?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Age-related changes in the dermis can be considered the result of intrinsic factors and the consequence of environmental damage, mainly due to ultraviolet (UV) radiation from the sun (responsible for skin photoaging). The great versatility of the mesotherapy “biorevitalization” lies in the synergy between different biological effects of the active injected substances, which treats the skin in a more complete way. Several studies about biorevitalization efficacy showed good results. To date, however, objective results supported by instrumental evaluation are very sparse.
Purpose
This study evaluated the efficacy of an injectable solution (32 mg of hyaluronic acid plus an antiaging antioxidant complex consisting of vitamins, minerals, and amino acids) in the treatment of skin aging and photoaging.
Methods
A total of 64 female volunteers (37–60 years) underwent four sessions of biorevitalization at 3-week intervals, involving multiple injections in the face (external corner of the eye and cheek), neck, décolletage, and back of the hands. The esthetic result was assessed at baseline and after 6, 9, and 12 weeks, and was established through the use of clinical and instrumental evaluations, supported by photographic documentation. Additionally, a phototest was performed to assess the effect of biorevitalization treatment on UVB-induced erythema.
Results
Instrumental assessment showed, as early as after the second biorevitalizing treatment, the antiaging efficacy of the tested product; there was a clinical and statistically significant improvement of profilometric parameters, skin brightness, pigmentation, and deep skin hydration. The study product induced a statistically significant decrease of the visual score of the UVB-induced erythema compared with baseline, which was statistically different from placebo.
Conclusion
The study confirmed the well-known efficacy of biorevitalization in skin rejuvenation. The positive difference between deep and superficial skin hydration registered at the end of the trial suggested improved skin moisture retention of the stratum corneum. Furthermore, the obtained results suggest that the injected product could intervene at different moments of the skin pigmentation process by activating an intrinsic photoprotective mechanism and improving skin pigmentation quality. It may be that these processes employ common mechanisms in which antioxidants could play a pivotal role. This last hypothesis deserves further investigation.
Introduction
Background
The increasing concern to maintain a youthful appearance is driving the growth of new dermatological procedures for treatment of skin aging. In recent years, there has been an increasing emphasis on minimally invasive treatments and techniques designed to treat volume loss, wrinkles, and skin photodamage.
Age-related changes in the dermis can be considered the result of intrinsic factors and the consequence of environmental damage, in particular ultraviolet (UV) radiation from the sun, which is responsible for skin photoaging. Both are cumulative processes that share common cellular and molecular pathways mediating skin damages, for example, reactive oxygen species (ROS) arising from oxidative cell metabolism.Citation1
The alterations of the dermal connective tissue, corresponding mainly to a reduction of the extracellular matrix (ECM), are highly responsible for the wrinkling and sagging of the skin since they determine deep modifications in its mechanical properties. Repeated studies have proven that aging processes affect the enzymatic activities related to synthesis, remodeling, and catabolism of the ECM components of the dermis (collagen, elastin, and glycosaminoglycans). As a result, not only do aging processes induce a reduction of the ECM density but also, its quality is affected; moreover, the less efficient biosynthetic activities make the newly formed collagen more easily attacked by collagenases and metalloproteinases, the key enzymes in matrix degradation.Citation2,Citation3 Today, intradermal injections of biological substances able to induce a revitalization of the dermis can stimulate qualitative and quantitative improvements in aging skin alterations. The most frequently used substance is natural non-cross-linked hyaluronic acid (HA). The levels of HA, the major nonsulfated glycosaminoglycan of the connective tissue scaffold, decrease with aging, an event that leads to a direct reduction in water content and skin turgor. Interestingly, injection of simple HA can, not only provide enrichment of one of the main ECM compounds and deep hydration of the skin but also, strongly stimulates fibroblasts, acting on specific receptors (CD44, RHAMM, and ICAM-1)Citation4 to synthesize new scaffold compounds.Citation5
Therefore, the goals of biorejuvenation are to increase the biosynthetic capacity of fibroblasts, inducing the reconstruction of an optimal physiologic environment; the enhancement of cell activity; hydration; and the synthesis of collagen, elastin, and HA. The desired effect – firm, bright, moisturized skin – can be achieved by microinjections in the superficial dermis of products containing only one active ingredient or “cocktails” of different compounds that are biocompatible and absorbable.Citation6
The great versatility of biorevitalization lies in the different biological effects of the injected active substances. The synergy of different functional ingredients can treat skin in a more complete way, acting on various age-related marks caused by both intrinsic and extrinsic aging factors, with a preventive and curative action.
Objective
Based on the considerations above, this study aimed to investigate the efficacy of an injectable solution containing 32 mg of nonreticulated HA of biotechnological origin plus an antiaging complex composed of nucleotides, amino acids, vitamins, and antioxidants (), in the treatment of skin aging and photoaging. The product (Viscoderm®Skinkò E) was commercially available (5 mL vial) and manufactured by IBSA Farmaceutici Italia Srl (Lodi, Italy). Patients underwent a cycle of four sessions of biorevitalization that was performed in different areas affected by skin aging, and a phototest was performed on dorsal skin, according to the COLIPA method,Citation7 to assess the possible photoprotective action of the study product.
Table 1 Formulation characteristics
Methods
A total of 64 female healthy volunteers, aged between 37 and 60 years (average age 52 years), were enrolled in this open clinical trial. Inclusion criteria were: patients in good health; accepting to maintain their current eating habits, physical activity, makeup use, and facial cleansing; and accepting to avoid strong UV radiation throughout the entire study period. Exclusion criteria for the study were: subjects who had undergone other medical aesthetical treatments during the previous 3 months; and pregnancy or breastfeeding. All subjects gave written consent for enrollment into the trial.
The objective of the study was to evaluate the tolerability and the antiaging and photoprotective efficacy of an injectable intradermal solution. The study was divided in two different parts: a separate single treatment and UVB exposure to evaluate photoprotective effects, followed by a cycle of four biorevitalizing treatments to examine overall effects. The study design was evaluated and approved by an independent ethics committee. Because of the high number of injections required by this esthetic procedure, the Derming Institues Independent Ethical Committee did not approve the use of a control group treated with placebo, except for the phototest.
Biorevitalization treatment
A total of five visits were carried out during the trial: at baseline (T0) and after 3 weeks, 6 weeks (T6W), 9 weeks (T9W), and 12 weeks (T12W). Each volunteer underwent four sessions of biorevitalization, one every 3 weeks starting from T0. The sessions involved multiple microinjections, with either a 0.40×4 mm/27 G or a 0.33×12 mm/23 G needle, in the face (external corner of the eye and cheek), neck, décolletage, and back of the hands. The amount of product injected corresponded to 3 mL on the face and 5 mL divided on the other treated areas; the microinjections were performed at a distance of 1–2 cm from each other.
The assessment of the esthetic result was carried out at T6W, T9W, and T12W and involved the use of clinical evaluations and instrumental measurements (using techniques previously described in the literature),Citation7–Citation18 supported by photographic documentation. Visual evaluations were performed monolaterally on the right or left side, according to a randomization list defined by the investigator before the subjects’ inclusion, except for the décolletage, which was evaluated in its entirety.
Biorevitalization treatments and clinical evaluations were performed by a dermatologist.
The following instrumental evaluations were performed on the cheek of each volunteer:
Tissue dielectric constant values of the superficial and deep skin layers, an index of hydration of the deep skin layers (assessed at 1.5 mm and 0.5 mm of depth) was obtained using a MoistureMeterD (Delfin Technologies, Kuopio, Finland). The MoistureMeterD generates a high-frequency, low-power electromagnetic wave to which the tissue is exposed. The reflected electromagnetic wave is registered by the device, and the obtained value is the dielectric constant, which is proportional to the water content of the measured tissue
Skin spectrophotometry was done using a visible-UV-infrared (IR) (λ from 300 to 900 nm) spectrophotometer (DH2000; TOP Sensor Systems BV, Eerbeek, the Netherlands), which uses a tungsten halogen lamp and a deuterium lamp compliant to CIE (Commission Internationale de l’Éclairage [International Commission on Illumination]) standards. Lamps were switched on 30 minutes before instrument use, in order to stabilize the lamp emissions. The inclination of the probe was 90° on the surface to be examined, on an area of approximately 2 mm2. The wavelength range was 380–780 nm, corresponding to the visible spectrum
Optical colorimetry was performed by a tristimulus colorimeter (Chroma Meter CR-200®; Konica Minolta, Osaka, Japan) equipped with three special filters to obtain red-green-blue (RGB) values in accordance with CIE. The CIE L*a*b* system (CIELAB) is the most complete color space specified by the CIE (1976).Citation19 It describes all the colors visible to the human eye. The three coordinates of L*a*b* represent the lightness of the color (L*=0 yields black, and L*=100 indicates diffuse white; specular white may be higher), its position between red/magenta and green (a* negative values indicate green, while positive values indicate magenta), and its position between yellow and blue (b* negative values indicate blue, and positive values indicate yellow). Following CIELAB, it was possible to assess skin color: the coordinate L* defined skin brightness; a* denoted skin erythema; and b* indicated the skin pigmentation
Wrinkles pictures were taken and profilometry was done. A picture of the malar region was taken using a Primos compact portable device (GFMesstechnik GmbH, Teltow, Germany). The device software is able to elaborate three-dimensional (3D) representations of skin wrinkles, to measure skin principal profilometric parameters, and to directly compare the obtained images. As a measuring method, the Primos compact uses a digital stripe projection based on micromirrors. The portable probe assures a constant distance from the skin as well as a fixed illumination angle of incidence; in this way, it is possible to acquire standardized and reproducible images. By defining an area within the image and tracing a segment of known length in a defined position across the wrinkle and perpendicular to it, it was possible to calculate the profilometric roughness parameters: average roughness of the analyzed profile; total wrinkle height; and maximum wrinkle depth.
At T0, and at 6, 9, and 12 weeks after the start of treatment, frontal and lateral (45°) pictures of treated areas were taken for each volunteer.
Clinical evaluations of each treated area were performed after the esthetic procedure and were recorded as follows:
Skin roughness of the periocular area was assessed according to Glogau’s reference photographic scale,Citation20 giving a visual score from 0= no wrinkles to 3= very marked wrinkles
Cheek ptosis was assessed according to the Facial Volume Loss Scale,Citation21 giving a visual score from 0 to 5
Neck wrinkles were assessed according to our internal reference clinical scale, from 0= no wrinkles to 5= very marked wrinkles ()
Décolletage and hand surface microrelief regularity grade was assessed according to our internal reference clinical scale, from 1= very regular to 4= very irregular ().
Table 2 Neck wrinkles: severity rating scale
Table 3 Décolletage and hand surface microrelief regularity scale
Phototest
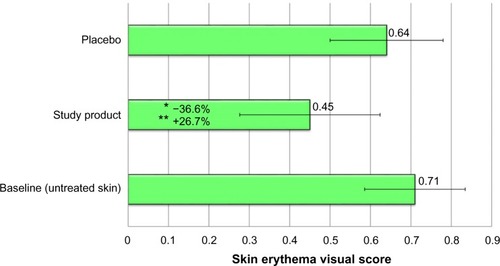
In basal conditions, before starting the aesthetic procedure with the test product, each volunteer will be exposed at the level of dorsal skin to six incremental doses of UVR (ultraviolet radiations) in order to determine the MED (minimal erythema dose of unprotected skin). To determine the protective efficacy of the tested product the UVR irradiation will be repeated 24 hours after a single anti-aging complex treatment performed on the small skin area of the back adjacent to the area where the basal UVR irradiation was performed. The induced erythema was graded 24±4 hours after irradiation, according to the COLIPA reference visual score: 0= no erythema, 1/2= perceptible redness reaction (MED), 1= moderate erythema, 2= severe erythema. Visual scores of every response to MED-testing were then compared with the ones obtained by irradiated skin previously injected with the study product and irradiated skin previously injected with a placebo (physiological solution for injection). UVB irradiation corresponding to 1 MED was performed 24 hours after the study product/placebo microinjection, and clinical evaluation of the induced erythema was scored 24±4 hours after irradiation. The photoprotective efficacy was expressed as a percentage of erythema visual score variation vs placebo.
Statistical analysis
The statistical evaluations of clinical and instrumental data (adjusted means and standard deviation) and relative graphs were produced using software provided with the statistics manual “Primer of Biostatistics”,Citation22 as follows:
Clinical data analyses included a comparison of results at different study times vs basal conditions, using the Friedman test followed by, in case of statistically significant result, the Dunnett test
Instrumental data analyses included a comparison of results at different study times vs basal conditions, using analysis of variance (ANOVA) for repeated-measures followed by, in case of statistically significant result, the Dunnett/Tukey test.
Results
There were five dropouts from the study: subject 47, for an adverse event related to the second injection procedure; subject 63 for protocol violation (UV light exposure); and subjects 45, 52, and 62 for personal problems unrelated to the study treatment. Therefore the statistical analysis was performed on the total of 59 subjects who completed the study.
Tolerability
Only one adverse event occurred during the trial: on the day after the second injection procedure, one subject showed edema on the lower eyelids, more marked on the right side, which resolved a few days after the application of an anti-inflammatory cream. To assess the relationship between the study product and the event, a small quantity was then injected intradermally on the right forearm of the patient. The redness disappeared normally within 3–4 hours, demonstrating that the adverse event was probably related to the particular sensitivity of the skin area in which the product was injected. At the end of the trial, the investigator judged the product tolerability good–excellent in 100% of subjects, as confirmed also by the subject self-assessments (29% as good and 71% as excellent). No adverse events were reported after the end of the trial.
Instrumental evaluations
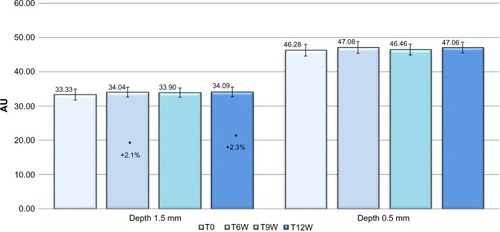
The tissue dielectric constant of the deep skin layers (registered at 1.5 mm of depth), starting from T6W to T12W, showed a statistically significant increase (Dunnett test P<0.05), of 2.1% and 2.3%, respectively, vs baseline. No significant variation was found in skin hydration measured at 0.5 mm of depth ().
Figure 1 Skin hydration at 0.5 and 1.5 mm of depth, variation vs baseline.
Spectrophotometry showed, at T9W and T12W, a statistically significant decrease (Dunnett test P<0.05), of 14.7% and 15.6%, respectively, of total visible spectrum area vs baseline, suggesting a general improvement of skin face radiance.
Optical colorimetry revealed improvements. The L* parameter data, which indirectly represents the skin brightness, significantly increased starting from the second treatment; moreover, a clinically and statistically significant decrease (Dunnett test P<0.05 vs T0) of the a* and b* parameters, representing, respectively, the skin redness and the skin pigmentation, confirmed the improvement of the face complexion and skin radiance highlighted by the spectrophotometric analysis ().
Table 4 Optical colorimetry: variation in LTable Footnote*, aTable Footnote* and bTable Footnote* parameters vs baseline
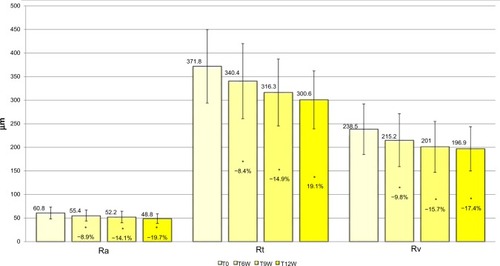
Image analysis of the periocular area (“crow’s feet”) showed, as early as the second biorevitalizing treatment, the antiaging efficacy of the tested product. Starting from T6W, evaluations showed a statistically and clinically significant reduction (Dunnett test P<0.05, for T6W, T9W, and T12W) of profilometric parameters vs baseline: average roughness of the analyzed profile decreased, by 8.9% at T6W, by 14.1% at T9W, and by 19.7% at T12W, suggesting that the area around the eyes was generally less wrinkled; total wrinkle height decreased, by 8.4% at T6W, by 14.9% at T9W, and by 19.1% at T12W, proving that wrinkles were less deep; and maximum wrinkle depth decreased by 9.8% at T6W, by 15.7% at T9W, and by 17.4% at T12W. These results demonstrate that wrinkles were less visible ().
Figure 2 Skin profilometric parameters (antiwrinkle efficacy), variation vs baseline.
Abbreviations: Ra, average roughness; Rt, total height; Rv, maximum depth.
Photoprotective efficacy
The study product demonstrated a clinically and statistically significant decrease in the erythema visual score compared with baseline (−36.6%) (Tukey test P<0.05). This result highlights a protective efficacy of the study product, statistically different from the area pretreated with placebo (–26.7% Tukey test P<0.05) (). The protective efficacy vs placebo, was calculated using the following formula:
(1)
Clinical evaluations and photographic documentation
The antiaging efficacy was also confirmed by clinical evaluations. Results highlighted, starting from T9W (after the third biorevitalizing session), a clinically and statistically significant improvement (Dunnett test P<0.05, at T9W and T12W) of at least one grade of the following items vs baseline: crow’s feet roughness, cheek ptosis, neck wrinkles (rings of Venus), décolletage, and hands skin surface microrelief. In particular, as soon as after the second biorevitalizing treatment, an improving trend in hand skin microrelief was highlighted in 47% of volunteers. The percentage of subjects who achieved an improvement of at least one grade from baseline for each item at T6W, T9W, and T12W are summarized in .
Table 5 Percentage of subjects, who achieved an improvement of at least one grade from baseline, for each item, at T6W, T9W and T12W
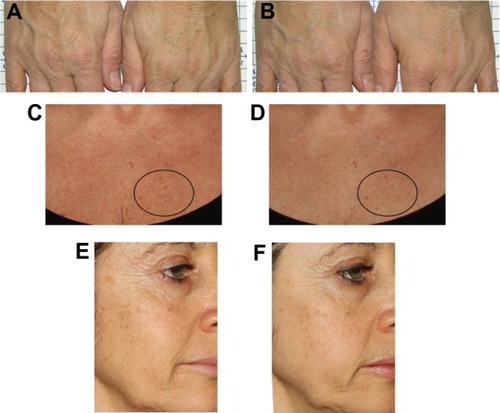
A surprising reduction in senile lentigines on the treated areas was observed in several volunteers, as reported by photographic documentation ().
Conclusion
Obtained data showed that the study product provided clinical and biophysical changes in the skin. Interesting quantitative and qualitative results were shown, especially, a multifunctional product activity. Thanks to its rich formulation, the injectable solution of hyaluronic acid plus an antiaging complex (Viscoderm®Skinkò E) demonstrated its effectiveness in the treatment of skin aging, confirmed by all the instrumental evaluations. The analysis of the profilometric parameters showed a statistically and clinically significant reduction of skin roughness around the eyes, as soon as after the second biorevitalizing treatment. The instrumental evaluations were confirmed by clinical evaluations (). In fact, compared with baseline, skin appeared younger after the third session, and a clinically and statistically significant improvement of at least one grade of crow’s feet and cheek ptosis was registered. The efficacy of the study product was observed, not only on the face but also, in the other treated skin areas, confirming the study product versatility. Moreover, an important reduction of the rings of Venus on the neck was highlighted, as was a relevant improvement of the décolletage and hand skin surface microrelief (an index of a redensifying activity).
Table 6 Summary table with an overview of the main results at T12W
The study confirmed the well-known efficacy of intradermal injections of HA on skin rejuvenation, which may have increased the biosynthetic capacity of fibroblasts and the synthesis of collagen, elastin, and HA in treated areas. It is generally assumed that when injected intradermally with others active ingredients, HA promotes fibroblasts to express collagen type 1, matrix metalloprotease, and tissue inhibitor of matrix metalloprotease-1,Citation23 gaining as a final effect, a skin renewal. Amino acids and vitamins are also important ingredients in a mesotherapeutic cocktail, thanks to properties essential for maintaining a youthful appearance. As documented, the vitamin B complex (vitamins B1 [thiamine], B2 [riboflavin], B5 [pantothenate], B6 [pyridoxine], B9 [folic acid], and B12 [cyanocobalamin]) plays a fundamental role in several metabolic processes, acting as free radical scavengers. Also important are vitamins H (biotin) and I (inositol), and the amino acids, which take part in the synthesis of polypeptides, forming the matrix of the cellular architecture.Citation24
More interesting, skin hydration results showed surprising data: assessment of skin hydration on the cheeks, in particular, showed a positive difference in the percentage hydration of deep and superficial skin layers at the end of the trial, suggesting improved skin moisture retention of the stratum corneum. The study product could have acted as a water content modulator in skin layers, thus improving the epidermal barrier function often affected by the aging mechanism.
In addition to the already known antiaging activity of intradermal injections of HA, Viscoderm®Skinkò E showed novel properties, such as photoprotective efficacy and activity on complexion homogeneity.
The study product showed a substantial protection against damage caused by 1 MED UVB exposure: skin erythema in the pretreated areas was significantly less marked than in untreated or placebo treated skin. This is related to the antioxidant activity of the ingredients, including lipoic acid. Lipoic acid is a powerful antioxidant within the cells and at the same time, a coenzyme that participates in complex reactions of cellular metabolism. It plays an important role as a topical photoprotectant thanks to its interesting antioxidant property; in fact, its use is proposed both for treatment of cutaneous aging and for the prevention of the erythema associated with exposure to UV radiation.Citation25
Spectrophotometric and optical colorimetric measurements showed an illuminating and brightening activity as soon as after the second treatment session, while the photographic documentation highlighted a clinically relevant and interesting reduction of senile lentigines in the treated areas of some volunteers. These hyperpigmented macules are a common component of photoaged skin,Citation26 caused by ROS, which deplete and damage nonenzymatic and enzymatic antioxidant defense systems.Citation27 This last evidence suggests that the injected product could intervene at different moments of the skin pigmentation process by activating an intrinsic photoprotective mechanism and improving skin pigmentation quality, with a clearing action on melanin aggregates. It is possible these processes employ common mechanisms, in which antioxidants could play a pivotal role. This last hypothesis deserves further investigation.
Disclosure
The study was supported by IBSA Farmaceutici Italia Srl, the company that distributes the product used in the trial. The authors report no other conflicts of interest in this work.
References
- PeresPSTerraVAGuarnierFACecchiniRCecchiniALPhotoaging and chronological aging profile: Understanding oxidation of the skinJ Photochem Photobiol B20111032939721356598
- VaraniJDameMKRittieLDecreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulationAm J Pathol200616861861186816723701
- WestMDThe cellular and molecular biology of skin agingArch Dermatol1994130187958285746
- EntwistleJHallCLTurleyEAHA receptors: regulators of signalling to the cytoskeletonJ Cell Biochem19966145695778806080
- GhersetichIManagement of aging skinJ Eur Acad Dermatol Venereol1997951 Abstract.
- IorizzoMDe PadovaMPTostiABiorejuvenation: theory and practiceClin Dermatol200826217718118472058
- Enterprise and Industry Directorate-GeneralStandardisation Mandate Assigned to CEN Concerning Methods for Testing Efficacy of Sunscreen ProductsBrusselsEuropean Comission2006 Available from: http://ec.europa.eu/consumers/sectors/cosmetics/files/doc/sunscreen_mandate_en.pdfAccessed December 31, 2014
- RiegerMMBattistaGWSome experiences in the safety testing of cosmeticsJ Soc Cos Chem1964153161172
- wma.net [homepage on the Internet]WMA declaration of Helsinki. Ethical principles for medical research involving human subjectsWorld Medical Association1964 (updated 2013). Available from: http://www.wma.net/en/30publications/10policies/b3/Accessed April 11, 2014
- MayrovitzHNBernalMBrlitFDesforRBiophysical measures of skin tissue water: variations within and among anatomical sites and correlations between measuresSkin Res Technol2013191475423046199
- MayrovitzHNBernalMCarsonSGender differences in facial skin dielectric constant measured at 300 MHzSkin Res Technol201218450451022142446
- TakiwakiHMeasurement of skin color: practical application and theoretical considerationsJ Med Invest1998443–41211269597799
- FullertonAFischerTLahtiAWilhelmKPTakiwakiHSerupJGuidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact DermatitisContact Dermatitis19963511108896947
- WesterhofWCIE ColorimetrySerupJJemecGBEHandbook of Non-Invasive Methods and the SkinBoca Raton, FLCRC Press1995385397
- FrankowskyGHainichRDPL-based 3D metrology by structured light or projected fringe technology for life sciences and industrial metrologyHornbeckLJDouglasMRProc SPIE 7210: Emerging Digital Micromirror Device Based Systems and ApplicationsBellingham, WASPIE Publications2009112
- HoppeUSauermannGLunderstadtRQuantitative analysis of the skin’s surface by means of digital signal processingJ Soc Cos Chem1985362105123
- HofCHopermannHComparison of replica- and in-vivo measurement of the microtopography of human skinSOFW Journal20001264047
- BergerDSSpecification and design of solar ultraviolet simulatorsJ Invest Dermatol19695331921995809822
- BaldevbhaiPJAnandRSColor image segmentation for medical images using L*a*b* color spaceIOSR Journal of Electronics and Communication Engineering2012122445
- GlogauRGAestethic and anatomic analysis of the aging skinSemin Cutan Med Surg1996531341388948530
- AscherBColemanSAlsterTFull scope of effect of facial lipoatrophy: a framework of disease understandingDermatol Surg20063281058106916918569
- GlantzSAPrimer of biostatistics5th edMcGraw-Hill, Medical Pub. Div.2002
- GaoFLiuYHeYHyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesisMatrix Biol201029210711619913615
- TostiADe PadovaMPAtlas of Mesotherapy in Skin RejuvenationLondonInforma Healthcare2007
- BastAHaenenGRLipoic acid: a multifunctional antioxidantBiofactors2003171–420721312897442
- SitumMBulatVBuljanMPuljizZSitumVBolancaZSenile lentigo – cosmetic or medical issue of the elderly populationColl Antropol201034Suppl 2S85S88
- WlaschekMTantcheva-PoórINaderiLSolar UV irradiation and dermal photoagingJ Photochem Photobiol B2001631–3415111684450