Abstract
Perianal Crohn’s disease affects a significant number of patients with Crohn’s disease and is associated with poor quality of life. The nature of the disease, compounded by presentation of various disease severities, has made the treatment of perianal Crohn’s disease difficult. The field continues to evolve with the use of both historical and contemporary solutions to address the challenges associated with it. The goal of this article is to review current literature regarding medical and surgical treatment, as well as the future directions of therapy.
Keywords:
Introduction
Perianal disease is commonly diagnosed in individuals with Crohn’s disease (CD). It is a marker of more severe disease and is associated with multiple surgical interventions and frequent relapses.Citation1,Citation2 The incidence of perianal Crohn’s disease (pCD) ranges from 17% to 43% of CD cases.Citation1,Citation3,Citation4 pCD is associated with more distal CD.Citation3 In 5% of individuals, however, CD will only manifest as perianal disease without associated luminal disease.Citation1,Citation5 Individuals will most commonly develop perianal disease prior to the diagnosis.Citation1
pCD is particularly difficult to manage, due to the complexity of its presentation. The incidence of pCD is similar in men and women. Women, however, have greater complications associated with the adjacent vaginal wall and risks associated with childbirth.Citation6 Patient-reported symptoms associated with it include pain with associated perianal swelling and fevers, drainage of pus, stool, or blood from the vagina, scrotum, or perineum. Some may report fecal incontinence. The disease may physically manifest as a perianal fistula, anal fissure, anal canal stricture, rectovaginal fistula, or abscess.Citation7 The etiology of the pCD is still unclear; theories suggest it arises from deep ulcers or anal gland abscesses. It is most likely a combination of genetic, microbiologic, and immunologic factors.Citation8
Current clinical classifications for pCD were proposed by the American Gastroenterological Association.Citation7 Fistulas are distinguished as simple and complex fistulas. Simple fistulas are low, below the dentate line, and include superficial, intersphincteric, or intrasphincteric fistulas, with a single external opening without other complications. Complex fistulas are high, arising above the dentate line, and may have multiple external openings. They may be associated with perianal abscesses, rectal stricture, proctitis, or connections with the bladder or vagina. This classification has been elaborated to include a clinical activity score using the Perianal Disease Activity Index, described by Irvine.Citation9 This classification includes the evaluation of five elements: fistula discharge, pain and restriction of activities, restriction of sexual activity, type of perianal disease, and degree of induration.Citation9 In addition, the Fistula Drainage Assessment Measure classifies fistulas as being either open and actively draining or closed.Citation9,Citation10
This article will provide a brief summary of diagnostic strategies, current medical and surgical therapies for pCD, and future directions for therapies, focusing on the use of stem cells.
Diagnosis
The first step in diagnosing of pCD is to obtain a thorough history and physical examination. History should include anorectal pain, purulent discharge, persistent drainage, rectal bleeding, recurrent urinary tract infection (UTI), or fecal incontinence. Exam under anesthesia (EUA) remains the standard for diagnosis and classification of perianal fistula with an accuracy of up to 90% when diagnosing pCD.Citation11 It should be performed by a specialized surgeon well-versed in the disease process. During an EUA, abscesses will be drained, fistula tracts will be delineated, and setons placed if indicated. During the examination, close attention is directed to the vaginal wall and scrotum to assess for complex fistulous tracts. EUA with abscess drainage and seton placement is considered the first step prior to antitumor necrosis factor (anti-TNF) intervention, and results in higher resolution and lower recurrence.Citation12 In combination with EUA, endoscopy may also facilitate the identification of luminal inflammation and the presence of internal openings, while strictures and cancer are excluded.Citation13
The above diagnostic strategies are aided by the addition of endoanal ultrasound (EUS) and magnetic resonance imaging (MRI). In EUS, a high frequency endoluminal probe that produces 2D and 3D ultrasound images is utilized to visualize all sphincter structures.Citation14 The addition of hydrogen peroxide during EUS also enhances the identification of fistula tracts.Citation15,Citation16 The most recent meta-analysis reported a sensitivity of 0.87 and specificity of 0.43 for EUS.Citation17 Pelvic MRI is now considered the noninvasive gold standard for perianal fistula assessment, and the most recent meta-analysis reports a sensitivity of 0.87 and specificity of 0.59.Citation17 T2-weighted sequence with fat suppression is the optimal technique for MR fistula imaging, while a gadolinium enhanced T1-weighted sequence is useful for the differentiation of fluid, pus, or granulation tissue.Citation18 MRI is also capable of identifying clinically silent abscesses and inflammation.Citation19 Schwartz et al compared pelvic MRI and EUS with a reported accuracy of 91% and 87%, respectively, compared to an EUA at 91%.Citation11 Currently, the higher diagnostic quality of EUS and pelvic MRI precludes the use of computed tomography and fistulography.
Treatment
Medical
Medical therapy is a critical adjunct in the treatment of pCD, and should be started once the diagnosis of active pCD is made. The main goal of therapy is to achieve and maintain disease remission.
Antibiotics
Antibiotics are used to treat perianal sepsis and act as an effective bridge to immunosuppressive therapy.Citation20 A positive clinical response within 6–8 weeks of initiation of treatment is observed in 70%–95% of patients.Citation21 In mild-to-moderate symptoms, treatment with oral metronidazole has been used as the initial therapy, with improved symptoms in 50% of patients.Citation22 Fistula healing rates from antibiotic therapy alone, however, are <50%, and the majority of cases will recur if antibiotics are withdrawn.Citation23
Immunosuppression
Immunosuppression is the definitive therapy for pCD. Azathioprine and 6-mercaptopurine (6-MP) are thiopurines that halt DNA replication, and are used for induction and maintenance of remission in fistulizing disease. A meta-analysis of five randomized controlled trials examined the efficacy of 6-MP and azathioprine, and demonstrated a 54% healing rate in patients versus 21% of controls.Citation24 Cyclosporine and tacrolimus have been used, but less frequently. Cyclosporine, a T-cell suppressant, when given intravenously has an excellent, rapid effect in up to 83% of patients,Citation25,Citation26 but is not as effective when administered orally. In a randomized controlled trial, tacrolimus, an interleukin-2 (IL-2) inhibitor, resulted in improvement of CD symptoms in 43% of patients versus 8% in the placebo arm.Citation27 Neither cyclosporine nor tacrolimus, however, were specifically studied in fistulizing pCD.
TNF antagonists
TNF antagonists are effective in achieving durable remission in pCD. First, infliximab is a chimeric monoclonal antibody to TNF-α, and was evaluated in the ACCENT 1 and 2 trials. ACCENT 1 demonstrated successful induction therapy with infliximab for fistulizing pCD: 68% of patients treated with infliximab had at least a 50% improvement in symptoms versus 26% with a placebo.Citation28 The ACCENT 2 trial documented longer time to recurrence of fistulas with infliximab maintenance therapy.Citation29 In addition, treatment with infliximab prevented additional surgeries and hospitalizations.Citation30 Interestingly, rectovaginal fistula healing had a poor response to infliximab therapy, compared to perianal fistula healing.Citation29,Citation31
Second, adalimumab is a fully humanized monoclonal antibody against TNF-α. In the CHOICE trial, 673 patients achieved complete fistula healing after 8 weeks of adalimumab therapy.Citation32 In a post hoc analysis of the CHARM trial, complete fistula closure was seen at 1 year in 39% of patients treated with adalimumab, versus 13% in the placebo arm. These results were shown to be durable at 2 years.Citation33 A retrospective cohort study comparing infliximab and adalimumab in achieving and maintaining closure of perianal fistulas in ambulatory CD patients demonstrated complete response in 77.0% of patients at 36 months of follow-up, with no difference between infliximab and adalimumab.Citation10
Third, certolizumab pegol has improved solubility and decreased immunogenicity compared with the other TNF antagonists.Citation34 Durable remission for at least 4 years and healing of perianal fistula in 36% of patients was reported.Citation35 All three major TNF antagonist antibodies are effective in pCD, but head-to-head comparisons have not yet been performed.
In summary, the medical approach to the treatment of pCD is evolving. Recently, Choi et al identified a significantly greater proportion of healing in septic perianal disease with the addition of anti-TNF interventions in a retrospective review of 114 patients. Their treatment algorithm appears to be quite successful.Citation36 In the past, therapy has been increased in a stepwise fashion to achieve remission, adding increasingly potent immunosuppressant medications. The value of early combination therapy to prevent disease progression may find a role in fistulizing pCD (REACT trial),Citation37 or the addition of anti-TNF agents earlier in therapy.
Surgical
Surgical interventions for pCD vary, depending on disease extent and severity and can be facilitated by concurrent use of medical therapy (). Although an attempt is made to be conservative with surgical interventions, the overarching goal is to manage perianal sepsis, drain any abscesses, and place setons in delineated fistulas. Treatments can be highly variable among practitioners, requiring a multidisciplinary approach.Citation38 Here, we have reviewed specific Crohn’s literature regarding surgical interventions (). Perianal abscesses, which commonly precede or accompany perianal fistulas, should be incised and drained when first identified.Citation39 Surgical drainage, as opposed to spontaneous drainage, minimizes the risk of further septic complications.Citation40 It is important to note that the surgical removal of skin tags associated with pCD is not recommended, due to the risk of poor wound healing, infection, and fistula formation.Citation41 If fistulas are identified at the time of intervention, noncutting seton placement is recommended, which will maintain patency of the fistula tracts and limit recurrent abscess formation.Citation42 Seton placement allows drainage without closure and increases effectiveness of medical therapy, while preserving external sphincter function.Citation42,Citation43 Fistulotomies at the time of perianal sepsis are contraindicated, as there is an increased risk of incontinence.Citation44 The rate of incontinence after seton placement is 12%.Citation45 One should also consider the addition of an anti-TNF antibody, which has been shown to improve response rate, recurrence rate, and time to recurrence.Citation12 The duration of seton placement is not clear; studies, however, have reported that effective treatment is observed after longer durations of infliximab, and setons may be removed.Citation40,Citation46 Our institution waits at least 6 weeks before any further surgical intervention is discussed.
Figure 1 Suggested algorithm for treatment and management of perianal Crohn’s disease.
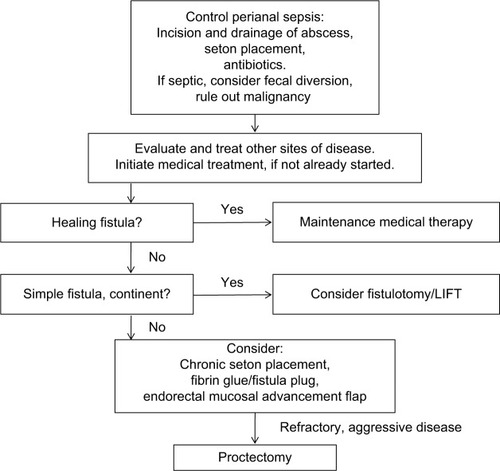
Table 1 Summary of the literature on perianal Crohn’s disease interventions
After resolution of perianal sepsis and remission of active distal disease, a fistulotomy is the preferred procedure, for simple, superficial fistulas. The tract is identified, the overlying tissue is divided, the base of the tract is curetted, and the wound is left open to close by secondary intention. In addition, marsupilization may improve the rate of wound healing.Citation47 Successful healing is reported in ~80% of patients with a 20% risk of recurrence over a follow-up between 2 and 20 years.Citation42,Citation48–Citation50 Fistulotomy is strongly discouraged in complex fistulas, due to high risk of fecal incontinence, decreased healing, and need for proctectomy.Citation50,Citation51 Minor continence issues following fistulotomy occur in 25% of patients.Citation52
Other interventions include the use of fibrin glue, which consists of two parallel syringes of fibrinogen and thrombin that facilitate healing, hemostasis, and angiogenesis. These syringes are injected together via a catheter to fill a fistula tract, resulting in clot formation, sealing the fistula. Current literature is discordant with the success of this intervention.Citation53,Citation54 One dedicated randomized controlled trialCitation55 found 38% of patients experienced remission with fibrin glue, which was twice than that for individuals with no intervention; this, however, was in 16 weeks of follow-up. These results echo smaller series previously performed that demonstrated healing in 31% of patients with pCD after fibrin glue over 26 months.Citation54
The Surgisis (COOK biotech, West Lafayette, IN, USA) fistula plug is a lyophilized porcine intestinal submucosa. It is inert, eliciting no foreign body or inflammatory reaction, and acts as a collagen scaffold, that is populated by a patient’s endogenous cells over the course of 3 months.Citation56 A recent systematic review of the fistula plug in normal patients demonstrated a closure rate of 58.4% after a median follow-up of 9 months. Most recently, a prospective randomized trial of 106 patients randomized to plug versus seton removal only; fistula closure was 31.5% versus 23.1%, respectively, over 12 weeks of follow-up.Citation57
The ligation of the intersphincteric fistula tract (LIFT) is designed to close complex fistulas with sphincter preservation. An incision is made in the intersphincteric groove at both the internal and external sphincters. The external tract is then curetted out, and the external opening is widened at the skin. Eventually, the skin is closed.Citation58 LIFT is a safe procedure that provides a mean healing rate of 70.6% with no reports of impairment of the sphincter function, based on a systemic review with follow-up between 4 weeks and 26 months (average 10.3 months).Citation59 In prospective studies, healing was seen in 67% of patients at 12-month followup.Citation60 A prospective observational study concluded the LIFT procedure has a high success rate (94.1% in 167 patients) in complex fistulae-in-ano. Recurrence is associated with diabetes, perianal abscesses, tract abscesses, and multiple tracts. A second LIFT procedure may be a feasible intervention if needed.Citation61
The endorectal mucosal advancement flap is a procedure that uses endogenous tissue to close the internal fistula opening. After complete excision of the fistula tract, the internal sphincter muscle is mobilized and approximated in the middle without tension. A flap is created consisting of mucosa, submucosa, and circular muscle, which is advanced and secured to cover the internal opening.Citation62 Endorectal mucosal advancement flaps are the preferred approach for complicated anorectal fistulae without incontinence. Prior to advancement flap, most patients undergo a period of infection control with a draining seton, with or without a diverting stoma. Relative contraindications to advancement flap are anal stenosis and active proctitis due to high complication and failure rates. van Koperen et alCitation63 reported that 52% of the population undergoing treatment with the mucosal advancement flap experienced healing after 11 months. A retrospective review of 127 patients with high anorectal fistulas had an overall recurrence rate of 26% with a mean follow-up of 13 months.Citation63 Mucosal advancement flaps with or without the addition of fibrin glue have been shown to result in healing rates as high as 62% in complex fistulas.Citation64
Fistulas that do not respond to aggressive medical and surgical management may require fecal diversion with the creation of an ileostomy.Citation65,Citation66 Diversion may result in relief of local inflammation.Citation67 In patients who underwent fecal diversion and drainage of local sepsis for their perianal disease, 81% went into early remission, although 68% of these relapsed at a median of 23 months after treatment. In this same group, a total of 25% of patients had long-term remission, but only 10% were able to restore intestinal continuity,Citation68 which had been reported by others.Citation19,Citation69
Patients with complex fistulas associated with abscesses, recurrent sepsis, colonic or perineal disease, refractory proctitis, and anal stenosis are candidates for proctectomy and permanent stoma.Citation19 Some authors recommend doing this in a two stage procedure to allow resolution of perianal sepsis.Citation44 Furthermore, after resolution of sepsis, there may be large subcutaneous tissue defects, which may require advanced tissue transfers, such as gluteal flaps and gracilis flaps.Citation70 Ileal pouch anal anastomosis has been attempted in these individuals; high rates of recurrence of pCD, however, have been reported. Therefore, we at our institution do not strongly recommend this procedure.Citation71 Colo-anal pull-through is another surgical option; fistula recurrence, however, has been reported up to 25%, although the study did not define which of those occurred in Crohn’s patients. Thirteen percent developed anal strictures, and only 38% returned to normal continence.Citation72 We do not perform this procedure at our institution.
Other interventions
Other interventions have been used to manage pCD. Local injections of anti-TNF alpha have been attempted. Two pilot studies were completed that demonstrated some improvement with injection of infliximab and adalimumab.Citation73,Citation74 Hyperbaric oxygen may also be utilized to facilitate healing.Citation75 In addition, topical tacrolimus has been attempted, but with little success.Citation76,Citation77 Although multiple avenues in treatment have been attempted, it appears surgical intervention remains the most successful.
Future directions
Promising evidence suggests that the injection of stem cells may improve healing in fistulizing pCD. They are nonhematopoietic precursors of connective tissue cells with anti-inflammatory and tissue regenerative properties, extracted from subdermal adipose tissue.Citation78 Peri- or intrafistula injection of autologous adipose-derived stem cells, as well as bone marrow-derived stem cells, is proven to be feasible and safe.Citation79,Citation80 Most recent trials have demonstrated closure rates over various lengths of follow-up from 37% to 85%, using a combination of autologous and allogenic mesenchymal and adipose-derived stem cells.Citation79–Citation85 Completion of Phase III trials is needed, but there is promising evidence that stem cells may aid in fistulizing pCD treatment.
Conclusion
Despite advancements in medical and surgical interventions for pCD, its treatment has remained challenging. Although current solutions for fistula management show varying degrees of success, additional research is needed to further the management of pCD. Current therapies, with the addition of a multidisciplinary team, will continue to improve the management of this difficult disease.
Acknowledgments
The authors would like to thank Mary Kwatkosky-Lawlor for her assistance with the review process and the preparation of the bibliography.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchwartzDALoftusEVJrTremaineWJThe natural history of fistulizing Crohn’s disease in Olmsted County, MinnesotaGastroenterology2002122487588011910338
- TarrantKMBarclayMLFramptonCMGearryRBPerianal disease predicts changes in Crohn’s disease phenotype–results of a population-based study of inflammatory bowel disease phenotypeAm J Gastroenterol2008103123082309319086959
- HellersGBergstrandOEwerthSHolmstromBOccurrence and outcome after primary treatment of anal fistulae in Crohn’s diseaseGut19802165255277429313
- WilliamsDRCollerJACormanMLNugentFWVeidenheimerMCAnal complications in Crohn’s diseaseDis Colon Rectum198124122247472097
- FaucheronJLSaint-MarcOGuibertLParcRLong-term seton drainage for high anal fistulas in Crohn’s disease – a sphincter-saving operation?Dis Colon Rectum19963922082118620789
- HatchQChampagneBJMaykelJACrohn’s disease and pregnancy: the impact of perianal disease on delivery methods and complicationsDis Colon Rectum201457217417824401878
- SandbornWJFazioVWFeaganBGHanauerSBAmerican Gastroenterological Association Clinical Practice CAGA technical review on perianal Crohn’s diseaseGastroenterology200312551508153014598268
- TozerPJWhelanKPhillipsRKHartALEtiology of perianal Crohn’s disease: role of genetic, microbiological, and immunological factorsInflamm Bowel Dis200915101591159819637358
- IrvineEJUsual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study GroupJ Clin Gastroenterol199520127327884173
- PresentDHRutgeertsPTarganSInfliximab for the treatment of fistulas in patients with Crohn’s diseaseN Engl J Med1999340181398140510228190
- SchwartzDAWiersemaMJDudiakKMA comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulasGastroenterology200112151064107211677197
- RegueiroMMardiniHTreatment of perianal fistulizing Crohn’s disease with infliximab alone or as an adjunct to exam under anesthesia with seton placementInflamm Bowel Dis2003929810312769443
- RegueiroMThe role of endoscopy in the evaluation of fistulizing Crohn’s diseaseGastrointest Endosc Clin N Am200212362163312486948
- LawPJBartramCIAnal endosonography: technique and normal anatomyGastrointest Radiol19891443493532680741
- CheongDMNoguerasJJWexnerSDJagelmanDGAnal endosonography for recurrent anal fistulas: image enhancement with hydrogen peroxideDis Colon Rectum19933612115811608253014
- Navarro-LunaAGarcia-DomingoMIRius-MaciasJMarco-MolinaCUltrasound study of anal fistulas with hydrogen peroxide enhancementDis Colon Rectum200447110811414719157
- SiddiquiMRAshrafianHTozerPA diagnostic accuracy meta-analysis of endoanal ultrasound and MRI for perianal fistula assessmentDis Colon Rectum201255557658522513437
- GecseKBBemelmanWKammMAWorld Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical TrialsWorld Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical TrialsA global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s diseaseGut20146391381139224951257
- BellSJWilliamsABWieselPWilkinsonKCohenRCKammMAThe clinical course of fistulating Crohn’s diseaseAliment Pharmacol Ther20031791145115112752351
- DejacoCHarrerMWaldhoerTMiehslerWVogelsangHReinischWAntibiotics and azathioprine for the treatment of perianal fistulas in Crohn’s diseaseAliment Pharmacol Ther20031811–121113112014653831
- BernsteinLHFrankMSBrandtLJBoleySJHealing of perineal Crohn’s disease with metronidazoleGastroenterology1980793599
- TurunenUMFarkkilaMAHakalaKLong-term treatment of ulcerative colitis with ciprofloxacin: a prospective, double-blind, placebo-controlled studyGastroenterology19981155107210789797360
- BrandtLJBernsteinLHBoleySJFrankMSMetronidazole therapy for perineal Crohn’s disease: a follow-up studyGastroenterology19828323833877084615
- JakobovitsJSchusterMMMetronidazole therapy for Crohn’s disease and associated fistulaeAm J Gastroenterol19847975335406741906
- PearsonDCMayGRFickGHSutherlandLRAzathioprine and 6-mercaptopurine in Crohn disease: a meta-analysisAnn Intern Med199512321321427778826
- HinterleitnerTAPetritschWAichbichlerBFickertPRannerGKrejsGJCombination of cyclosporine, azathioprine and prednisolone for perianal fistulas in Crohn’s diseaseZ Gastroenterol19973586036089297775
- EganLJSandbornWJTremaineWJClinical outcome following treatment of refractory inflammatory and fistulizing Crohn’s disease with intravenous cyclosporineAm J Gastroenterol19989334424489517654
- GuruduSRGriffelLHGialanellaRJDasKMCyclosporine therapy in inflammatory bowel disease: short-term and long-term resultsJ Clin Gastroenterol199929215115410478875
- SandbornWJPresentDHIsaacsKLTacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trialGastroenterology2003125238038812891539
- FeaganBGRochonJFedorakRNMethotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group InvestigatorsN Engl J Med199533252922977816064
- FeaganBGFedorakRNIrvineEJA comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group InvestigatorsN Engl J Med2000342221627163210833208
- AroraSKatkovWCooleyJMethotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trialHepatogastroenterology199946271724172910430331
- Mate-JimenezJHermidaCCantero-PeronaJMoreno-OteroR6-Mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel diseaseEur J Gastroenterol Hepatol200012111227123311111780
- SandsBEAndersonFHBernsteinCNInfliximab maintenance therapy for fistulizing Crohn’s diseaseN Engl J Med2004350987688514985485
- ParsiMALashnerBAAchkarJPConnorJTBrzezinskiAType of fistula determines response to infliximab in patients with fistulous Crohn’s diseaseAm J Gastroenterol200499344544915056083
- ChoiCSBergASSangsterWCombined medical and surgical approach improves healing of septic perianal Crohn’s diseaseJ Am Coll Surg20162233506514.e127266825
- KhannaRBresslerBLevesqueBGREACT Study InvestigatorsEarly combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trialLancet2015386100061825183426342731
- LeeMJHeywoodNSagarPMBrownSRFearnheadNSp CD CollaboratorsSurgical management of fistulating perianal Crohn’s disease – a UK surveyColorectal Dis Epub2016716
- SolomonMJFistulae and abscesses in symptomatic perianal Crohn’s diseaseInt J Colorectal Dis19961152222268951512
- HyderSATravisSPJewellDPMcCMNJGeorgeBDFistulating anal Crohn’s disease: results of combined surgical and infliximab treatmentDis Colon Rectum200649121837184117041753
- KeighleyMRAllanRNCurrent status and influence of operation on perianal Crohn’s diseaseInt J Colorectal Dis1986121041073611935
- BuchananGNOwenHATorkingtonJLunnissPJNichollsRJCohenCRLong-term outcome following loose-seton technique for external sphincter preservation in complex anal fistulaBr J Surg200491447648015048751
- WhiteRAEisenstatTERubinRJSalvatiEPSeton management of complex anorectal fistulas in patients with Crohn’s diseaseDis Colon Rectum19903375875891694476
- SchwartzDAGhaziLJRegueiroMCrohn‘s & Colitis Foundation of America IncGuidelines for the multidisciplinary management of Crohn’s perianal fistulas: summary statementInflamm Bowel Dis201521472373025751066
- RitchieRDSackierJMHoddeJPIncontinence rates after cutting seton treatment for anal fistulaColorectal Dis200911656457119175623
- TanakaSMatsuoKSasakiTClinical advantages of combined seton placement and infliximab maintenance therapy for perianal fistulizing Crohn’s disease: when and how were the seton drains removed?Hepatogastroenterology201057973720422862
- RakinicJAnal fissureClin Colon Rectal Surg200720213313720011388
- HalmeLSainioAPFactors related to frequency, type, and outcome of anal fistulas in Crohn’s diseaseDis Colon Rectum199538155597813346
- ScottHJNorthoverJMEvaluation of surgery for perianal Crohn’s fistulasDis Colon Rectum1996399103910438797656
- TaxoneraCSchwartzDAGarcia-OlmoDEmerging treatments for complex perianal fistula in Crohn’s diseaseWorld J Gastroenterol200915344263427219750568
- NordgrenSFasthSHultenLAnal fistulas in Crohn’s disease: incidence and outcome of surgical treatmentInt J Colorectal Dis1992742142181293243
- van TetsWFKuijpersHCContinence disorders after anal fistulotomyDis Colon Rectum19943712119411977995143
- LindseyISmilgin-HumphreysMMCunninghamCMortensenNJGeorgeBDA randomized, controlled trial of fibrin glue vs. conventional treatment for anal fistulaDis Colon Rectum200245121608161512473883
- LoungnarathRDietzDWMutchMGBirnbaumEHKodnerIJFleshmanJWFibrin glue treatment of complex anal fistulas has low success rateDis Colon Rectum200447443243614978618
- GrimaudJCMunoz-BongrandNSiproudhisLGroupe d’Etude Thérapeutique des Affections Inflammatoires du Tube DigestifFibrin glue is effective healing perianal fistulas in patients with Crohn’s diseaseGastroenterology20101387227522812281 e227120178792
- ChampagneBJO’ConnorLMFergusonMOrangioGRSchertzerMEArmstrongDNEfficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-upDis Colon Rectum200649121817182117082891
- SenejouxASiproudhisLAbramowitzLGroupe d’Etude Thérapeutique des Affections Inflammatoires du tube Digestif [GETAID]Fistula plug in fistulising ano-perineal Crohn’s disease: a randomised controlled trialJ Crohns Colitis201610214114826351393
- RojanasakulAPattanaarunJSahakitrungruangCTantiphlachivaKTotal anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tractJ Med Assoc Thai200790358158617427539
- Zirak-SchmidtSPerdawoodSKManagement of anal fistula by ligation of the intersphincteric fistula tract – a systematic reviewDan Med J20146112A497725441733
- GingoldDSMurrellZAFleshnerPRA prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn’s diseaseAnn Surg201426061057106124374520
- ParthasarathiRGomesRMRajapandianSLigation of the intersphincteric fistula tract for the treatment of fistula-in-ano: experience of a tertiary care centre in South IndiaColorectal Dis201618549650226476011
- KobayashiHSugiharaKSuccessful management of rectovaginal fistula treated by endorectal advancement flap: report of two cases and literature reviewSpringerPlus201542125694858
- van KoperenPJBemelmanWAGerhardsMFThe anal fistula plug treatment compared with the mucosal advancement flap for cryptoglandular high transsphincteric perianal fistula: a double-blinded multicenter randomized trialDis Colon Rectum201154438739321383557
- MarchesaPHullTLFazioVWAdvancement sleeve flaps for treatment of severe perianal Crohn’s diseaseBr J Surg19988512169516989876077
- GrantDRCohenZMcLeodRSLoop ileostomy for anorectal Crohn’s diseaseCan J Surg198629132353940583
- ZelasPJagelmanDGLoop illeostomy in the management of Crohn’s colitis in the debilitated patientAnn Surg198019121641687362285
- HarperPHTrueloveSCLeeECKettlewellMGJewellDPSplit ileostomy and ileocolostomy for Crohn’s disease of the colon and ulcerative colitis: a 20 year surveyGut19832421061136852621
- YamamotoTAllanRNKeighleyMREffect of fecal diversion alone on perianal Crohn’s diseaseWorld J Surg2000241012581262 discussion 1262–126311071472
- LeeESplit ileostomy in the treatment of Crohn’s disease of the colonAnn R Coll Surg Engl1975562941021119787
- HurstRDGottliebLJCrucittiPMelisMRubinMMichelassiFPrimary closure of complicated perineal wounds with myocutaneous and fasciocutaneous flaps after proctectomy for Crohn’s diseaseSurgery20011304767772 discussion 772–77311602910
- SagarPMDozoisRRWolffBGLong-term results of ileal pouch-anal anastomosis in patients with Crohn’s diseaseDis Colon Rectum19963988938988756845
- PatsourasDYassinNAPhillipsRKClinical outcomes of colo-anal pull-through procedure for complex rectal conditionsColorectal Dis201416425325824344638
- PoggioliGLauretiSPierangeliFLocal injection of infliximab for the treatment of perianal Crohn’s diseaseDis Colon Rectum200548476877415768185
- TonelliFGiudiciFAsteriaCREffectiveness and safety of local adalimumab injection in patients with fistulizing perianal Crohn’s disease: a pilot studyDis Colon Rectum201255887087522810472
- SoltaniAKaiserAMEndorectal advancement flap for cryptoglandular or Crohn’s fistula-in-anoDis Colon Rectum201053448649520305451
- CassonDHEltumiMTomlinSWalker-SmithJAMurchSHTopical tacrolimus may be effective in the treatment of oral and perineal Crohn’s diseaseGut200047343644010940284
- HartALPlamondonSKammMATopical tacrolimus in the treatment of perianal Crohn’s disease: exploratory randomized controlled trialInflamm Bowel Dis200713324525317206671
- Garcia-OlmoDGarcia-ArranzMHerrerosDPascualIPeiroCRodriguez-MontesJAA phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantationDis Colon Rectum20054871416142315933795
- ChoYBLeeWYParkKJKimMYooHWYuCSAutologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical studyCell Transplant201322227928523006344
- CiccocioppoRBernardoMESgarellaAAutologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s diseaseGut201160678879821257987
- LeeWYParkKJChoYBAutologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistulaStem Cells201331112575258123404825
- de la PortillaFAlbaFGarcia-OlmoDHerreriasJMGonzalezFXGalindoAExpanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multi-center phase I/IIa clinical trialInt J Colorectal Dis201328331332323053677
- ChoYBParkKJYoonSNLong-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistulaStem Cells Translat Med201545532537
- Garcia-OlmoDGuadalajaraHRubio-PerezIHerrerosMDde-la-QuintanaPGarcia-ArranzMRecurrent anal fistulae: limited surgery supported by stem cellsWorld J Gastroenterol201521113330333625805941
- MolendijkIBonsingBARoelofsHAllogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s diseaseGastroenterology20151494918927e626116801
- HobbissJHSchofieldPFManagement of perianal Crohn’s diseaseJ Royal Soc Med1982756414417
