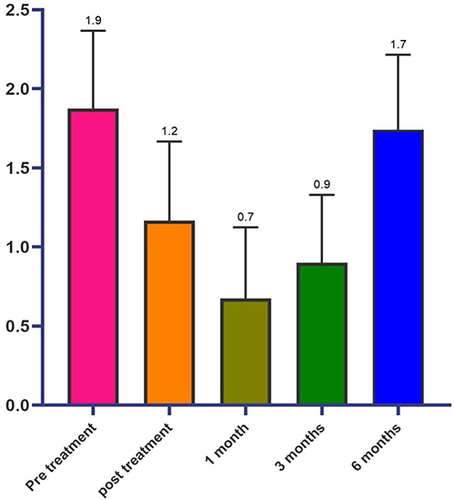
In the first analysis, shows the average values of the scores obtained from the subministration of MFWS before the treatment, immediately and 1, 3, and 6 months after the injection.
The responder patients, ie for whom an increase of 0.5 points compared to the baseline value was observed, were 96.6% immediately after treatment (), 76.6% at one month, 48.3% after 3 months and 28.3% at 6 months (). The MFWS shows a sensible improvement of the tissue depression immediately after the injection with a substantial improvement that is maximum 1 month (p < 0.001) after the implant and it is maintained till the end of the observation time () (p < 0.001), as shown in .
Table 1 Percentage of Responding Patients as Defined in the Protocol
Table 2 Statistical Significance of the Values Measured in the Different Visits
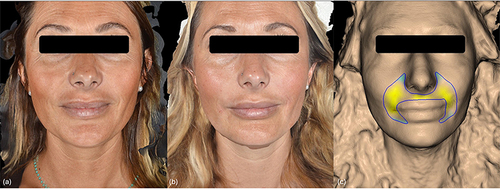
Figure 2 (a and b) Participant prior to receiving treatment as part of the study and immediately after. (c) Improvement of NLF with 3D facial reconstructions generated by QuantifiCare® software immediately following treatment.
In analyzing the frequency of MFWS during different temporal assessments shown in and , it was found that patients in the pre-treatment condition mostly had scores in level 1.5 (55%), level 2 (23.3%), level 2.5 (13.4%), and level 3 (8.3%). However, after the injection treatment, there was a significant change in the frequency distribution of the MFWS scores. The majority of patients were at level 1.0 (43.3%), followed by level 1.5 (25%), level 0.5 (16.7%), level 2 (11.7%), and level 0.0 and 2.5 (1.7%). Four weeks post-treatment, the number of patients at level 1.0 decreased to 38.3%, while level 0.5 increased to 36.7%. Patients at level 0.0 decreased to 18.3%, level 1.5 decreased to 5%, and level 2 decreased to 1.7%. After twelve weeks from the post-treatment injection, the distribution of the patients was found to be as follows: level 0.5 (43.3%), level 1 (38.4%), level 1.5 (13.3%), and level 2 (5%). Finally, after six months from the treatment carried out, the results showed a greater percentage of patients at level 1.5 (53.4%), followed by level 2 (25%), then levels 1.5 and 2.5 (8.3%), and finally level 3 (5%).
Table 3 Frequencies of MFWS
The changes in the mean values detected in the checks are carried out immediately after the injection, and in the subsequent controls, they are always statistically significant (Anova p < 0.001; Wilcoxon test p 0.01) as reported in .
Table 4 Statistical Significance of MFWS Rates Measured at the Different Evaluation Steps
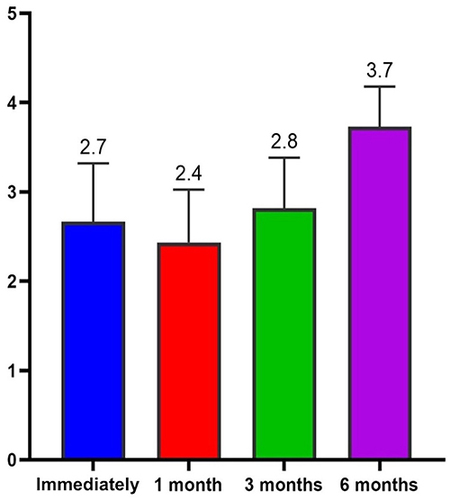
The Average GAIS calculation of the population in the different evaluation time steps (0, 30, 90, 180 days) showed us an interesting trend with the maximum improvement of the correction in the time point of 30 and 90 days ().
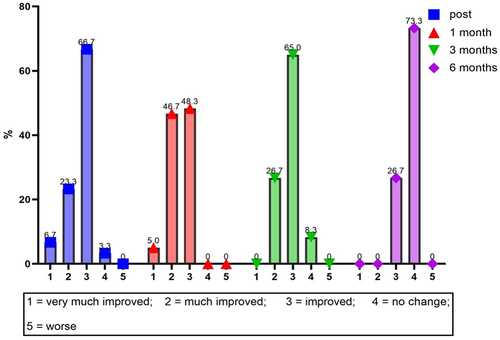
The results of the investigator’s GAIS assessment at 4 weeks post-treatment showed that the majority of patients were “improved” (29 patients [48.3%]) or “much improved” (28 patients [46.7%]). Three patients were classified as “very much improved” and 0 patients were classified as “no change” or “worse” (0%).
Again, 12 weeks after treatment, the investigator assessed that the majority of subjects had “improved” (39 patients [65%]) and 16 patients [26.7%] had “much improved”. Zero patients were rated as ‘very much improved,’ 5 patients were rated as ‘no change,’ and 0 were rated as ‘worse’ (0%).
Finally, 24 weeks after the injection treatment, the investigator declared that the most significant number of those treated “had no change” (44 patients [73.3%]) and 16 [26.7%] had “improved”. No patient was classified as “very much improved”, “much improved” or “worse” (, ).
Table 5 Mean Values of GAIS Rate at the Different Evaluation Steps
The statistical analysis carried out on repeated administrations of the GAIS highlights statistical significance (Wilcoxon p < 0.01 and Anova Friedman p < 0.01) at one month and at 6 months and overall (ANOVA p < 0.001) ().
Table 6 GAIS Statistical Analysis
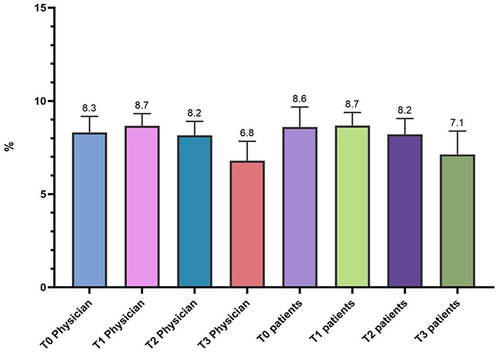
The satisfaction score was based on VAS; the score is determined by measuring the distance on the 10-cm line between the “no satisfaction” and “high satisfaction” and the patients’ mark, providing a range of scores from 0 to 10. A higher score indicates greater satisfaction. shows the values of the VAS score as expressed by the Physician in charge and by the patient himself, based on their evaluation experience. The scores were expressed immediately after treatment, after 1 month, after 3 months and after 24 weeks after the injection event.
Table 7, Description of VAS Score for Doctors and Patients
displays the average value of the patient and doctor’s satisfaction in different time points, as previously reported. The comparison between the VAS scores recorded for the doctor and for the patients highlights an overlap in reference to values recorded at T1 and T2, with minimal differences in the other measurements (T0 and T3). As can be seen from and , the fluctuations found in the average scores obtained from the administration of the scales are statistically significant for the doctors at 4 and 24 weeks after treatment but not at 12 weeks, while they are not for the patients, except for what concerns the decrease observed but 24 weeks after treatment (Anova p < 0.001; Wilcoxon p < 0.05). Throughout the entire follow-up duration of the patients enrolled in the study, no adverse effects related to the use of the product were observed.
Table 8 Statistical Analysis of VAS Score
Discussion
In this comprehensive investigation, we delved into the efficacy of NEAUVIA Intense, a PEG cross-linked polymeric hydrogel specifically designed as a subcutaneous filler for addressing NLF. The study’s approach allowed treating physicians to exercise clinical judgment in determining crucial aspects of the intervention, such as access points, needle/cannula selections, injection techniques, and volume administration. The injectable filler was strategically applied through bolus or linear retrograde injection, employing varying needle/cannula dimensions and effecting tissue correction at both subcutaneous and supraperiosteal levels.
A cohort of 60 selected patients participated in this prospective evaluation, characterized predominantly by females (96.7%), with an average age of 56.3 years. The assessment, facilitated by both the MFWS and the GAIS, occurred at distinct intervals—immediate and 1 month, 3 months, and 6 months post-injection. Our findings illuminated a notable initial responsiveness that gradually diminished over the observational period.
The MFWS outcomes revealed a substantial enhancement in tissue depression immediately post-injection, a benefit that endured up to the 6-month mark. A nuanced analysis of the frequency distribution of MFWS scores illustrated variations at different time points, with the most pronounced improvement occurring notably at level 1.5 after the 6-month follow-up.
The GAIS assessments unveiled the pinnacle of improvement at 30- and 90-days post-injection, with a significant proportion of patients consistently categorized as either “improved” or “much improved”. This positive categorization persisted, fortifying the outcomes up to the 24-week evaluation. Noteworthy was the fact that the satisfaction scores, gauged using the VAS, exhibited unwavering consistency between the evaluations of physicians and patients alike. Despite statistically significant fluctuations over the observation period, both medical practitioners and patients expressed heightened satisfaction immediately after the treatment, with a gradual but sustained decline at the 6-month milestone.
Injectable fillers, such as the one under scrutiny in our study, play a pivotal role in contemporary aesthetic medicine. Beyond the surface-level improvements observed in this investigation, the broader significance of injectable fillers lies in their capacity to provide non-invasive and customizable solutions for individuals seeking facial rejuvenation. The nuanced variations in our findings underscore the importance of tailoring treatment approaches to the unique needs of patients, emphasizing the dynamic nature of facial aesthetic interventions. Our study contributes not only to the understanding of the specific hydrogel filler evaluated but also underscores the broader context of injectable fillers as integral components of comprehensive aesthetic strategies, reflecting the evolving landscape of facial aesthetic medicine.
Conclusion
The findings from our study confirm that NEAUVIA Intense is an effective solution for correcting moderate-to-severe nasolabial folds, offering significant and lasting improvements in both tissue depression and overall aesthetic appearance. The study underlines the importance of ongoing evaluation and adaptation in aesthetic treatments to meet evolving patient expectations and achieve optimal long-term results. Our research contributes to a deeper understanding of this specific hydrogel filler and its role within the broader context of facial aesthetic strategies,Citation39,Citation40 emphasizing the dynamic nature of patient satisfaction in aesthetic medicine.
Disclosure
UB-CARE S.R.L. is part of the Matex Lab Group. Dr. Nicola Zerbinati is the scientific director of MatexLab. The authors report no other conflicts of interest in this work.
Acknowledgments
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Fallacara A, Manfredini S, Durini E, et al. Hyaluronic Acid Fillers in Soft Tissue Regeneration. Facial PlastSurg. 2017;33:87–96. doi:10.1055/s-0036-1597685
- Bogdan Allemann I, Baumann L Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. ClinInterv Aging 2008;3(4):629–634.
- Funt D, Pavicic T Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 2013;6 (1):295–316.
- Fagien S, Monheit G, Jones D, et al. Hyaluronic Acid Gel With (HARRL) and Without Lidocaine (HAJU) for the Treatment of Moderate-to-Severe NLF: a Randomized, Evaluator-Blinded, Phase III Study. DermatolSurg 2017;10:1–8.
- Sattler G, Philipp-Dormston WG, Van Den Elzen H, et al. A Prospective, Open-Label, Observational, Postmarket Study Evaluating VYC-17.5L for the Correction of Moderate to Severe NLF Over 12 Months. DermatolSurg 2017;43:238–245.
- Monheit G, Beer K, Hardas B, et al. Safety and Effectiveness of the Hyaluronic Acid Dermal Filler VYC-17.5L for NLF: Results of a Randomized, Controlled Study. DermatolSurg 2018;44:670–678.
- Philipp-Dormston WG, Eccleston D, De Boulle K, et al. A prospective, observational study of the volumizing effect of open-label aesthetic use of Juvéderm ® VOLUMA ® with Lidocaine in mid-face area. J Cosmet Laser Ther, 2014; 16: 171–179. doi:10.3109/14764172.2014.910079
- Dayan S, Corey S, Maas CS, et al. Safety and Effectiveness of VYC-17.5L for Long-term Correction of NLF. Aesth Surg J, 2019;1–11.
- Liu MH, Beynet DP, Gharavi NM Overview of Deep Dermal. Facial PlastSurg;35:224–229.
- Sobanko JF, Dai J, Gelfand JM, et al. Prospective cohort study investigating changes in body image, quality of life, and self-esteem following minimally invasive cosmetic procedures. DermatolSurg 2018;44 (08):112.
- Rauso R, Sesenna E, Fragola R, et al. Skin Necrosis and Vision Loss or Impairment After Facial Filler Injection. J Craniofac Surg 2020; 31(8):2289–2293. doi:10.1097/SCS.0000000000007047
- Ortiz AE, Ahluwalia J, Song SS, et al. Analysis of U.S. Food and Drug Administration Data on Soft-Tissue Filler Complications. DermatolSurg 2019;00:1–5.
- Rauso R, Zerbinati N, R F, et al. Transvascular hydrolysis of hyaluronic acid filler with hyaluronidase: An Ex vivo study. Dermatol Surg 2021; 47(3):370–372. doi:10.1097/DSS.0000000000002773
- Zerbinati N, Lotti T, Monticelli D. In Vitro Evaluation of the Sensitivity of a Hyaluronic Acid PEG Cross-Linked to Bovine Testes Hyaluronidase. Open Acc Maced J Med Sci.2018; 6(1):20–24. doi:10.3889/oamjms.2018.046
- Zerbinati N, Mocchi R, Galadari H, et al. In Vitro Evaluation of the Biological Availability ofHyaluronic Acid Polyethylene Glycols-Cross-Linked Hydrogels to Bovine Testes Hyaluronidase. Biomed Res Int. 2019; 2019: 3196723. doi:10.1155/2019/3196723
- Pignatti M, Pedone A, Baccarani A, et al. High-density Hyaluronic Acid for the Treatment of HIV-related Facial Lipoatrophy. AesthPlastSurg 2012; 36:180–185.
- Hausauer AK, Jones DH Long-Term Correction of Iatrogenic Lipoatrophy With Volumizing Hyaluronic Acid Filler. DermatolSurg. 2017;10:1–3.
- Bertossi D, Dell’Acqua I, Albanese M, et al.Face treatment Using Nonsurgical Mini-Invasive Techniques as Postsurgical Procedure for Traumatic Injury. Aesthet Surg J. 2019; 39(7):NP266–NP278.
- Bisson JI, Shepherd JP, Dhutia M, Dhutia M Psychological sequelae of facial trauma. J Trauma. 1997;43(3):496–500. doi:10.1097/00005373-199709000-00018
- Cooper JS, Lee BT Treatment of facial scarring: lasers, filler, and nonoperative techniques. FacialPlastSurg. 2009;25(5):311–315.
- Smith L, Cockerham K Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer Adherence. 2011; 5: 133–139. doi:10.2147/PPA.S11251
- Tripodo G, Trapani A, Torre ML, et al. Hyaluronic acid and its derivatives in drug delivery and imaging: recent advances and challenges. Eur J PharmBiopharm. 2015 (Pt B):400–416. 97 doi:10.1016/j.ejpb.2015.03.032
- Monticelli D, Martina V, Mocchi R, et al. Chemical Characterization of Hydrogels Crosslinked with Polyethylene Glycol for Soft Tissue Augmentation. Open Access Maced J Med Sci.2019; 7(7):1077–1081. doi:10.3889/oamjms.2019.279
- Zerbinati N, Esposito C, Cipolla G, et al. Chemical and mechanical characterization of hyaluronic acid hydrogel cross-linked with polyethylen glycol and its use in dermatology. Dermatol Ther 2020; 33(4):e13747. doi:10.1111/dth.13747
- Zerbinati N, Sommatis S, Maccario C, et al. Toward physicochemical and rheological characterization of different injectable hyaluronic acid dermal fillers cross-linked with polyethylene glycol diglycidyl ether. Polymers 2021; 13(6):948. doi:10.3390/polym13060948
- Zerbinati N, Lotti T, Monticelli D, et al. In Vitro Evaluation of the Biosafety of Hyaluronic Acid PEG Cross-Linked with Micromolecules of Calcium Hydroxyapatite in Low Concentration. Open Access Maced J Med Sci 2018; 6(1):15–19. doi:10.3889/oamjms.2018.044
- Zerbinati N, D’Este E, Parodi PC, et al. Microscopic and ultrastructural evidences in human skin following calcium hydroxylapatite filler treatment. Arch Dermatol Res 2017; 309(5):389–396. doi:10.1007/s00403-017-1734-3
- Zerbinati N, D’Este E, Farina A, et al. Morphological evidences following pegylated filler treatment in human skin. J Biol Regul Homeost Agents 2017; 31(2 Suppl. 2):79–85.
- Rauso R, Zerbinati N, Franco R, et al. Cross-linked hyaluronic acid filler hydrolysis with hyaluronidase: Different settings to reproduce different clinical scenarios. Dermatol Ther 2020; 33(2):e13269. doi:10.1111/dth.13269
- Fruijtier-Pölloth C Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005; 214: 1–38. doi:10.1016/j.tox.2005.06.001
- Lam J, Truong N F,Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. ActaBiomater. 2014; 10: 1571–1580.
- Marino F, Cosentino M, Legnaro M, et al. Immune profile of hyaluronic acid hydrogel polyethylene glycol crosslinked: an in vitro evaluation in human polymorphonuclear leukocytes. Dermatologic Ther 2020; 33(3):e13388. doi:10.1111/dth.13388
- Shoshani D, Markovitz E, J. MS, et al. The modified Fitzpatrick Wrinkle Scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. DermatolSurg, 2008; 341, S85–91
- Kopera D, Palatin M, Bartsch R, et al. An Open-Label Uncontrolled, Multicenter Study for the Evaluation of the Efficacy and Safety of the Dermal Filler Princess VOLUME in the Treatment of Nasolabial Folds. Biomed Res Int 2015; 2015:195328. doi:10.1155/2015/195328
- Brokelman RBG, Haverkamp D, van Loon C, et al. The validation of the visual analogue scale for patient satisfaction after total Hip arthroplasty. Eur Orthop Traumatol 2012; 3:101–105. doi:10.1007/s12570-012-0100-3
- Mousavi SV Does Cosmetic Rhinoplasty Improve Self-Concept and Patient’s Satisfaction with Nose Fitness? Testing the Differences Before and After Surgery with 3 To 6 Months Follow-Up. Caspian J Neurol Sci 2016; 2(6):25–32 doi:10.18869/acadpub.cjns.2.6.25
- The jamovi project. jamovi. (Version 2.2) [Computer Software]. Available from https://www.jamovi.org. Accessed June 5, 2024.
- R Core Team (2021). R: A Language and environment for statistical computing. (Version 4.0) [Computer software]. Available from https://cran.r-project.org. Accessed June 5, 2024
- Zerbinati N, Rauso R, Protasoni M, et al. Pegylated hyaluronic acid filler enriched with calcium hydroxyapatite treatment of human skin: collagen renewal demonstrated through morphometric computerized analysis. J Biol Regul Homeost Agents 2019; 33(6):1967–1971. doi:10.23812/19-250-L
- Zerbinati N, Rauso R, Gonzalez P, et al. In vitro evaluation of collagen production on human fibroblasts treated with hyaluronic acid peg cross-linked with micromolecules of calcium hydroxyapatite in low concentration. J Biol Regul Homeost Agents 2017; 31(Suppl 2):87–90.