Abstract
Background
Acne vulgaris is an inflammatory disorder of the pilosebaceous unit.
Aim
To confirm that BGM (bakuchiol, Ginkgo biloba extract, and mannitol) complex increases the established clinical efficacy of adapalene 0.1% gel in patients with acne.
Methods
A clinical trial was conducted in acne patients. A total of 111 subjects received adapalene 0.1% gel and BGM complex or vehicle cream for 2 months. Assessments comprised Investigator Global Assessment (IGA), global efficacy, seborrhea intensity, inflammatory and non-inflammatory lesions, and subject perception, as well as overall safety and local tolerance and quality of life.
Results
At the end of the trial, inflammatory and non-inflammatory lesions, IGA, global efficacy, and seborrhea intensity had significantly improved in both treatment groups. Differences were statistically significant (P<0.05) in favor of BGM complex for inflammatory lesions as well as IGA and seborrhea intensity. Global efficacy assessments and subject perception confirmed the superiority of BGM complex-including treatment over the comparative combination. Quality of life had improved more with the active combination than with the vehicle combination. In the active group, four subjects had to interrupt temporarily BGM complex and 12 adapalene compared to seven subjects interrupting the vehicle and eleven adapalene in the vehicle group. One subject withdrew from the trial due to an allergy to adapalene. The majority of all events were mild.
Conclusion
BGM complex improves the treatment outcome of adapalene 0.1% gel in patients with acne vulgaris. Overall, safety and local tolerance of BGM complex were good.
Introduction
Acne vulgaris is a multifactorial, inflammatory disorder of the pilosebaceous unit. It presents with ductal hyperkeratinization and increased cohesiveness of keratinocytes, increased sebum production, alteration of sebum lipid composition, dysregulation of the hormone microenvironment, interaction with neuropeptides, hypercolonization by Propionibacterium acnes, and inflammation.Citation1,Citation2
Changes in the activity of the sebaceous gland not only result in seborrhea but also in alterations of the sebum fatty acid composition.Citation3 Sebum lipid fractions with proinflammatory properties and inflammatory tissue cascades have been associated with the process of the development of acne lesions, while follicular keratinocytes and sebocytes, the major components of the pilosebaceous unit, act as immune-active cells capable of microbial recognition and abnormal lipid presentation.Citation4 The latter may be activated by P. acnes and recognize altered lipid content in sebum, followed by the production of proinflammatory cytokines.
There is no single topical antiacne medication that acts against all four major pathophysiologic acne features. Retinoids, such as adapalene, are well known for targeting comedones, including microcomedos and precursors of both inflammatory and non-inflammatory lesions, and exerting anti-inflammatory activity on the innate immunity.Citation5–Citation7 They are more and more often applied in combination with topical antibacterials, including antibiotics and benzoyl peroxide (BPO).Citation8,Citation9 The latter is known to cause irritation, while the use of topical antibiotics is less and less recommended due to the increased development of antimicrobial-resistant bacteria.
Laboratoire Bioderma, France, developed a new dermocosmetic cream containing a triple complex (hereafter BGM complex, Sébium Global cream). This complex mimics the effect of vitamin E, allowing protection from potentially harmful effects of squalene oxidation products, such as hyperkeratinization and inflammation.Citation10 The product contains a triple complex consisting of bakuchiol, Ginkgo biloba extract, and mannitol.Citation11–Citation14 Bakuchiol regulates seborrhea, is antibacterial, and has antiacne properties.Citation15–Citation19 In addition to its excipients, the active formulation contains enoxolone (18β-glycyrrhetinic acid), salicylic acid, alpha hydroxy acid esters, and zinc gluconate, all of which are all well-recognized pharmacological active agents used in the treatment of acne.Citation20–Citation23
The aim of the present trial was to assess the potential of BGM complex to improve the established clinical efficacy of adapalene 0.1% gel.
Methods
Settings
This multicenter and comparative double-blind clinical trial was conducted according to the Declaration of Helsinki of 1973 and all its amendments and its successive updates, to good clinical practices for the conduct of clinical trials and to local regulatory requirements. The trial was performed from January to June 2013 at 13 sites in Slovakia.
Trial population
Subjects had to be 12–25 years of age, present with mild-to-moderate acne (grades 2–3 on the Investigator Global Assessment [IGA] scale), and had to be prescribed adapalene 0.1% gel for at least 2 months.
Treatments
Subjects were randomized at investigational sites in a 1:1 ratio to either an association of BGM complex cream and adapalene 0.1% gel, hereafter active association, or to an association of vehicle cream and adapalene (hereafter vehicle association).
Adapalene was prescribed by the investigators on the day of inclusion and had to be used as per the instructions.
BGM complex or the vehicle, containing water (aqua), glycerin, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, titanium dioxide, phenoxyethanol, chlorphenesin, polysorbate 60, alumina, and stearic acid, was to be applied on the entire face in the morning, after the face had been cleaned with a gentle cleanser. Adapalene was to be applied in the evening on the cleaned skin.
Make-up, except foundation cream, was permitted, while no other topical skin care product or treatment was authorized on the face during the trial. Oral contraception for women of childbearing potential had to remain unchanged during the course of the trial.
Assessments
Clinical evaluations of inflammatory and non-inflammatory lesions count, seborrhea intensity on a visual scale (from 0: nil to 3: high), global acne severity using the IGA score (from 0: none to 4: severe), and global efficacy assessed on a scale (from 0 [lowest score] to 10 [highest possible score]) were performed on D0, D28, and D56 (D standing for day). Quality of life using the Cardiff Acne Disability IndexCitation24 was assessed on D0 and D56.
Safety was assessed through adverse event reporting; local tolerance signs and symptoms comprising erythema, desquamation, pruritus, burning, prickling, tightness, vesicles, papules, and edema were assessed on D28 and D56 by the investigator on a scale from 0 to 3 (0= none, 1= light, 2= average, 3= high).
Statistics
Statistical analyses were performed on data from subjects who completed the trial. Quantitative criteria were expressed by sample size, mean, and standard deviation. Qualitative criteria were expressed in percentage. The Student’s t-test on paired data or paired Wilcoxon test was applied to compare the number of non-inflammatory and inflammatory lesions, IGA and intensity of seborrhea between D0 and D28, and D0 and D56 in each group. Differences between groups for lesion count, intensity of seborrhea and IGA were calculated using the Student’s t-test or Mann–Whitney test. The chi-square test was used to compare global efficacy between groups. A two-sided significance level of 0.05 was chosen. IGA was compared between each time point.
Results
Demographics and baseline data
A total of 111 subjects participated in this trial, 55 in the active association and 56 in the vehicle association group (). Three patients presented with an acne severity of less than mild (IGA grade 1) at inclusion. The investigator decided to maintain these subjects in the trial.
Table 1 Demographics and baseline data
Efficacy
Lesion count
After 56 days of treatment, the mean number of non-inflammatory lesions had decreased from 34.4±23.5 to 15.2±13.5 lesions in the active association group and from 29.3±20.3 to 14.6±8.5 lesions in the vehicle association group. The percent decrease at D56 from D0 reached 56% and 50.2% in the active association and the vehicle association groups, respectively (). These reductions were statistically significant (P<0.005) for each group and sustained from D28. The difference between both groups was not statistically significant.
Figure 1 Percent decrease over time of acne lesions: (A) non-inflammatory and (B) inflammatory lesions.
Abbreviation: D, day.
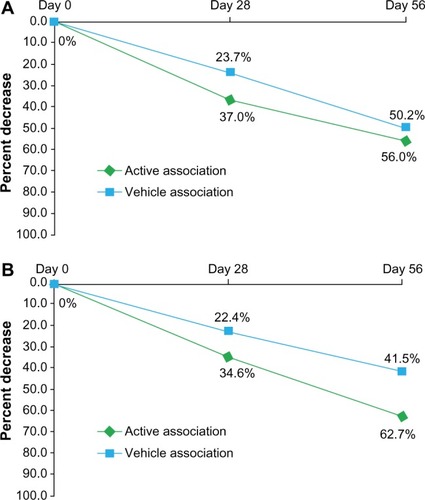
At D56 compared to D0, the mean number of inflammatory lesions had decreased in both groups: from 18.4±10.9 to 6.9±6.6 and from 17.3±11.5 to 10.1±10.4 lesions in the active association and vehicle association groups, respectively. At the end of the trial, the percent decrease from D0 was 62.7% for the active association group and 41.5% for the vehicle association group (). These reductions were statistically significant (P<0.005) and sustained from D28. The difference between both groups was statistically significant (P<0.05) and in favor of the active association group.
shows results for seborrhea intensity, IGA, global efficacy evaluation, and quality of life at D0 and D56.
Table 2 Results for seborrhea intensity, IGA, global efficacy evaluation, and quality of life after 56 days of use of BGM complex or vehicle in combination with adapalene 0.1% gel
Safety
In the active association group, 23 subjects reported at least one local adverse event (burning sensation, erythema, desquamation, and pruritus). In 15 subjects, they were considered related to adapalene and in four subjects, related to BGM complex. The majority of all local adverse events were mild in intensity.
A total of 12 subjects had to stop adapalene and four had to stop BGM complex. The interruption of applications was temporary in all subjects except for one subject who experienced an allergy considered to be related to adapalene.
In the vehicle association group, 17 subjects reported at least one local adverse event (erythema, burning sensation, pruritus, and desquamation). Of those, 12 were related to adapalene. The majority of all local adverse events were mild in intensity.
In eleven subjects, adapalene had to be interrupted temporarily; vehicle had to be interrupted temporarily in seven subjects.
Discussion and conclusion
After 2 months of combined use with adapalene, BGM complex improved significantly the clinical outcome for inflammatory lesions when compared to the vehicle. The fact that BGM complex enhances the anti-inflammatory potential of adapalene may be due to its antibacterial properties on P. acnes as well as its anti-irritation potential. Results observed for the non-inflammatory and inflammatory lesion counts were confirmed by the IGA, the seborrhea intensity, and the global efficacy rated by the investigator and the subject, which at the end of the trial were all significantly in favor of the BGM complex/adapalene regimen; quality of life of subjects had significantly improved. Even though results are not shown, the excellent subjects’ perceptions of BGM complex may have contributed to making them adhere to their treatment, especially in the light of acne being a chronic disease.Citation25 One may argue that subjective evaluation is secondary to objective clinical assessments. However, disfigurement from inflamed lesions, post-inflammatory hyper-pigmentation, and scars often causes embarrassment and frequently undermines confidence and lowers self-esteem. Hence, adherence to treatment, reducing sequels from acne is crucial for a positive treatment outcome.
The fact that bakuchiol, one of BGM complex agents, has antibacterial properties may open new perspectives in the issue of P. acnes strains, becoming more and more resistant to antibiotics. Indeed, to limit the use of topical antibiotics, retinoids such as adapalene are more and more combined with BPO. However, BPO may cause irritation and is hence contraindicated in a certain number of patients. As for BPO, bakuchiol does not lead to antibiotic resistance. Hence, in such patients, BGM complex may be considered an alternative to BPO. To confirm this, future clinical trials may be conducted to evaluate the efficacy of BGM complex combined to a topical retinoid compared to a combination of topical retinoids and topical antibacterials.
A similar number of subjects reported mild and transient local adverse events with BGM complex and with the vehicle cream; the majority of local adverse events were caused by adapalene, even though known to be the less-irritating topical retinoid.Citation26,Citation27
A missing treatment arm with adapalene in monotherapy and the short treatment duration, treatment of adapalene should last 3 months, may raise concerns for limitations. However, we feel that the results obtained from the active and the vehicle groups confirmed the expected treatment outcome of adapalene. Therefore, we consider that the trial is robust enough to show that BGM complex improves the treatment outcome of adapalene in patients with acne vulgaris.
In conclusion, the dermocosmetic containing BGM complex improves the treatment outcome of adapalene in patients with acne vulgaris and is well tolerated.
Acknowledgments
The authors acknowledge the contribution of the investigators and staff members of the trial centers, the scientific advice provided by Prof Dr Christos C Zouboulis, Germany, and the writing support provided by Patrick Göritz, SMWS – Scientific and Medical Writing Services, France. The trial was funded by Laboratoire Bioderma, France.
Disclosure
Dr Katarína Poláková has no financial interest to disclose. Eric Jourdan, Aurélie Fauger, and Michèle Sayag are employees of Laboratoire Bioderma, France.
References
- ZouboulisCCEadyAPhilpottMWhat is the pathogenesis of acne?Exp Dermatol200514214315215679586
- KurokawaIDanbyFWJuQNew developments in our understanding of acne pathogenesis and treatmentExp Dermatol2009181082183219555434
- ZouboulisCCJourdanEPicardoMAcne is an inflammatory disease and alterations of sebum composition initiate acne lesionsJ Eur Acad Dermatol Venereol201428552753224134468
- KoreckAPivarcsiADobozyAKemenyLThe role of innate immunity in the pathogenesis of acneDermatology200320629610512592074
- LeydenJAdapalene in clinical practiceCutis2001684 Suppl7911845950
- BershadSPoulinYPBersonDSTopical retinoids in the treatment of acne vulgarisCutis1999642 Suppl820 quiz 21–2310472493
- ThielitzASidouFGollnickHControl of microcomedone formation throughout a maintenance treatment with adapalene gel, 0.1%J Eur Acad Dermatol Venereol200721674775317567301
- BabaeinejadSHFouladiRFThe efficacy, safety and tolerability of adapalene versus benzoyl peroxide in the treatment of mild acne vulgaris; a randomized trialJ Drugs Dermatol20131291033103824002152
- BettoliVBorghiAZauliSMaintenance therapy for acne vulgaris: efficacy of a 1 2-month treatment with adapalene-benzoyl peroxide after oral isotretinoin and a review of the literatureDermatology201322729710224029411
- ChibaKYoshizawaKMakinoIKawakamiKOnoueMComedogenicity of squalene monohydroperoxide in the skin after topical applicationJ Toxicol Sci2000252778310845185
- FengXZhangLZhuHComparative anticancer and antioxidant activities of different ingredients of Ginkgo biloba extract (EGb 761)Planta Med200975879279619288403
- LiuJHChenMMHuangJWTherapeutic effects and mechanisms of action of mannitol during H2O2-induced oxidative stress in human retinal pigment epithelium cellsJ Ocul Pharmacol Ther201026324925720565311
- GoldieMDolanSBilobalide, a unique constituent of Ginkgo biloba, inhibits inflammatory pain in ratsBehav Pharmacol201324429830623838965
- CavoneLCalosiLCinciLMoroniFChiarugiATopical mannitol reduces inflammatory edema in a rat model of arthritisPharmacology2012891–2182122236612
- KaulRIn vitro Kinetik der anti-Staphylococcus Aktivität von Bakuciol. [Kinetics of the anti-staphylococcal activity of bakuchiol in vitro (author’s transl)]Arzneimittelforschung1976264486489 German989003
- KatsuraHTsukiyamaRISuzukiAKobayashiMIn vitro antimicrobial activities of bakuchiol against oral microorganismsAntimicrob Agents Chemother200145113009301311600349
- FerrándizMLGilBSanzMJEffect of bakuchiol on leukocyte functions and some inflammatory responses in miceJ Pharm Pharmacol19964899759808910867
- PaeHOChoHOhGSBakuchiol from Psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor-kappaB in RAW 264.7 macrophagesInt Immunopharmacol200119–101849185511562076
- ChaudhuriRKBojanowskiKBakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effectsInt J Cosmet Sci201436322123024471735
- MesserschmidtASchultheisKOchsendorfFTopische Benhandlung von Infektionen, Tumoren und Hyperkeratosen. [Topical treatment of infections, tumors and hyperkeratotic disorders]Hautarzt2014653207217 German24500043
- PoiraudCQuereuxGKnolACAllixRKhammariADrenoBZinc gluconate is an agonist of peroxisome proliferator-activated receptor-alpha in the epidermisExp Dermatol201221534735122509831
- LodenMRole of topical emollients and moisturizers in the treatment of dry skin barrier disordersAm J Clin Dermatol200341177178814572299
- IshidaTMizushinaYYagiSInhibitory effects of glycyrrhetinic acid on DNA polymerase and inflammatory activitiesEvid Based Complement Alternat Med2012201265051421785649
- MotleyRJFinlayAYPractical use of a disability index in the routine management of acneClin Exp Dermatol1992171131424249
- ZouboulisCCAcne as a chronic systemic diseaseClin Dermatol201432338939624767186
- DosikJSHomerKArsonnaudSCumulative irritation potential of adapalene 0.1% cream and gel compared with tretinoin microsphere 0.04% and 0.1%Cutis200575423824315916222
- DosikJSHomerKArsonnaudSCumulative irritation potential of adapalene 0.1% cream and gel compared with tazarotene cream 0.05% and 0.1%Cutis200575528929315984630
