Abstract
Melanoma is a lethal skin disease with a mostly predictable clinical course according to a known constellation of clinical and pathologic features. The distinction of melanoma from benign melanocytic nevus is typically unequivocol; however, there is a subset of tumors known for its diagnostic challenges, development of late metastases, and difficulties in treatment. Several melanocytic tissue biomarkers are available that can facilitate the histopathologic interpretation of melanoma as well as provide insight into the biologic potential and mutational status of this disease. This review describes the clinical application of some of these established and emerging tissue biomarkers available to assess melanocytic differentiation, vascular invasion, mitotic capacity, and mutation status. The selected tissue biomarkers in this review include MiTF, Sox10, D2-40, PHH3, H3KT (anti-H3K79me3T80ph), anti-BRAFV600E, and anti-BAP-1.
Introduction
Melanoma is a deadly skin disease known for its aggressive clinical behavior and high propensity for lethal metastasis.Citation1,Citation2 High throughput genomic analysis with next-generation sequencing technology has allowed us to interrogate whole exomes and genomes in melanoma, which have provided insight to the key oncogenic pathways involved in melanomagenesisCitation3,Citation4 RAS/mitogen-activated protein kinase (MAPK) pathway and PI3 kinase/AKT pathways continue to emerge as key driver mutations in melanomas.Citation5,Citation6 The most frequent oncogenic driver mutation identified in melanoma is in BRAF, which exists in approximately 50%–60% of patients with this malignancy.Citation7,Citation8 Molecular techniques available for evaluation of BRAFV600E mutations in melanoma are further reviewed in detail elsewhere.Citation9,Citation10
Regulation of melanogenesis in melanocytes involves a series of complex biochemical steps, with conversion of L-tyrosine to L-3,4 dihydroxyphenylalanine (L-DOPA) by tyrosinase to final melanin product.Citation11 In fact, it is now clear that L-tyrosine and L-DOPA can act as positive regulators of melanogenesis and melanocyte function.Citation12 Tyrosinase is central to melanogenesis, and transcriptional regulation of tyrosinase involves several MiTF-binding sites, further reviewed by Slominski et al.Citation11 Melanogenic activity is dependent on binding of MiTF to melanogenesis-related proteins; thus, the development of melanocytes in the epidermis and in the follicle is conserved as well as melanin production in melanocytes and melanoma cells.Citation12,Citation13
Corticotropin releasing hormone and proopiomelanocortin are known to be produced in the skin in response to environmental stress.Citation14 Melanocytes and melanoma cells predominantly express the α isoform of corticotropin releasing hormone receptor-1 (CRHR-1). Upon CRHR-1α activation, cyclic adenosine monophosphate is produced, and through release of proopiomelanocortin peptides (ACTH, α-MSH, β-endorphin), stimulates melanin production in melanocytes.Citation15 Activation of CRHR-1 inhibits melanocytes and melanoma cell proliferation, making selective antagonists a rational target for future melanoma targeted therapy.Citation15
The treatment for localized primary melanoma is surgical excision with adequate margins, and for a subset of patients with high-risk tumors, lymphatic mapping and sentinel lymph node biopsy is recommended. Adjuvant interferon alfa therapy and chemotherapy have been the standard treatment for patients with advanced stage disease. However, more recently, targeted therapy with small molecule inhibitors (eg, vemurafenib) has revolutionized melanoma treatment. In a Phase III trial (BRIM-3), patients who harbor BRAFV600E mutant melanomas who were treated with vemurafenib had better clinical response and overall survival rates than did patients treated with chemotherapy.Citation16 Selective targeted therapy against other intracellular molecules (eg, NRAS, MEK, KIT) are under clinical trials and hold promise for future melanoma therapy. Several publications review current melanoma-targeted therapy.Citation17–Citation19
Clinical response to vemurafenib therapy may be dramatic, with complete shrinkage of tumor burden in patients; however, the duration of response has been limited and eventual disease progression frequently occurs within months of therapy.Citation16 Resistant mechanisms have curtailed long-term therapeutic benefit from vermurafenib therapy; thus, targeting multiple pathways or combined therapy with immune check point blockade (eg, anti-CTLA4 and anti-PD-L1) are under clinical investigation.Citation20–Citation23 Further review of resistant mechanisms via protective effects of insulin on melanoma cells or by activation of the PI3K/AKT pathway can be examined in a study by Chi et al.Citation24 Future application of nanotechnology in melanoma to improve therapeutic efficacy is further reviewed by Chen et al.Citation25
Accurate diagnosis of melanoma remains critical to further clinical management. Melanoma can demonstrate a wide range of morphologic features and may be misinterpreted as other human malignancies (eg, sarcomas, squamous cell carcinomas, Paget’s disease, and lymphomas). Thus, melanoma is known as the “great mimicker.”Citation26 Diagnosis of melanoma can be further complicated since a subset of ambiguous melanocytic lesions may demonstrate features overlapping with melanoma and benign nevi (in particular, Spitz nevi).Citation27 These characteristics make the histologic diagnosis of melanoma challenging for even the most experienced dermatopathologists.
To help distinguish melanoma from its imitators, a variety of tissue biomarkers and ancillary techniques (eg, immunohistochemical [IHC] analysis or fluorescence in situ hybridization) are currently available. In fact, hundreds of tissue biomarkers are available in clinical laboratories for diagnosing melanoma and determining the prognosis and mutation status of this devastating skin disease. This review provides an update on the clinical applications of some of the established and emerging melanoma tissue biomarkers used at The University of Texas MD Anderson Cancer Center. Specifically, we will review the following melanoma tissue biomarkers () :1) melanocytic differentiation [MiTF and Sox10]; 2) vascular invasion [D2-40 and dual IHC marker with MiTF/D2-40 and Sox10/D2-40]; 3) mitotic figures [PHH3, dual IHC marker with Mart-1/PHH3 and H3KT (anti-H3K79me 3T80ph)]; and 4) mutation status [anti-BRAFV600E, anti-BAP-1].
Table 1 List of selected tissue biomarkers in melanoma
Markers of melanocytic differentiation
MiTF
MiTF (microphthalmia-associated transcription factor) functions in the development and differentiation of a variety of cell types, including melanocytes.Citation28 There are ten isoforms of MiTF, with the M isoform specifically expressed in melanocytes.Citation29 MiTF regulates the transcription of genes (eg, tyrosinase, tyrosinase-related protein 1 and 2) involved in melanin synthesis and survival of postnatal melanocytes.Citation28,Citation30,Citation31 Thus, MiTF is critical for pigment synthesis and melanocyte differentiation. MiTF protein functions in the nucleus of melanocytes and can be recognized with antibodies directed against it. The D5 antibody recognizes human MiTF.Citation32
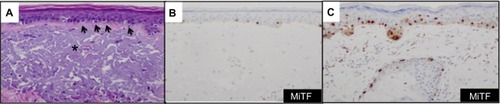
The sensitivity of MiTF in melanocytic lesions exceeds 80% and is similar to that of HMB45.Citation33 In desmoplastic melanomas, however, the sensitivity of MiTF dramatically decreases to less than 55% according to some studies.Citation32,Citation34 The low sensitivity of MiTF in desmoplastic melanoma is comparable to that of HMB45, a marker of premelanosomal glycoprotein 100. Therefore, MiTF appears to exhibit sensitivity comparable to that of HMB45 in melanocytic neoplasms. MiTF and HMB45 differ, however, in their specificity. HMB45 is a highly specific marker with greater than 97% specificity for melanocytic differentiation.Citation35,Citation36 In contrast, the specificity of MiTF in melanocytic lesions is less, and the widespread use of this biomarker alone in evaluating melanocytic lesions is an important pitfall. MiTF has been shown to highlight cells other than melanocytes and nonmelanocytic neoplasms. In particular, MiTF reactivity can be seen in macrophages, fibroblasts, and mast cells and in a variety of spindle cell tumors in the differential diagnosis of melanoma (eg, dermal scar, dermatofibrosarcoma, leiomyosarcoma, neurofibroma, malignant peripheral nerve sheath tumor).Citation37 In our experience, MiTF has clinical utility if incorporated with a panel of biomarkers in certain clinical scenarios:Citation38 first, to enumerate melanocytes in the epidermis on chronic sun-damaged skin; second, to detect melanocytic differentiation in tumors negative for more conventional melanocytic markers (HMB45, anti-tyrosinase, and anti-Mart1); and third, to confirm melanocytic differentiation in rare isolated cells in vascular channels and the presence of lymphovascular invasion (LVI).
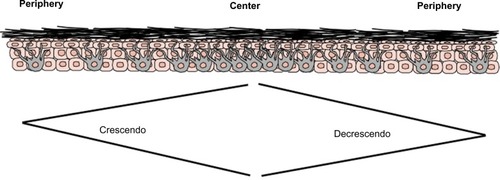
Since MiTF is expressed in the nucleus of melanocytes, it facilitates and accurately highlights the number of melanocytes seen in the epidermis () and lesions of lentigo maligna ().Citation39 This is particularly useful in evaluating melanocytes on atrophic sun-damaged skin that typically harbors increased numbers of melanocytes in the epidermis, where perceived melanocytes numbers may be overestimated and their presence may be misinterpreted as lentigo maligna with Melan A since this marker labels the cytoplasm of melanocytes.Citation40 One factor contributing to the overestimation of melanocytes with Melan A is the cytoplasmic labeling of dendritic processes; also, Melan A has been shown to label occasional keratinocytes as “pseudomelanocytic nests.”Citation41 When we evaluate Melan A in this clinical setting, attention to labeling of only the cell bodies helps prevent overestimation of the number of melanocytes. A study by Nybakken et alCitation39 demonstrated that the use of Melan A resulted in an estimated 23.3% higher melanocyte count in the epidermis compared with use of hematoxylin and eosin (H&E)-stained sections in atypical intraepithelial melanocytic proliferations (lesions of concern for diagnosis of melanoma in situ); use of MiTF resulted in estimates of melanocytes similar to those observed with H&E-stained sections. Thus, MiTF can be useful to distinguish pigmented actinic keratosis from melanoma in situ.Citation42 In fact, compared with Melan A and HMB45, MiTF allowed greater distinction of melanocytes from keratinocytes with melanin pigment.Citation43 The mean density of intraepithelial melanocytes in a 1.0 mm segment of epidermis was reported to be 27 positive MiTF cells in solar lentigines compared to 111.9 in melanoma in situ.Citation43 Morphometric analysis identified greater than ten MiTF-positive nuclei per 200 μm segment of H&E-stained, section-favored melanoma in situ over solar lentigo.Citation44 Although quantitative assessment is valuable, we also find it helpful to compare the background melanocytes with the central lesion in skin ellipse specimens. Typically, there should be crescendo-decrescendo density of melanocytes in the epidermis from the periphery–center of the lesion–periphery in examined cross-sections (either by H&E or IHC) (). Discrimination from background increase density of melanocytes in chronic sun damage skin from lentigo maligna may become more challenging in en face margin evaluation.
Figure 1 MiTF accurately enumerates the melanocytes in the epidermis of chronic sun exposed skin and in lesions of lentigo maligna.
Abbreviations: H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Sox10
The Sox (Sry-related box) family of genes is involved in the development processes of testis, oligodendrocytes, the central nervous system, and chodrocytes.Citation45 Sox10 plays a pivotal role in neural crest cell development, and mutations in Sox10 result in Waardenburg–Shah syndrome. Afflicted individuals exhibit pigmentary alterations and nervous system defects.Citation46 Sox10 directly regulates expression of MiTF and other genes essential in melanin synthesis.Citation47,Citation48 A study by Bondurand et alCitation49 showed that Sox10 in synergy with PAX3 bind directly to the MiTF promoter; thus, regulating MiTF expression. Consequently, Sox10 has emerged as a useful tissue biomarker in melanocytic neoplasms.Citation50,Citation51 Similar to MiTF, Sox10 protein is expressed in the nucleus, and the utility of Sox10 in evaluation of melanocytic lesions parallels that of MiTF. In particular, like MiTF, Sox10 facilitates the enumeration of melanocytes in the epidermis on chronic sun-damaged skin, and minimizes the overestimation of melanocytes. Sox10 also facilitates the distinction of pigmented actinic keratosis and melanocytic hyperplasia from lentigo maligna.Citation42
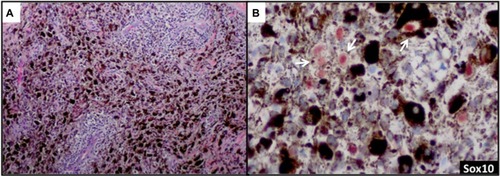
Sox10 is expressed in Schwann cells, myoepithelial cells, granular cells, adnexal structures, and salivary glands. Since the expression of Sox10 has been limited to tumors of melanocytic, Schwannian, or myoepithelial differentiation, Sox10 has become useful in differential diagnosis of melanoma. The sensitivity of Sox10 in melanoma with various morphologies and patterns (eg, spindle, nodular, desmoplastic, metastatic) is comparable to that of S100 and is greater than 95%.Citation50,Citation52,Citation53 Sox10 and MiTF differ, however, in specificity. Sox10 expression is absent in fibroblasts and macrophages and thus can aid in evaluating excision specimens for residual tumor.Citation54 Sox10 has also been reported to be a reliable marker in evaluating sentinel lymph nodes for metastatic melanoma.Citation55 In addition to these uses for Sox10, we have found this tissue biomarker to be helpful in identifying rare tumor cells in a heavily regressed area of metastatic melanoma with abundant melanophages (). The nuclear labeling of Sox10 (preferably with red chromogen to contrast melanin pigment in the cytoplasm of the macrophages) confirms the presence of isolated melanoma cells () but not macrophages, and thus may have important clinical implications in staging.
Figure 3 Sox10 identifies rare tumor cells in heavily regressed areas of melanoma.
Abbreviations: H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Markers of vascular invasion
D2-40
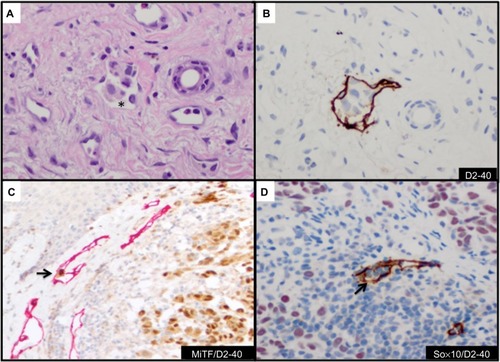
The endothelial marker D2-40 (podoplanin) is a highly specific monoclonal antibody for lymphatic endothelium but does not react with blood vessel endothelium; thus, D2-40 is a selective marker to highlight LVI by tumor cells.Citation56 Use of D2-40 in cutaneous melanoma, compared with use of HE stain alone, increased the frequency of both intratumoral and peritumoral LVI detection.Citation57,Citation58 In fact, detection of LVI with D2-40 in cutaneous melanoma correlated with positive sentinel lymph nodes and worse overall and disease-free survival ().Citation58,Citation59 The importance of LVI detection with D2-40 is not limited to cutaneous melanomas but offers prognostic value in other human cancers such as endometrial, breast, esophageal, colorectal, and Merkel cell cancers.Citation60–Citation63
Figure 4 Detection and confirmation of lymphovascular invasion.
Abbreviations: H&E, hematoxylin and eosin; IHC, immunohistochemistry; LVI, lymphovascular invasion.
CD34 and CD31 are panvascular markers that label both lymphatic and blood vessel endothelium. The sensitivity of CD34 and CD31 in identifying lymphatic endothelium is lower than that of D2-40.Citation64,Citation65 In addition, CD31 is reactive in macrophages. These qualities make CD34 and CD31 suboptimal markers in the detection of LVI in cutaneous melanoma.
Dual IHC marker with MiTF/D2-40 or Sox10/D2-40
The use of double IHC stains to evaluate melanocytic lesions is generally available in academic settings and some private laboratories.Citation66 At our institution, to confirm histologic suspicion of LVI in tumors that histologically harbor poor prognostic attribute(s) (eg, ulceration, mitotic figures, thick tumors) in patients with cutaneous melanomas, we routinely evaluate for LVI with D2-40 in lesions with adequate dermal component amenable to further IHC studies. Since a vascular collection of inflammatory cells (eg, lymphocytes or monocytes) highlighted by D2-40 may mimic melanoma LVI, we have developed a dual IHC protocol that allows us to identify lymphatic endothelium with D2-40 and confirm melanocytic differentiation in cells within the highlighted lymphatic vessel with either MiTF or Sox10 (). Both dual IHC markers with MiTF/D2-40 or Sox10/D2-40 have clinical utility. An advantage of using Sox10 in some instances will be in lesions negative for MiTF (eg, spindle/desmoplastic melanomas) or if there is concern for MiTF reactivity with macrophages.
Markers of mitotic figures
PHH3
Modifications of histone tails are critical events in melanoma cells, and condensation of chromatin is an essential biologic process in preparation for cell division.Citation67 Detection of mitotic figures is an important histologic feature in both the diagnosis and prognosis of melanoma. The presence of mitotic figures in melanoma correlates with worse prognosis and defines a subset of thin melanomas (Breslow thickness ≤1.0 mm) classified as T1b (American Joint Committee on Cancer [AJCC]).Citation2 PHH3 (phosphorylation of histone H3 at serine 10) has been shown to be associated with chromatin condensation at G2 and M phases of the cell cycle.Citation68
Immunodetection of mitotic figures in cutaneous melanoma with PHH3 has been shown to facilitate the mitotic figure count, demonstrate increased risk of metastasis, and was correlated with worse survival.Citation69–Citation71 PHH3 is a sensitive and specific mitotic-associated histone marker; it is reactive in all phases of the mitotic cycle and is absent in apoptotic cells.Citation70 The clinical utility of PHH3 at our institution is to confirm/detect mitotic figures in melanocytic lesions on a case-by-case basis. Since the AJCC-TNM (tumor, nodes, metastasis) melanoma classification system was based on detection of mitotic figures from a data set where mitotic figures were reported on “routine” H&E sections, exhaustive examination of multiple levels to search only for mitotic figures is not warranted.Citation2,Citation72,Citation73 The application of PHH3 in search for mitotic figures for all melanomas is not currently part of the standard operating procedure at our institution. This further ensures that we do not exhaust the tissue block in search for mitotic figures in situations where further molecular studies may become necessary.
Dual IHC marker with Mart1/PHH3
A variety of proliferative cells in the skin may be reactive with PHH3, including lymphocytes and macrophages that may make detection of tumor mitotic figures a challenge. A dual IHC stain with Mart1/PHH3 allows identification of mitotic figures in melanoma cells (). Dual IHC staining with Mart1/PHH3, compared with PHH3 staining alone, improved specificity and time in the detection of mitotic figures in melanoma cells.Citation70,Citation74 Misinterpretation of PHH3-positive inflammatory cells can be avoided with anti-Mart1/PHH3, and this marker is typically used in our clinical practice to confirm mitotic figures in melanoma cells.
Figure 5 Detection of mitotic figures and G2+ tumor nuclei with histone markers.
Abbreviation: IHC, immunohistochemistry.
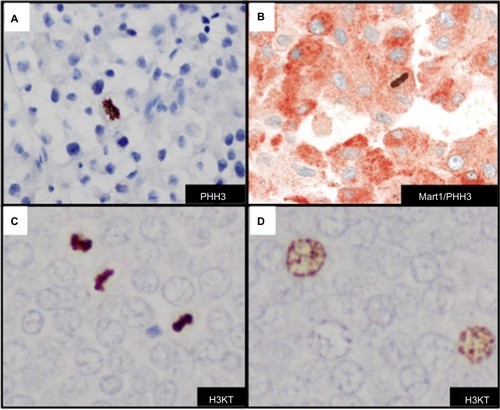
Anti-H3K79me3T80ph
Dynamic structural changes in chromatin are critical in the stage of the cell cycle. Chromatin condensation is important for proper cell division. A dual modified phosphohistone H3 marker anti-H3K79meT80ph (H3KT) is associated with mitotic figures in cutaneous melanoma.Citation75 H3KT was found significantly in G2/M phase of the cell cycle in asynchronous HeLA cells and essentially undetectable levels in G0/G1 and S phases of the cell cycle.Citation75 More recently, studies indicate that H3KT may in fact recognize dual modified histone H3K9me3S10ph (H3KS).Citation76 The detection of mitotic figures and G2+ tumor nuclei with H3KT identified a subset of cutaneous melanomas with a more aggressive clinical course. Furthermore, the combined mitotic figure count and G2+ tumor nuclei correlated with worse survival in a cohort of patients with Merkel cell carcinoma ().Citation77 H3KT or anti-H3KS is an emerging mitotic-associated histone marker that in a subset of tumors highlights the prognostic value of detection of G2+ tumor nuclei in human cancers (). Since H3KT identifies mitotic figures in any proliferating cell, the type of cell with mitosis may be further identified with incorporation of another IHC marker (eg, Mart-1/H3KT for proliferating melanocytes or CK20/H3KT for proliferating Merkel cells). Additional studies with H3KS and H3KT and double IHC marker studies are warranted for further clinical application.
Marker of mutation status
Anti-BRAFV600E
Analysis of the aberrant activation of the MAPK signaling pathway in a subset of melanomas with BRAF mutations coupled with the development of selective RAF inhibitors that target mutated BRAF protein has revolutionized therapy for patients with advanced-stage disease. Approximately 50%–60% of cutaneous melanomas harbor BRAF mutations that involve a thymine to adenine DNA base point mutation that replaces the amino acid valine with glutamic acid at the 600 position (BRAF V600E).Citation7 A variety of molecular platforms exist for determining the presence of BRAFV600E mutation in formalin-fixed paraffin-embedded (FFPE) tissue samples of melanoma, including pyrosequencing, the cobas BRAF V600 mutation test developed by Roche, and next-generation sequencing platforms.Citation10 Molecular testing, however, often requires a specialized laboratory, may be technically demanding, and is associated with increased costs and time for completing the test. Alternatively, circulating plasma DNA with BRAF mutation reported by Denis et alCitation78 may aid in predicting patients who may respond to BRAF inhibitor therapy.
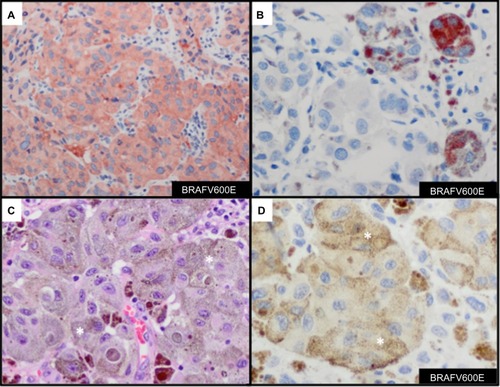
Results from detecting BRAFV600E mutations in melanoma with use of molecular tests with IHC methods are highly correlated with results from IHC staining with anti-BRAFV600E (clone VE1), with a sensitivity of 97%–100% and a specificity of 98%–100%.Citation79–Citation81 IHC expression of mutant BRAFV600E is typically diffuse throughout the cytoplasm of melanoma cells (). At times, focal cytoplasmic labeling of melanoma cells occurs with BRAFV600E IHC (), which has also been shown to correlate with the presence of the BRAFV600E mutation.Citation82 These findings suggest that IHC analysis with anti-BRAFV600E is important to identify a subset of tumors that harbor BRAFV600E tumor heterogeneity. One should be aware of the chance of misinterpreting the presence of positive BRAFV600E stain in heavily pigmented tumor samples (), especially when the developing agent is a brown chromogen (eg, diaminobenzidine) (). An alternative would be to use a red chromogen (eg, 3-amino-9-ethylcarbazole or Texas red) as the developing agent, which may allow better discrimination of positive or negative staining. Reactivity of the developing agent in macrophages is another possible misinterpretation of BRAFV600E stain. Absence of the BRAFV600E mutation is seen as a negative stain. Nuclear labeling with the absence of cytoplasmic staining should be interpreted as negative. Our experience with BRAFV600E IHC stain (in preparation for publication) demonstrates high correlation with BRAFV600E mutation status and protein expression with anti-BRAFV600E antibody.
Figure 6 Immunohistochemcial evaluation of BRAFV600E mutation status in melanoma.
Abbreviations: AEC, 3-amino-9-ethylcarbazole; DAB, diaminobenzidine; H&E, hematoxylin and eosin; IHC, immunohistochemistry.
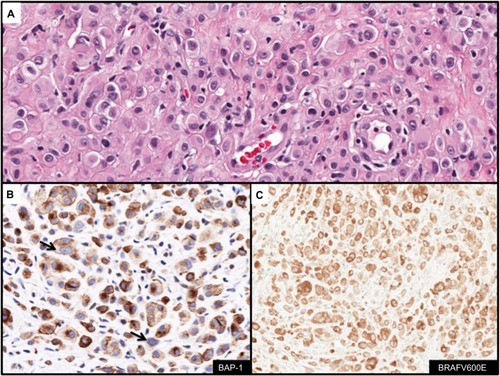
Anti-BAP-1
BAP-1 (BCRA1 associated protein-1) protein is an ubiquitin hydrolase that binds to BRCA1 and functions as a tumor suppressor.Citation83 Germline mutations in BAP-1 have been described that predispose affected family members to uveal and cutaneous melanomas and development of melanocytic lesions with distinct clinical and histologic features.Citation84,Citation85 Affected individuals present with multiple (range 5–50 lesions) well-circumscribed dome-shaped papules near the second decade of life.Citation84,Citation85 Histologically, the melanocytic lesions are located predominantly in the dermis and are composed of epithelioid melanocytes with abundant cytoplasm and distinct nucleoli. The lesions demonstrate similar cytologic features seen with Spitz nevi (). Similar lesions develop in the context of somatically acquired BAP-1 loss. IHC analysis with anti-BAP-1 demonstrates loss of nuclear staining in melanocytic lesions with BAP-1 mutations (). Furthermore, a significant proportion of BAP-1 lesions also frequently harbor a BRAFV600E mutation (). The combined features of BRAFV600E expression and loss of BAP-1 constitutes a distinct subset of Spitz nevi analogous to those harboring gains in chromosome 11p together with HRAS mutation.Citation86
Figure 7 Immunohistochemcial evaluation of BAP-1 and BRAFV600E status in melanocytic lesions.
Abbreviations: H&E, hematoxylin and eosin; IHC, immunohistochemistry.
Summary of emerging clinical utility of biomarkers in melanoma
MiTF and Sox10 are both useful biomarkers in identification of melanocytic differentiation and to enumerate the melanocytes along the dermal–epidermal junction in setting of chronic sun-damaged skin. These markers may aid in margin control and in the diagnosis of melanoma. Sox10 has further utility in the distinction between melanoma cells and macrophages in extensively regressed nodules of melanoma and in examination of lymph nodes since this marker exhibits greater melanocytic specificity. D2-40 exposes vascular invasion in a subset of melanomas, and use of double IHC marker MiTF/D2-40 or Sox10/D2-40 confirms the presence or absence of melanoma cells in the vascular lumen. PHH3 identifies mitotic figures and Mart-1/PHH3 double IHC stain will confirm mitosis in cells with melanocytic differentiation. Detection and confirmation of mitotic figures in melanocytes may have clinical impact on T stage of thin melanomas (Breslow thickness ≤1.0 mm). H3KT identified mitotic figures and G2+ tumor nuclei and may have prognostic implications in melanoma as well as Merkel cell carcinoma. Anti-BRAFV600E identifies melanomas that harbor BRAFV600E mutation and is a useful surrogate marker in the evaluation of BRAF mutation status in melanoma patients. Anti-BAP-1 allows for identification of melanocytic neoplasms with BAP-1 mutations and distinction from Spitz nevi and BAPomas when combined BRAFV600E and BAP-1 mutations coexist. Finally, next-generation sequencing and gene expression profiling may have some future diagnostic and prognostic utility in a subset of melanocytic lesions.Citation87,Citation88
Conclusion
Melanoma is a deadly skin disease that provides clinical challenges in diagnosis and treatment. However, the use of established and emerging tissue biomarkers will aid in the interpretation of difficult melanocytic lesions, provide prognostic information, identify mutation status, and allow the selection of patients who may benefit from targeted therapy.
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- BalchCMGershenwaldJESoongSJFinal version of 2009 AJCC melanoma staging and classificationJ Clin Oncol200927366199620619917835
- KozarewaIRosa-RosaJMWardellCPA modified method for whole exome resequencing from minimal amounts of starting DNAPLoS One201273e3261722403682
- HodisEWatsonIRKryukovGVA landscape of driver mutations in melanomaCell2012150225126322817889
- IbrahimNHaluskaFGMolecular pathogenesis of cutaneous melanocytic neoplasmsAnnu Rev Pathol2009455157919400696
- KoJMFisherDEA new era: melanoma genetics and therapeuticsJ Pathol2011223224125021125678
- DaviesHBignellGRCoxCMutations of the BRAF gene in human cancerNature2002417689294995412068308
- XiaJJiaPHutchinsonKEA meta-analysis of somatic mutations from next generation sequencing of 241 melanomas: a road map for the study of genes with potential clinical relevanceMol Cancer Ther20141371918192824755198
- WoodmanSELazarAJAldapeKDDaviesMANew strategies in melanoma: molecular testing in advanced diseaseClin Cancer Res20121851195120022275506
- CurryJLTorres-CabalaCATetzlaffMTBowmanCPrietoVGMolecular platforms utilized to detect BRAF V600E mutation in melanomaSemin Cutan Med Surg201231426727323174497
- SlominskiATobinDJShibaharaSWortsmanJMelanin pigmentation in mammalian skin and its hormonal regulationPhysiol Rev20048441155122815383650
- SlominskiAZmijewskiMAPawelekJL-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functionsPigment Cell Melanoma Res2012251142721834848
- SlominskiAWortsmanJPlonkaPMSchallreuterKUPausRTobinDJHair follicle pigmentationJ Invest Dermatol20051241132115654948
- SlominskiAWortsmanJLugerTPausRSolomonSCorticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stressPhysiol Rev2000803979102010893429
- SlominskiATZmijewskiMAZbytekBTobinDJTheoharidesTCRivierJKey role of CRF in the skin stress response systemEndocr Rev201334682788423939821
- ChapmanPBHauschildARobertCBRIM-3 Study GroupImproved survival with vemurafenib in melanoma with BRAF V600E mutationN Engl J Med2011364262507251621639808
- KeeDMcArthurGTargeted therapies for cutaneous melanomaHematol Oncol Clin North Am201428349150524880943
- MillerDMFlahertyKTTsaoHCurrent status and future directions of molecularly targeted therapies and immunotherapies for melanomaSemin Cutan Med Surg2014332606725085663
- KwongLNDaviesMATargeted therapy for melanoma: rational combinatorial approachesOncogene20143311923416974
- SolitDBRosenNResistance to BRAF inhibition in melanomasN Engl J Med2011364877277421345109
- BucheitADDaviesMAEmerging insights into resistance to BRAF inhibitors in melanomaBiochem Pharmacol201487338138924291778
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- CooperZAJunejaVRSagePTResponse to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockadeCancer Immunol Res20142764365424903021
- ChiMYeYZhangXDChenJInsulin induces drug resistance in melanoma through activation of the PI3K/Akt pathwayDrug Des Devel Ther20148255262
- ChenJShaoRZhangXDChenCApplications of nanotechnology for melanoma treatment, diagnosis, and theranosticsInt J Nanomedicine201382677268823926430
- KossLGMelamedMRKoss’ Diagnostic Cytology and Its Histopathologic Bases15th edPhiladelphia, PALippincott Williams & Wilkins2006
- TetzlaffMTWangWLMillessTLAmbiguous melanocytic tumors in a tertiary referral center: the contribution of fluorescence in situ hybridization (FISH) to conventional histopathologic and immunophenotypic analysesAm J Surg Pathol201337121783179624061523
- HemesathTJSteingrímssonEMcGillGmicrophthalmia, a critical factor in melanocyte development, defines a discrete transcription factor familyGenes Dev1994822277027807958932
- FuseNYasumotoKSuzukiHTakahashiKShibaharaSIdentification of a melanocyte-type promoter of the microphthalmia-associated transcription factor geneBiochem Biophys Res Commun199621937027078645245
- BentleyNJEisenTGodingCRMelanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiatorMol Cell Biol19941412799680067969139
- YasumotoKYokoyamaKShibataKTomitaYShibaharaSMicrophthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase geneMol Cell Biol19941412805880707969144
- GranterSRWeilbaecherKNQuigleyCFletcherCDFisherDEMicrophthalmia transcription factor: not a sensitive or specific marker for the diagnosis of desmoplastic melanoma and spindle cell (non-desmoplastic) melanomaAm J Dermatopathol200123318518911391097
- MiettinenMFernandezMFranssilaKGatalicaZLasotaJSarlomo-RikalaMMicrophthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma: comparison with four other melanoma markersAm J Surg Pathol200125220521111176069
- OrdóñezNGValue of melanocytic-associated immunohistochemical markers in the diagnosis of malignant melanoma: a review and updateHum Pathol201445219120523648379
- WickMRSwansonPERocamoraARecognition of malignant melanoma by monoclonal antibody HMB-45. An immunohistochemical study of 200 paraffin-embedded cutaneous tumorsJ Cutan Pathol19881542012073053811
- OhsieSJSarantopoulosGPCochranAJBinderSWImmunohistochemical characteristics of melanomaJ Cutan Pathol200835543344418399807
- BusamKJIversenKCoplanKCJungbluthAAAnalysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanomaAm J Surg Pathol200125219720411176068
- IvanDPrietoVGUse of immunohistochemistry in the diagnosis of melanocytic lesions: applications and pitfallsFuture Oncol2010671163117520624128
- NybakkenGESargenMAbrahamRZhangPJMingMXuXMITF accurately highlights epidermal melanocytes in atypical intraepidermal melanocytic proliferationsAm J Dermatopathol2013351252922668579
- PrietoVGSheaCRImmunohistochemistry of melanocytic proliferationsArch Pathol Lab Med2011135785385921732774
- BeltraminelliHShabrawi-CaelenLEKerlHCerroniLMelan-a-positive “pseudomelanocytic nests”: a pitfall in the histopathologic and immunohistochemical diagnosis of pigmented lesions on sun-damaged skinAm J Dermatopathol200931330530819384076
- BuonaccorsiJNPrietoVGTorres-CabalaCSusterSPlazaJADiagnostic utility and comparative immunohistochemical analysis of MITF-1 and SOX10 to distinguish melanoma in situ and actinic keratosis: a clinicopathological and immunohistochemical study of 70 casesAm J Dermatopathol201436212413023782678
- KimJTaubeJMMcCalmontTHGlusacEJQuantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situJ Cutan Pathol2011381077577921797920
- BlackWHTharejaSKBlakeBPChenRCherpelisBSGlassLFDistinction of melanoma in situ from solar lentigo on sun-damaged skin using morphometrics and MITF immunohistochemistryAm J Dermatopathol201133657357821697700
- KieferJCBack to basics: Sox genesDev Dyn200723682356236617584862
- PingaultVBondurandNKuhlbrodtKSOX10 mutations in patients with Waardenburg-Hirschsprung diseaseNat Genet19981821711739462749
- LeeMGoodallJVerasteguiCBallottiRGodingCRDirect regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2J Biol Chem200027548379783798310973953
- LudwigARehbergSWegnerMMelanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factorsFEBS Lett20045561–323624414706856
- BondurandNPingaultVGoerichDEInteraction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndromeHum Mol Genet20009131907191710942418
- NonakaDChiribogaLRubinBPSox10: a pan-schwannian and melanocytic markerAm J Surg Pathol20083291291129818636017
- OrdóñezNGValue of SOX10 immunostaining in tumor diagnosisAdv Anat Pathol201320427528323752089
- AgnarsdóttirMSoomanLBolanderASOX10 expression in superficial spreading and nodular malignant melanomasMelanoma Res201020646847820890226
- KaramchandaniJRNielsenTOvan de RijnMWestRBSox10 and S100 in the diagnosis of soft-tissue neoplasmsAppl Immunohistochem Mol Morphol201220544545022495377
- Ramos-HerberthFIKaramchandaniJKimJDadrasSSSOX10 immunostaining distinguishes desmoplastic melanoma from excision scarJ Cutan Pathol201037994495220653825
- BlochinENonakaDDiagnostic value of Sox10 immunohistochemical staining for the detection of metastatic melanoma in sentinel lymph nodesHistopathology200955562662819912373
- KahnHJBaileyDMarksAMonoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomasMod Pathol200215443444011950918
- NiakosariFKahnHJMarksAFromLDetection of lymphatic invasion in primary melanoma with monoclonal antibody D2-40: a new selective immunohistochemical marker of lymphatic endotheliumArch Dermatol2005141444044415837861
- PeterssonFDiwanAHIvanDImmunohistochemical detection of lymphovascular invasion with D2-40 in melanoma correlates with sentinel lymph node status, metastasis and survivalJ Cutan Pathol200936111157116319222695
- DoedenKMaZNarasimhanBSwetterSMDetmarMDadrasSSLymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasisJ Cutan Pathol200936777278019032379
- WeberSKSauerwaldAPölcherMDetection of lymphovascular invasion by D2-40 (podoplanin) immunoexpression in endometrial cancerInt J Gynecol Cancer20122281442144822964524
- LupinacciRMMelloESPinheiroRSIntrahepatic lymphatic invasion but not vascular invasion is a major prognostic factor after resection of colorectal cancer liver metastasesWorld J Surg20143882089209624663482
- ImamuraYWatanabeMNagaiYLymphatic vessel invasion detected by the D2-40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinomaJ Surg Oncol2012105327728322271500
- LeeJABaeJWWooSUKimHKimCHD2-40, Podoplanin, and CD31 as a prognostic predictor in invasive ductal carcinomas of the breastJ Breast Cancer201114210411121847404
- KahnHJMarksAA new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumorsLab Invest20028291255125712218087
- RoseAEChristosPJLackayeDClinical relevance of detection of lymphovascular invasion in primary melanoma using endothelial markers D2-40 and CD34Am J Surg Pathol201135101441144921881483
- NielsenPSRiber-HansenRSteinicheTImmunohistochemical double stains against Ki67/MART1 and HMB45/MITF: promising diagnostic tools in melanocytic lesionsAm J Dermatopathol201133436137021610457
- RichardsHWMedranoEEEpigenetic marks in melanomaPigment Cell Melanoma Res2009221142919040501
- JuanGTraganosFJamesWMHistone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosisCytometry199832271779627219
- SchimmingTTGrabellusFRonerMpHH3 immunostaining improves interobserver agreement of mitotic index in thin melanomasAm J Dermatopathol201234326626922197861
- TetzlaffMTCurryJLIvanDImmunodetection of phosphohistone H3 as a surrogate of mitotic figure count and clinical outcome in cutaneous melanomaMod Pathol20132691153116023558574
- LadsteinRGBachmannIMStraumeOAkslenLAPrognostic importance of the mitotic marker phosphohistone H3 in cutaneous nodular melanomaJ Invest Dermatol201213241247125222297638
- GershenwaldJESoongSJBalchCMAmerican Joint Committee on Cancer (AJCC) Melanoma Staging Committee2010 TNM staging system for cutaneous melanoma … and beyondAnn Surg Oncol20101761475147720300965
- ThompsonJFSoongSJBalchCMPrognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging databaseJ Clin Oncol201129162199220521519009
- IkenbergKPfaltzMRakozyCKempfWImmunohistochemical dual staining as an adjunct in assessment of mitotic activity in melanomaJ Cutan Pathol201239332433022335591
- MartinezDRRichardsHWLinQH3K79me3T80ph is a novel histone dual modification and a mitotic indicator in melanomaJ Skin Cancer2012201282353423227340
- HammondSLByrumSDNamjoshiSMitotic phosphorylation of histone H3 threonine 80Cell Cycle201413344045224275038
- HendersonSATetzlaffMTPattanaprichakulPDetection of mitotic figures and G2+ tumor nuclei with histone markers correlates with worse overall survival in patients with Merkel cell carcinomaJ Cutan Pathol Epub9262014
- DenisMGKhammariAValleeADetection of BRAF mutations in the plasma of melanoma patients as an early marker of treatment efficiencyJ Clin Oncol2014325s (Suppl; abstr 9069)
- CapperDBerghoffASvon DeimlingAPreusserMClinical neuropathology practice news 2-2012: BRAF V600E testingClin Neuropathol2012312646622385786
- LongGVWilmottJSCapperDImmunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanomaAm J Surg Pathol2013371616523026937
- ColombaEHélias-RodzewiczZVon DeimlingADetection of BRAF p.V600E mutations in melanomas: comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencingJ Mol Diagn20131519410023159108
- BusamKJHedvatCPulitzerMvon DeimlingAJungbluthAAImmunohistochemical analysis of BRAF(V600E) expression of primary and metastatic melanoma and comparison with mutation status and melanocyte differentiation antigens of metastatic lesionsAm J Surg Pathol201337341342023211290
- JensenDERauscherFJBAP1, a candidate tumor suppressor protein that interacts with BRCA1Ann N Y Acad Sci199988619119410667217
- AoudeLGWadtKBojesenAA BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancersPLoS One201388e7214423977234
- WiesnerTObenaufACMuraliRGermline mutations in BAP1 predispose to melanocytic tumorsNat Genet201143101018102121874003
- WiesnerTMuraliRFriedIA distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expressionAm J Surg Pathol201236681883022367297
- ClarkeLEBessEMoyersKKolquistKARockCThe influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologistsJ Clin Oncol2014325s (Suppl; abstr TPS9111)
- SiroyAEBolandGMMiltonDRBeyond BRAF: clinical mutation panel testing by next-generation sequencing in advanced melanomaJ Invest Dermatol Epub8222014