Abstract
Background and aims
Patients with advanced systemic illness or critically ill patients may present with upper gastrointestinal tract (GIT) bleeding which may need endoscopic intervention; however, this may expose them to unnecessary endoscopy. The aim was to validate a novel scoring system for risk stratification for urgency of GIT endoscopy in critically ill patients.
Methods
This is an observational study conducted from January 2013 to January 2016 to analyze 300 patients with critical medical conditions and presenting with upper gastrointestinal bleeding. Meticulous clinical, laboratory, and sonographic evaluations were performed to calculate Glasgow Blatchford score (GBS) and variceal metric score for risk stratification and prediction of the presence of esophageal varices (OV). Finally, this score was applied on a validation group (n=100).
Results
The use of GBS and variceal metric scores in critically ill patients revealed that patients who showed a low risk score value for OV (0–4 points) and GBS <2 can be treated conservatively and discharged safely without urgent endoscopy. In patients with a low risk for varices but GBS >2, none of them had OV on endoscopy. In patients with intermediate risk score value for OV (5–8 points) and with GBS >2, 33.33% of them had varices on endoscopy. In patients with high risk score value for varices (9–13) and GBS >2, endoscopy revealed varices in 94.4% of them. Finally, in patients with very high risk score for varices (14–17), endoscopy revealed varices in 100% of them.
Conclusion
GBS and variceal metric score were highly efficacious in identifying critically ill patients who will benefit from therapeutic endoscopic intervention.
Introduction
Patients with advanced systemic illness could be presented with an acute attack of hematemesis which may need endoscopic intervention; however, this may expose the brittle patients to serious life threatening adverse effects if performed unnecessarily.
Cardiopulmonary complications related to sedation account for nearly 60% of endoscopic adverse events;Citation1 patients at high risk include those who are old and with a preexisting cardiopulmonary disease. Endoscopy-related risk factors for hypoxia include difficult intubation, a prolonged procedure, and a patient in the prone position.Citation2 Transient bacteremia and infective endocarditis rates are low.Citation3,Citation4
Clinically significant bleeding after endoscopy is a rare adverse event. Diagnostic endoscopy can be performed when platelet count is at least 20,000/mL, and a count of at least 50,000/mL should be considered before taking biopsies.Citation5
Causes of upper gastrointestinal bleeding (UGIB) in critically ill patients include variceal and acid-related disorders. The risk of bleeding increases with an age ≥65 years, prolonged use of nonsteroidal anti-inflammatory drugs (NSAIDs),Citation6 polymorphism of CYP 2C9,Citation7 associated Helicobacter pylori infection, and concomitant use of steroids, anticoagulants, and bisphosphonates.
Stress-related mucosal damage is the most common cause of UGIB in critically ill patients; the risk is increased in mechanical ventilation >48 hours, platelets <50.000/mm3, international normalized ratio (INR) >1.5, sepsis, shock, severe burns, and malnutrition.Citation8
Bleeding esophageal varices (OV) is the second most common cause of UGIB.Citation9 In Egypt, OV represented 70% of UGIB followed by nonvariceal causes (26%) and obscure causes (4%);Citation10 other less common cases of UGIB are Mallory-Weiss syndrome (15%) and vascular lesions such as aortoenteric fistula and Dieulafoy lesion (2%–3%).Citation6
As regards to endoscopy in special situations such as pregnancy, endoscopy is not contraindicated. Variceal hemorrhage is uncommon, although it may occur in patients with underlying cirrhosis, and endoscopic band ligation is the favored intervention.Citation11,Citation12
As regards to patients on anticoagulants or antiplatelet therapy, hemorrhage may occur immediately at the time of endoscopy or delayed up to 2 weeks following the procedure.Citation13 So, in diagnostic procedures without biopsy such as biliary or pancreatic stenting and diagnostic endosonography, clopidogrel can be continued; warfarin can be also be continued if INR is within therapeutic range but if ≤5 the daily dose is reduced.Citation14
In variceal therapy, in high-risk patients (metal mitral valve replacement, valve replacement with atrial fibrillation [AF], AF with mitral stenosis, venous thromboembolism <3 months), warfarin should be stopped 5 days before the procedure and low-molecular-weight heparin (LMWH) need to be started 2 days after warfarin withdrawal and then stopped the day of procedure, with warfarin introduced at the evening after the completion of the procedure; LMWH should be started the next day till adequate INR is achieved.Citation14
However, regarding patients with coronary artery disease (CAD), peptic ulcer and bleeding OV are the most common causes of UGIB. Ventricular arrhythmias and myocardial ischemia were common complications after endoscopy in patients with CAD, especially with concomitant congestive heart failure.Citation15
In patients on dialysis, endoscopy is not contraindicated when it is highly indicated, and common causes are gastric antral vascular ectasia (GAVE) followed by gastroduodenal ulcer. GAVE was improved when hemodialysis was changed into continuous ambulatory peritoneal dialysis.Citation16,Citation17
In elderly patients, risks arising during sedation are mainly due to hypotension, hypoxia, arrhythmias, and aspiration.Citation18
In patients with chronic obstructive pulmonary disease (COPD), due to inadequate sedation, patients may be exposed to the risk of elevated blood pressure, angina, and myocardial infarction.Citation19
The study aimed to propose a noninvasive scoring system to identify OV in critically ill patients allowing prompt endoscopic intervention for the patients in urgent need for this intervention. At the same time; exclusion of nonvariceal causes to avoid unnecessary endoscopy and its related adverse events till they pass their medical critical illness safely.
Methods
Patient selection
This is an observational study carried out during the period from January 2013 to January 2016; out of 853 patients, we selected 300 patients with other comorbidities presented with UGIB. They were admitted to the intensive care unit (ICU) unit, Gastroenterology Section, Internal Medicine Department, Zagazig University, which is a tertiary referral center. Inclusion criteria were patients with UGIB and concomitant medical critical illness such as cardiopulmonary and renal diseases which make endoscopy difficult and risky. Patients on nonselective beta blockers, portal vein thrombosis, and previous endoscopic or surgical intervention for portal hypertension were excluded.
The study was approved by the ethical review board of Zagazig University. Although the study depended on investigations which were mandatory for patients presenting with UGIB, an oral consent was obtained from all patients, and written consent was obtained from patients who were able or from the relatives of the other patients.
Patients were evaluated and prepared for 12 hours before endoscopy by thorough clinical examination, including vital signs, signs of portal hypertension, signs of liver cell failure, and any signs of renal, cardiac, or respiratory diseases were documented. Also, routine investigations were performed for them including liver function tests, coagulation profile, renal function tests, and complete blood count. For each patient Child-Turcotte-Pugh score (CTP) was calculated.Citation20
Diagnostic procedures
Glasgow Blatchford score (GBS) was used for risk stratification of UGIB, as shown in . A low-risk category is defined as GBS of <2. A score of 6 or more was associated with a greater than 50% risk which needs endoscopic intervention.Citation21
Abdominal ultrasonography was performed for all patients, stressing on the presence of cirrhotic echo pattern or the presence of ascites. Also, portal vein diameter (PVD) >13 mm indicated portal hypertension.Citation22 The spleen was evaluated for its bipolar diameter. A length >130 mm indicated enlargement with associated splenic vein diameter >10 mm denoted portal hypertension.Citation23,Citation24
Color Doppler ultrasound was performed by real-time portable ultrasound equipment (SonoScape S9) consisting of a color Doppler and a pulsed Doppler device working at 3.5 MHz frequency.Citation25
Congestive index of the portal vein (PVCi) was calculated as the ratio between the portal vein cross-sectional area (cm2) and the blood flow velocity (cm/s). A cutoff value >0.14 cm×sec was selected, which has a sensitivity of 70% and specificity of 64.9% for the risk of bleeding from OV.Citation26,Citation27
The intraparenchymal renal artery resistance index (RARI) was calculated as follows: A–B/A, where A is the peak systolic velocity and B is the end diastolic velocity. A cutoff value >0.7 was selected as 70% probability for the existence of OV.Citation28
Variceal metric score composed of clinical, laboratory, and radiological variables in order to minimize the hazards of unnecessary exposure to endoscopy in high-risk patients, as shown in . The total score is 17: low risk (≤4), intermediate risk (5–8), high risk (9–13), and very high risk (14–17).
Table 2 OV metric score for noninvasive prediction of esophageal varices
Upper gastrointestinal (GI) endoscopy was done for patients enrolled in the study. If OV were present, their size was graded as I–IV using the Paquet grading system.Citation29
Validation
Further, we aimed to assess the predictive power of the scoring model clinically through evaluation in another cohort composed of 100 patients in the period from January 2016 to January 2017 who presented with other comorbidities and UGIB. The methods used for selection were similar to those of the study patients.
Statistical analysis
Data were analyzed using PASW Statistics 18. Continuous variables were summarized as mean ± standard deviation and standard of error (SE) when appropriate. Chi-square test was used for categorical variables. The Student’s t-test and analysis of variance were appropriately used. Correlation of risk factors associated with OV was determined using Pearson rank correlation.
A scoring system was postulated for noninvasive prediction of OV,Citation30 and points were assigned to each variable based on the magnitude of its regression coefficient; the one with the smallest β coefficient was given 1 point and others were given points according to the strength of their β coefficient when compared to the smallest. The collected points were grouped into predefined categories for the risk of OV: low (<15%), intermediate (15%–49%), high (50%–79%), and very high (>80%).Citation31
A receiver operating characteristic (ROC) analysis was carried out to examine the diagnostic utility of the constructed scoring model. The cutoff point for each variable is the point with the highest sensitivity and specificity in ROC curve analysis.
Results
Three hundred critically ill patients with UGIB were evaluated for priority of upper GI endoscopy.
They were 108 females (36%) and 192 males (64%), with a mean age of 53.7±12 years. A total of 144 patients had hepatic diseases (48%), mostly due to hepatitis C virus (HCV, n=120), hepatitis B virus (HBV, n=16), and combined HCV and HBV (n=8). A total of 78 patients had cardiac diseases (25.8%), such as Acute coronary syndrome (n=65), cardiogenic shock due to dilated cardiomyopathy (n=3), stuck artificial valve (n=6), and four patients with aortic and mitral valve replacement presented with melena due to warfarin toxicity. Sixty patients had chronic renal disease (20%). Ten females were pregnant and six of them had hepatic disorders ().
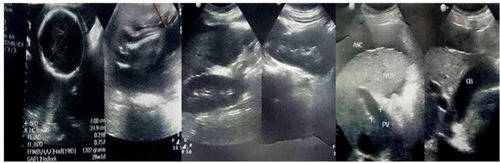
Figure 1 Abdominal ultrasonography of young adult pregnant female presenting with hematemesis showed cirrhotic liver with moderate ascites and intrauterine pregnancy.
On initial clinical evaluation, the mean systolic blood pressure was 106.8±22.7 mmHg. Splenomegaly by palpation was documented in 144 patients (47.4%), ascites and signs of parenchymatous liver cell failure were seen in 78 patients (25.8%).
The laboratory data included a mean hemoglobin of 8.47±2.3 g/dL, platelet count 128±59×103/µL, serum albumin 3.07±0.78 g/dL, total bilirubin 1.42±0.56 mg/dL, creatinine 1.74±1.6 mg/dL, and urea 52.1±23.7 mg/dL. Mean value of the CTP score was 5.8±1.8. CTP class B was seen in 96 patients (31.8%), and class C was seen in 14 patients (4.6%).
Bedside portable ultrasonography including color Doppler evaluation confirmed liver cirrhosis and splenomegaly in 144 patients, with a mean PVD of 12.4±2.3 mm, PVD >13 mm (n=144, 47.4%); SVD 8.46±2.7 mm, SVD >10 mm (n=138, 45.7%); PVCi 0.116±0.05, PVCi >0.14 (n=132, 43.7%); RARI 0.698±0.126, RARI > 0.7 (n=150, 49.7%) ( and ).
Figure 2 Colour Doppler sonography of the portal vein with maximum flow velocity of 12.7 cm/s, minimum flow velocity of 9.2 cm/s, and mean flow velocity of 11 cm/s. Portal vein cross-sectional area equals 20.4 mm.
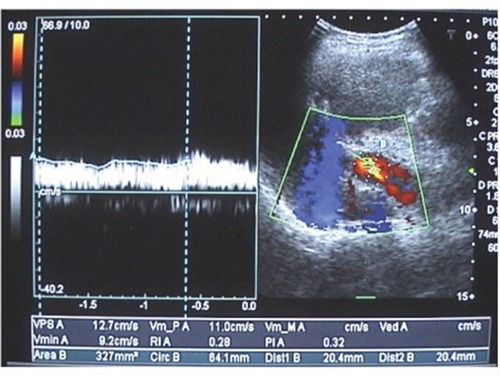
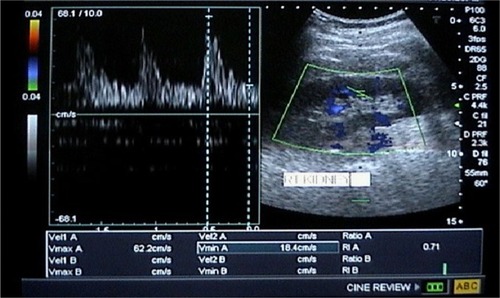
Figure 3 Doppler sonography of the right kidney showing increased renal arteriolar resistive index with a value of 0.71.
GBS was calculated to determine the priority for intensive care management and endoscopy. The mean GBS was 11.6±6.5. Score was 0 in six female patients (2%) and two females were pregnant in the first trimester, and all of them discharged safely as outpatients because their GBS was 0.
Thirty (10%) patients with UGIB showed GBS score 1, with a mean age 50.6±11.5 years. They were followed for 3 days in ICU and discharged for outpatient management and follow-up.
A score of 3–6 was seen in 48 patients (16%). GBS >6 was seen in 216 patients (72%), and their characteristics are described in .
Table 3 Baseline clinical, laboratory, ultrasonographic, and Doppler criteria of the patients under study stratified by their GBS value
The study patients were classified into four subgroups according to their GBS score: GBS 0 (n=6), GBS 1–2 (n=30), GBS 3–6 (n=48), GBS >6 (n=216). There was a significant statistical difference among the four subgroups with regard to systolic blood pressure, radial pulse, hemoglobin, platelet count, INR, albumin, total bilirubin, AST, creatinine, and blood urea. The occurrence of melena and pre-syncope were more frequent in the subgroup with GBS >6 (p=0.000) (). The ultrasonographic and Doppler criteria in the four subgroups showed a significant statistical difference with higher values in patients with GBS >6 and with mean OV score 8.82±5.7 (p=0.000), as shown in .
Approach to the critically ill patients according to GBS and OV metric score
Patients with GBS 0 and <2 and with OV metric score mean value of 2.5±0.7, i.e., no risk of hemodynamic instability by GBS and low risk for OV, were followed up for 2 days in ICU and kept on fluid therapy and proton pump infusion with initial loading dose of 80 mg and then continuous infusion at 8 mg per hour for 72 hours. Then the patients were discharged safely and followed up as outpatients for 1 month without adverse clinical events. Endoscopy was performed again after 1 month and revealed no abnormalities.
Patients with GBS 3–6 and OV score mean value of 3±0.9 were at low risk for OV but with high priority for endoscopic intervention as GBS >2, hence they were kept on fluid therapy, blood transfusion, and proton pump infusion. Endoscopy in these patients was done within 12 hours and revealed peptic ulcer disease (PUD) (n=34), vascular ectasia (n=8), and GAVE (n=6) with no OV, and they were treated with argon plasma coagulation.
Patients with GBS >6 and with OV score mean value of 8.82±5.7, i.e., intermediate to high risk for OV and high priority for endoscopy according to GBS, were given fluid therapy and blood transfusion. Endoscopy was done within 12 hours which revealed OV (n=144) secured with rubber band ligation, portal hypertensive gastropathy (PHG) grade II–III (n=40) treated with argon plasma and beta blockers, PUD (n=14), vascular ectasia (n=2), and GAVE (n=16) managed with argon plasma coagulation, as shown in .
Table 4 Outcome of the patients under study according to GBS risk stratification
Evaluation of the patients under study according to their current illness, OV, and GBS scores
A total of 144 hepatic patients (48%) presented with UGIB. All of them showed GBS >6, i.e., high risk of hemodynamic instability, and OV score 12.7±2.2, i.e., high risk for OV, so endoscopy was performed which showed OV in 136 patients (94.4%) (p=0.000).
Seventy-eight cardiac patients (26%) presented with UGIB, 18 patients with GBS 3–6 and 60 patients with GBS >6, and OV score 2.1±1.2 (SE). Endoscopy was performed which showed OV in only six patients (7.6%) as the OV score indicated low risk (p=0.000).
Sixty patients with chronic renal failure (20%) presented with UGIB, six patients with GBS 3–6 and 54 patients with GBS >6, and OV score 7.7±3.1. Endoscopy was performed which showed OV in 30 patients (50%).
Eight patients with COPD (2.6%) presented with UGIB, four patients with GBS 0 and four patients with GBS 1–2, and OV score 1.7±0.2, i.e., low risk. They were observed in ICU for 2 days and discharged as outpatients and were followed up for 1 month without adverse clinical events. Endoscopy was done 1 month after stabilization of their medical condition and it revealed no abnormalities.
Ten pregnant female patients (3.3%) presented with UGIB, two patients with GBS 0, six patients with GBS 3–6, and two patients with GBS >6, and OV score 8.7±0.7. Endoscopy was performed in eight patients due to intermediate risk of OV and high priority for endoscopy. The results showed OV in six patients (75%) (p=0.03) which were managed with rubber band ligation as shown in .
Table 5 Correlation of GBS score with endoscopy among patients with critical illness
The predictive power of the OV score
The predictive power of OV scoring system was evaluated in the study population by using ROC curve analysis. The area under the curve (AUC) was 0.993. The cutoff value of the score associated with highest sensitivity and specificity was 8 with a sensitivity of 98.5% and specificity of 76%.
A total of 156 patients (52%) showed an OV score value of 0–4); of them 6 and 30 patients showed GBS score 0 and <2, respectively, and OV score 2.3±0.7. Therefore, they were followed up in the ICU and discharged without endoscopy and followed up for 1 month as outpatients without any complications. The other 120 patients (77%) showed GBS >2, so we performed GI endoscopy and as the OV score was 2.5±0.9), none of them showed OV, magnifying the predictive power of the score.
Six patients (2%) showed an intermediate risk for OV (score 5–8). All of them showed GBS >6 and upper GI endoscopy revealed grade II OV in two patients (33.3%).
Seventy-two patients (24%) showed high risk for OV (score 9–13) and all of them showed GBS >6. Endoscopy was done which revealed OV in 68 patients (94.4%); of them 20 patients showed grade II OV (29.4%), 36 patients (53%) grade III OV, and 12 patients (17.6%) grade IV OV.
Sixty-six patients (22%) had a very high risk for OV (score 14–17) and all of them showed GBS >6. Upper GI endoscopy was done and revealed OV in 66 (100%) patients; of them 32 patients showed grade III OV (48.4%) and 34 patients (51.6%) showed grade IV OV, i.e., higher score values were associated with large size of OV.
There was a highly significant correlation between the GBS grade and the presence and size of varices only when associated with high OV metric score (Pearson’s chi-square t-value =53.85, p=0.000), as shown in . The clinical score was highly efficacious in predicting OV presence (Pearson’s chi-square t-value =175.3, p=0.000), as shown in .
Figure 4 Correlation between OV presence and grade with GBS score value.
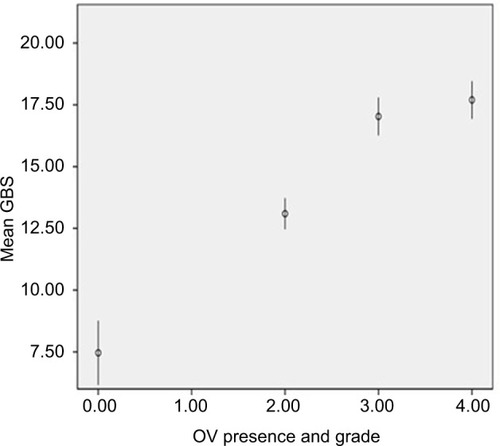
Table 6 Prevalence of OV in study population according to summed OV cirrhometric score
The presence of OV is negatively correlated with age (r=–0.171, p=0.037) and platelet count (r=–0.835, p=0.000). It is positively correlated with the presence of splenomegaly (r=0.910, p=0.000), GBS (r=0.711, p=0.000), CTP score (r=0.790, p=0.000), and OV metric score (r=0.939, p=0.000).
Validation
From a total of 300 patients who presented with UGIB, 100 patients (20 females, 80 males) were selected. Their main age was 46.2±3.4 years. Thirty patients were suffering from hepatic diseases (30%) due to HCV (n=28) and HBV (n=2); 50 patients from cardiac diseases (50%); 10 patients from chronic renal failure (10%); and 10 patients from COPD (10%).
On clinical evaluation; the mean systolic blood pressure was 95±15 mmHg. Splenomegaly by palpation was detected in 50 patients (50%); ascites and signs of parenchymatous liver cell failure were documented in 20 patients (20%).
The laboratory data included mean hemoglobin 7.45±1.7 g/dL, platelet count 135±43×103/µL, albumin 3.34±0.23 g/dL, total bilirubin 1.22±0.34 mg/dL, and creatinine 1.6±0.7 mg/dL. Bedside portable ultrasonography included color Doppler which revealed liver cirrhosis and splenomegaly in 50 patients with the mean PVD 11.7±1.9 mm, SVD 8±2.2 mm, PVCi 0.122±0.1, and RARI 0.679±0.13.
The OV score was applied on the validation group, and endoscopy was done in all patients to confirm the predictive role.
Among the 20 patients with GBS =0 and OV metric score 2.35±1.1, endoscopy showed no abnormalities in eight patients, linear antral erosions in 10 patients, and small pre-pyloric ulcers in two patients and no OV were detected. They were managed by pantoprazole infusion and cytoprotective agents.
Among the 11 patients with GBS ≥6 and OV metric score 2.8±0.8, endoscopy revealed bleeding gastric ulcer in six patients, corporeal vascular ectasia in two patients, subcardiac Dieulafoy lesion in one patient, GAVE in two patients and no one showed OV. They were managed with argon plasma coagulation and pantoprazole infusion for gastric ulcers.
Among the 19 patients who showed GBS ≥6 and OV metric score 6.9±1.1, endoscopy revealed bleeding gastric ulcer in four patients, vascular ectasia in one patient, PHG in six patients, and eight patients (44.4%) showed OV which were managed with rubber band ligation.
Among the 50 patients who showed GBS ≥6 and OV metric score 12.3±1.9, endoscopy revealed PHG grade III (mainly fundic) in three patients which is the cause of bleeding, and 47 patients (94%) showed OV grade III–IV with variable grades of PHG. They were managed with band ligation and argon plasma coagulation where appropriate.
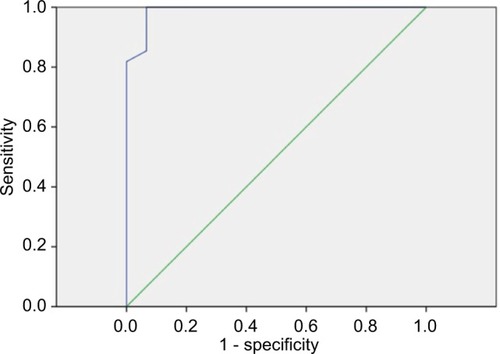
The accuracy of the scoring system including GBS and OV metric score in risk stratification and prediction of OV in the validation group was evaluated using ROC curve analysis as shown in . The AUC value was 0.989 (95% CI: 0–1, p=0.000) at GBS ≥6 and OV metric score ≥6.5. It showed a sensitivity of 99% and a specificity of 97.2% in predicting OV presence and grade. GBS alone was not able to predict the presence of OV with the AUC value of 0.254, p=0.23.
Discussion
Although patients with UGIB should perform upper GI endoscopy to declare the cause of bleeding, many patients showed that trivial symptoms such as gastric mucosal abrasions, congestion, or minute ulcers are the cause and could be managed medically, but may expose them to the hazards of endoscopy, especially, if there are associated systemic comorbidities, a critical issue discussed by Adamopoulos et al.Citation32
It is a common situation for the high-risk patients to be presented with UGIB either due to their morbidity or the drugs given for their illness. It remains a challenge to select patients who will have the utmost therapeutic intervention benefit from upper GI endoscopy, the endoscopic triage.
Many patients with noncurable diseases, such as cardiopulmonary, renal, and neurological diseases, and elderly patients may be treated for long periods due to their illness without giving the attention to the underlying liver disease until they present with the first episode of UGIB.
The incidence of chronic liver disease has been growing, which is mainly due to viruses, alcohol, and fatty liver disease, so it is of major interest to apply noninvasive predictive tools to narrow the scope for identification of cirrhotic patients with significant portal hypertension. For example, as we experienced in patients presented with UGIB with prolonged INR due to the use of oral anticoagulants after valve replacement when presented with UGIB; after apply ing GBS and OV metric score, the patients got benefit from endoscopic therapeutic intervention rather than deferring endoscopy depending on the usual belief that side effects of oral anticoagulants are a cause of bleeding.
Variceal metric score composed of clinical, laboratory, ultrasonographic, and Doppler data was accurate and precise in identifying patients with varices. The cutoff value of the score associated with the highest sensitivity and specificity was 8 in the study population (with a sensitivity of 98.5% and a specificity 76%) and when applied to the validation group composed of critically ill patients who presented with UGIB.
GBS alone is useful in determining the patients who will need hospital-based intervention and is equivalent to the full Rockall score in predicting mortality and the need for endoscopic therapy, surgery, or blood transfusion.Citation33 However, GBS alone cannot predict OV presence unless combined with OV metric score. A GBS ≥6 and OV metric score ≥6.5 showed a sensitivity of 99% and specificity of 97.2% in predicting OV.
Our study included a sufficient number of patients and a validation group. The clinical, laboratory and radiological variables selected in this study are easily collected and applicable, where the constellation of which has an accurate prediction of OV and when combined with GBS score make it novel way in critically ill patients and this enabled us to pick up the occult cases of bleeding due to OV or any serious underlying pathology and select with high degree of accuracy critically ill patients who will benefit from urgent endoscopy, thus avoiding many unnecessary risks on this category of patients.
Previous studies investigated noninvasive parameters such as splenomegaly, ascites and spider angiomata,Citation34 CTP class,Citation35 platelet count, platelet count/spleen diameter ratio,Citation36,Citation37 serum albumin, and serum bilirubin;Citation38 however, they searched for limited number of variables with AUC (0.75–0.81). Our score showed an AUC value of 0.989; in addition, no one investigated the power of the noninvasive parameters for OV in critically ill patients.
Application of GBS and OV metric score in critically ill patients can be performed during the initial 12 hours of preparation before endoscopy.
A particular conclusion of our study is that GBS score >2, OV score >4, and cutoff value of 8 necessitates performing upper GI endoscopy whatever may be the associated medical illness, since endoscopy will be life saving as the cause of bleeding can be controlled. One direction for the future could be the integration of this approach in multicenters for the benefit of the critically ill patients and to minimize the burden on endoscopy units.
Acknowledgments
The authors extend special thanks to residents of Internal Medicine and diagnostic radiology specialists – (Zagazig University) and Prof Ahmed Khaled Tawfik for their help and support in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- SharmaVKNguyenCCCrowellMDLiebermanDAde GarmoPFleischerDEA national study of cardiopulmonary unplanned events after GI endoscopyGastrointest Endosc200766273417591470
- GangiSSaidiFPatelKJohnstoneBJaegerJShineDCardiovascular complications after GI endoscopy: occurrence and risks in a large hospital systemGastrointest Endosc20046067968515557942
- AllisonMCSandoeJATigheRAntibiotic prophylaxis in gastrointestinal endoscopyGut20095886988019433598
- VogelSBRoutWRMartinTDAbbittPLEsophageal perforation in adults aggressive, conservative treatment lowers morbidity and mortalityAnn Surg200524110161021 discussion 1021–102315912051
- British Society of GastroenterologyGuidelines on Complications of Gastrointestinal Endoscopy2006 Available from: http://www.bsgorg.uk/clinical-guidelinesAccessed November 14, 2017
- StabileBEStamosMJSurgical management of gastrointestinal bleedingGastroenterol Clin North Am200029118922210752022
- MartínezCBlancoGLaderoJMGenetic predisposition to acute gastrointestinal bleeding after NSAIDs useBr J Pharmacol2004141220520814707031
- PimentelMRobertsDEBernsteinCNHoppensackMDuerksenDRClinically significant gastrointestinal bleeding in critically ill patients in an era of prophylaxisAm J Gastroenterol2000952801280611051351
- Garcia-TsaoGSanyalAJGraceNDCareyWPractice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastresophageal varices and variceal hemorrhage in cirrhosisHepatology200746392293817879356
- ElwakilRRedaMAAbdelhakamSMGhorabaDMIbrahimWACauses and outcome of upper gastrointestinal bleeding in Emergency Endoscopy Unit of Ain Shams University HospitalJ Egypt Soc Parasitol201141245546721980783
- CappellMSHepatic disorders mildly to moderately affected by pregnancy: medical and obstetric managementMed Clin North Am20089271773718570940
- TanJSurtiBSaabSPregnancy and cirrhosisLiver Transpl2008141081109118668664
- CappellMSAbdullahMManagement of gastrointestinal bleeding induced by gastrointestinal endoscopyGastroenterol Clin North Am20002912516710752020
- VeitchAMBaglinTPGershlickAHHarndenSMTigheRCairnsSGuidelines for the management of anticoagulant and antiplatelet therapy in patients undergoing endoscopic proceduresGut2008571322132918469092
- TsengPHLiouJMLeeYCEmergency endoscopy for upper gastrointestinal bleeding in patients with coronary artery diseaseAm J Emerg Med200927780280919683108
- LiangCCWangSMKuoHLUpper gastrointestinal bleeding in patients with CKDClin J Am Soc Nephrol2014981354135924903385
- YoriokaNHamaguchiNTaniguchiYGastric antral vascular ectasia in a patient on hemodialysis improved with CAPDPerit Dial Int19961621771789147554
- ClarkeGAJacobsonBCHammettRJCarr-LockeDLThe indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohortEndoscopy20013358058411473328
- RomagnuoloJCottonPBEisenGVargoJPetersenBTIdentifying and reporting risk factors for adverse events in endoscopy. Part I: cardiopulmonary eventsGastrointest Endosc20117357958521353857
- ChildCTurcotteJSurgery and portal hypertensionChildCGThe Liver and Portal HypertensionPhiladelphiaSaunders196450
- StanleyAJAshleyDDaltonHROutpatient management of patients with low-risk upper-gastrointestinal hemorrhage: multicentre validation and prospective evaluationLancet2009373424719091393
- GoyalAKPokharnaDSSharmaSKUltrasonic measurements of portal vasculature in diagnosis of portal hypertension: a controversial subject reviewedJ Ultrasound Med1990945482404133
- SenecalBSonographic anatomy of the normal spleen, normal anatomic variants, and pitfallsBrunetonJNUltrasonography of the SpleenBerlinSpringer-Verlag1988113
- ZhouHYChenTWZhangXMThe diameter of the originating vein determines esophageal and gastric fundic varices in portal hypertension secondary to posthepatitic cirrhosisClinics (Sao Paulo)201267660961422760900
- SchepisFCemmàCNiceforoDWhich patients should undergo endoscopic screening for esophageal varices detection?J Hepatol200133333338
- MoriyasuFNishidaOBanNUchinoHCongestion Index of the portal veinAJR Am J Roentgenol19861467357393485345
- PlestinaSPulanicRKralicMPlestinaSSamarzijaMColor Doppler ultrasonography is reliable in assessing the risk of esophageal variceal bleeding in patients with liver cirrhosisWien Klin Wochenschr200511771171716416372
- ColliAFraquelliMPomettaRCoccioloMVisentinSConteDRenovascular impedance and esophageal varices in patients with Child-Pugh Class ARadiology200121971271511376259
- PaquetKJProphylactic endoscopic sclerosing treatment of esophageal wall in varices: a prospective controlled trialEndoscopy198214457035153
- El-SayedAMNoninvasive Prediction of Esophageal Varices in Patients with Compensated Cirrhosis [master’s thesis]2005 Available from: srv4.eulc.edu.eg/eulc_v5/LibrariesAccessed November 14, 2017
- SullivanLMMassaroJMAgostinoBDPresentation of multivariate data for clinical use: the Framingham Study risk score functionsStat Med2004231631166015122742
- AdamopoulosABBaibasNMEfstathiouSPDifferentiation between patients with acute upper gastrointestinal bleeding who need early urgent upper gastrointestinal endoscopy and those who do not. A prospective studyEur J Gastroenterol Hepatol200315438138712655258
- StanleyAJDaltonHRBlatchfordOMulticentre comparison of the Glasgow Blatchford and Rockall scores in the prediction of clinical end-points after upper gastrointestinal haemorrhageAliment Pharmacol Ther20113447047521707681
- ThomopoulosKCLabropoulou-KaratzaCMimidisKPKatsakoulisECIconomouGNikolopoulouVNNon-invasive predictors of the presence of large esophageal varices in patients with cirrhosisDig Liver Dis20033547347812870732
- ZamanABeckerTLapidusJBennerKRisk factors for the presence of varices in cirrhotic patients without a history of variceal hemorrhageArch Intern Med20011612564257011718587
- GianniniEZamanAKreilAPlatelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation studyAm J Gastroenterol20061012511251917029607
- Abd-ElsalamSHabbaEElkhalawanyWCorrelation of platelets count with endoscopic findings in a cohort of Egyptian patients with liver cirrhosisMedicine20169523e385327281094
- BresslerBPintoREl-AshryDHeathcoteEJWhich patients with primary biliary cirrhosis or primary sclerosing cholangitis should undergo endoscopic screening for esophageal varices detectionGut20055440741015710991