Abstract
Esophageal cancer (EC) is an extremely aggressive cancer with one of the highest mortality rates. The cancer is generally only diagnosed at the later stages and has a poor 5-year survival rate due to the limited treatment options. China and South Africa are two countries with a very high prevalence rate of EC. EC rates in South Africa have been on the increase, and esophageal squamous cell carcinoma is the predominant subtype and a primary cause of cancer-related deaths in the black and male mixed ancestry populations in South Africa. The incidence of EC is highest in the Eastern Cape Province, especially in the rural areas such as the Transkei, where the consumption of foods contaminated with Fusarium verticillioides is thought to play a major contributing role to the incidence of EC. China is responsible for almost half of all new cases of EC globally. In China, the prevalence of EC varies greatly. However, the two main areas of high prevalence are the southern Taihang Mountain area (Linxian, Henan Province) and the north Jiangsu area. In both countries, environmental toxins play a major role in increasing the chance that an individual will develop EC. These associative factors include tobacco use, alcohol consumption, nutritional deficiencies and exposure to environmental toxins. However, genetic polymorphisms also play a role in predisposing individuals to EC. These include single-nucleotide polymorphisms that can be found in both protein-coding genes and in non-coding sequences such as miRNAs. The aim of this review is to summarize the contribution of genetic polymorphisms to EC in South Africa and to compare and contrast this to the genetic polymorphisms observed in EC in the most comprehensively studied population group, the Chinese.
Introduction
Esophageal cancer (EC) is a malignant tumor in the epithelial cells padding the esophagus.Citation1 Worldwide, EC is the third most frequently diagnosed malignancy of the upper digestive tract. EC is responsible for >400,000 deaths each year, making it the seventh leading cause of cancer-related deaths.Citation2–Citation4 The regions with the highest number of cases and most deaths are the south and the east of Africa, central Asia, Turkey, Iran, Kazakhstan and China ().Citation5 The disease has a poor prognosis with a 5-year survival rate of <10%.Citation6 The two most important histological forms of EC are etiologically and pathologically unrelated. These are esophageal squamous cell carcinoma (ESCC), which predominates in non-white populations, and adenocarcinoma, which is more common in white population groups.Citation4,Citation7,Citation8 EC occurrence and type are strongly influenced by the geographic area and ethnicity.Citation4,Citation7,Citation8 Most incidences of esophageal malignancy are ESCC, which makes up >95% of all cases.Citation9 However, the number of adenocarcinoma cases is on the rise, with the proportion of adenocarcinomas increasing from 3.5% in 1985 to 17.0% in the year 2000. This is especially true in western countries, where it now accounts for 30%–50% of all diagnosed ECs.Citation10–Citation12 One of the main environmental factors that has been investigated as an associative factor contributing to the development of ESSC is nutrition. Nutritional factors that may play a role in the development of ESSC include a diet low in dietary vitamins and minerals, the consumption of hot beverages and pickled food and exposure to foods contaminated with or containing nitrosamines.Citation13,Citation14 However, while these environmental risk factors may be factors that strongly contribute to the development of ESCC, they alone may require a genetic component which would predispose an individual to ESCC.Citation4,Citation15
Figure 1 Incidence and mortality rates of EC.
Abbreviation: EC, esophageal cancer.
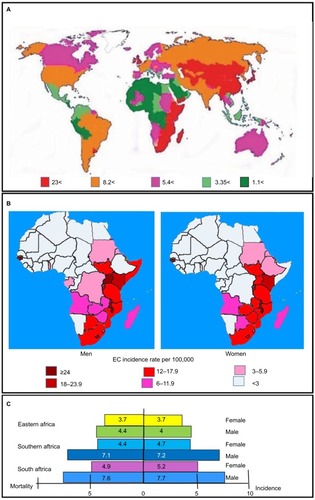
Cancer of the esophagus was an uncommon disease in South African during the 1920s and 1930s.Citation16 This was followed by a rapid rise in the number of diagnosed cases of the disease.Citation17–Citation19 EC has become the third most commonly diagnosed cancer in Black South Africa. Age occurrence values are 22.3 per 100,000 in black males and 11.7 per 100,000 in black females, respectively.Citation20 It is also the fourth highest cause of death in males of the colored or mixed ancestry group.Citation21 EC rates increase noticeably in South Africa between the average age of 60 and 70 years, along with cigarette fuming and extreme alcohol use ().Citation22 The Transkei region is considered as the center of the malady in South Africa,Citation23 with a standard incidence rate of 46.7/100,000 for males and 19.2/100,000 for females.Citation24 The increase in EC rates in South Africa over the last decades can largely be attributed to changes in environmental factors and exposure to carcinogens.Citation23 Two of the most important factors are increased alcohol intake ()Citation16 and increased tobacco use ().Citation25 However, other weak carcinogens also play a role, such as the exposure to environmental smokeCitation26 mainly in the form of cooking fires, the use of wild herbs such as Solanum nigrum, human papillomavirus (HPV) infectionCitation23 and the consumption of traditional beer brewed with maize that may contain fungal mycotoxins or nitrosamines.Citation16 Another important factor contributing to the increase in EC rates is the adoption of a western diet. This includes increased consumption of fats and animal protein as well as a decrease in the vitamin content of traditional beer that is now brewed almost exclusively with maize.Citation16
Table 1 Alcohol consumption and tobacco use in China and South Africa
Another region of the world with a high incidence rate of EC is China. Over half of all new cases of EC are diagnosed in China. In South Africa, EC is the eighth most common cancer in men and the eleventh in women, while in China, it is the fourth most frequently diagnosed cancer.Citation4 Like South Africa, the areas in China with the highest incidence rates are the rural areas.Citation27 Areas such as Henan, Hebei and Shanxi in Central North China have the highest incidence rates in the world (over 100 per 100,000).Citation28,Citation29 Additionally, the provinces of Sichuan, Anhui, Jiangsu, Hubei, Fujian, Guangdong and Xinjiang have age-adjusted rates >30 per 100,000 ().Citation30 Overall, EC is the fourth leading cause of cancer-related deaths in China.Citation4 However, in Xi’a city in Shaanxi Province, it is the leading cause of cancer-associated mortality with a mortality rate of 24 per 100,000.Citation31 Once again, environmental factors similar to those suspected of playing a role in South African cases of EC are also thought to be important contributing factors in China. These include nutritional factors such as the consumption of fatty meat, salted and pickled vegetables and moldy food, as well as nutritional deficiencies. Lifestyle factors include passive smoking, esophageal lesions, infection with Helicobacter pylori, low socioeconomic status and poor oral hygiene. However, a family history of cancer is also an important risk factor associated with an increased risk of ESCC,Citation32 and this points to a genetic component.
Figure 2 Geographic locations of the four high-risk areas in China.
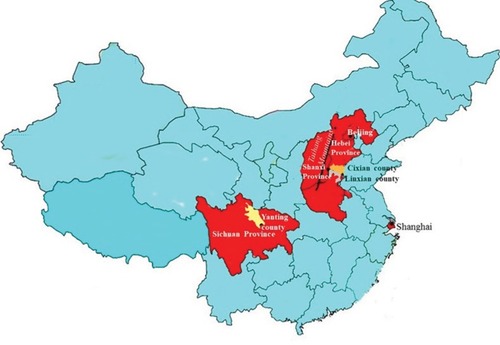
Despite the known dominant role played by environmental factors in increasing the rates of EC, the occurrence of high rates of EC in members of the same blood family observed in both China and South Africa suggests that genetics may also play an important role.Citation26
Environmental risk factors for EC
Environmental risk factors for EC are complex and have a complicated relationship with genetic and etiological elements concerning the development of ESCC and adenocar-cinoma.Citation33 The influence of these different factors on the risk of developing adenocarcinoma and ESCC is given in . The geographic variation in the occurrence of EC is likely caused by genetic polymorphism and unstable environmental factors.Citation6 Worldwide, the primary risk factors for ESCC are alcohol use and tobacco smoking. Other factors rely on the region of the world. These include hot brews in Iran and South America, and smokeless tobacco and alimentary deficits in China, Central Asia and South Africa.Citation34 Other risk factors include the extensive utilization of wood and charcoal for cooking and heating,Citation35,Citation36 along with nutritional factors that are related to the growth of the EC.Citation6
Table 2 Comparison of the risk factors for adenocarcinoma and ESCC
Analyses carried out within the areas of the Transkei region and KwaZulu-Natal Province have demonstrated that people at highest risk are commonly poorer, have inadequate diets and consume some class of alcohol and tobacco.Citation31,Citation32,Citation37 Furthermore, bacteria from the family Helicobacteraceae were discovered in roughly 50% of South African patients with EC.Citation38 The use of any form of tobacco was found to increase the risk of developing EC by as much as four times in the Eastern Cape Province of South Africa.Citation39 Currently, the prevalence of tobacco consumption among adult South Africans is 17.6%, with males being four times more likely (29.2%) than females (7.3%) to use tobacco products.Citation40 Cigarette smoke is composed of over 4,000 chemical compounds, and >60 of these are recognized to cause diseases, including cadmium, nicotine/cotinine and benzo[a]pyrene.Citation41 In South Africa, the consumption of >53 g of ethanol per day led to a risk of developing EC five times greater in comparison to non-drinkers, while a combination of both alcohol, consumption and smoking led to an 8.5 times greater risk of developing EC. The attributable fractions in high-risk populations in South Africa were 58% for smoking, 48% for alcohol consumption and 64% for both factors combined.Citation39 In comparison, tobacco use in China is much higher in Chinese males than females. Currently, the prevalence of smoking in China is 56.1% among males and 2.2% among females in rural areas. In urban regions, the occurrence was 49.2% among males and 2.6% among females.Citation42 The attributable fraction for both tobacco and alcohol consumption was lower in high-risk Chinese populations compered to their South African counterparts. The attributable fractions of esophageal patients who were smokers were 17.9% in Chinese men and 1.9% in Chinese women,Citation43 and for alcohol consumption they were 15.2% in Chinese men and 1.3% in Chinese women.Citation44 This higher tobacco use and alcohol consumption among males in both South Africa and China () is most likely the major factor contributing to the higher incidence of EC observed in males.
In the Eastern Cape of South Africa, an increase in the risk of developing EC is associated with a diet low in green leafy vegetables and fruit.Citation45 When examining the diet of those in high-risk, remote rural areas of South Africa, it was found that low plasma concentrations of vitamins A, E, B12, folic acid and selenium were also associated with an increased risk of developing EC.Citation46 Similarly, reports in Linxian, China showed that general undernourishment, as well as deficiencies in selenium, zinc, folate, riboflavin and vitamins A, C, E and B12 were linked with amplified risk of ESCC.Citation47 Another dietary cause of EC in South Africa is the production of carcinogenic nitrosamines resulting from fungal contamination of food. The shift in the diet of the black population of South Africa from sorghum to maize is one of the factors that have been identified as contributing to the current high levels of EC in this population group. Unlike sorghum, maize is frequently contaminated with fungi such as Fusarium verticillioides. These fungi produce toxins such as fumonisins, which reduce nitrates to nitrites and synthesize nitrosamines.Citation48 Homemade traditional beer is commonly consumed in these regions of South Africa. This beer was once brewed mainly from sorghum, but is now generally brewed from maize. This traditional beer has been found to contain high amounts of carcinogenic N-nitrosamines.Citation49 The maize-rich diet also leads to high levels of prostaglandin E2 (PGE2) in gastric fluid. This enzyme activates the Wnt signaling pathway. PGE2 also stimulates proliferation and may predispose esophageal cells exposed to gastric fluid to developing ESCC.Citation50 Linoleic acid is used to synthesize prostaglandins, and therefore, high levels of linoleic acid lead to increased PGE2 activity in the stomach.Citation51 PGE2 represses gastric acid secretion and leads to a reduction in the tone of the muscles that control pyloric and lower esophageal sphincters. This results in chronic hyperchlorhydria duodenogastroesophageal reflux (DGOR).Citation52 Chronic alkali DGOR is thought to lead to the denaturation of proteases due to the altered pH. The inhibition of proteases leads to increased growth factor activity as they are no longer broken down by proteases. This leads to an increase in the risk of developing ESCC.Citation53 The cyclooxygenase enzyme (COX-2) is required to convert a linoleic acid derivative into PGE. Like PGE2, the level of COX-2 is elevated in EC.Citation36,Citation54–Citation57 It has previously been confirmed that gastric fluid out of specimens from Transkei contained higher levels of PGE2.Citation50 In China, analysis was performed on local sources of drinking water and samples and in food samples, in order to determine the level of nitrates and nitrites in these samples. It was determined that these concentrations were abnormally high and, therefore, indicated that high levels of nitrosamine were present.Citation58 Another common causative agent is infection with H. pylori and HPV. However, case studies in South Africa found no association between EC and an increase in H. pylori infection.Citation59 However, in Chinese populations, the relationship between H. pylori infection and ESCC was significant.Citation60 This shows an important difference between environmental factors that may lead to ESCC in Chinese and western populations. In terms of HPV infection and its relationship to EC in South Africa, HPV DNA could only be isolated from 9% of ESCC patients. HPV infection appears to only play a minor role in EC.Citation61 However, looking for HPV biomarkers (E6, E8 and p16) in ESCC tissue samples from China led to the conclusion that there was no association between HPV infection and ESCC.Citation62
Epidemiologic studies in Chinese populations have studied the link between EC and other factors such as the consumption of warm beverages in the form of green tea. While there seemed to be no relationship between drinking tea and ESCC, either directly or inversely, there was a relationship between drinking hot green tea and the development of ESCC. This implies that consumption of any high-temperature beverage could increase the chances of developing ESCC.Citation63 Another factor that has been studied is gastric atrophy. This refers to dying of gastric cells and their replacement with intestinal tissues. Gastric atrophy is indicated by lower serum ratios of pepsinogen I/pepsinogen II. In Chinese populations, gastric atrophy increases the risk of ESCC as indicated by lower Pepsinogen I to Pepsinogen II ratios being positively associated with ESCC.Citation13
These risk factors have the capacity to influence the stability of fragile sites.Citation64 Tobacco amplifies the expression of regular sensitive locations;Citation65 HPV fuses within fragile site loci.Citation66 Alcohol and fumonisin each influence folate levels, which facilitates the expression of fragile sites.Citation64,Citation67,Citation68 The addition of fumonisin to cell cultures increases the frequency of chromosomal damage.Citation69 The combination of these risk factors leads to the initiation of genetic instability at the early stages of carcinogenesis, with the fragile sites being prime targets.Citation64 It would be of great benefit, for diagnostic and prognostic purposes, if the genes within the most common fragile sites could be determined in South African cases of ESCC.Citation64
Genetic polymorphisms
The importance of genetic factors in contributing to increased risk of developing ESCC was demonstrated by a study undertaken in the Tenwek Mission Hospital in Western Kenya. Kenya is a very high-risk area for ESCC and is part of the ESCC corridor (). In this study, ESCC patients younger than 30 were found to have a high incidence of cancer within their family with 20 out of 60 patients having a family history of EC. All these patients also originated from the same community (Kipsigis).Citation70 Many of the genes that have been linked to the development of ECCitation71,Citation72 were found to be associated with DNA maintenance and repair, alcohol folate and carcinogen metabolism, cell cycle regulation and apop-tosis.Citation73 Nevertheless, just a few of these genes have shown to be associated with disease vulnerability.Citation72 Therefore, some additional genetic factors assist in predisposing individuals to EC.Citation71 The most common genetic polymorphisms are single-nucleotide polymorphisms (SNPs). A large percentage of the genetic variations that occur in the human genome are due to SNPs.Citation74
A xenobiotic is any chemical substance that is foreign to an organism in its normal physiological function. Humans are exposed to multiple environmental conditions that may result in the buildup of xenobiotics within the body tissues. Many dietary and environmental xenobiotics would require metabolic activation by the Phase I or Phase II enzymes to exert their carcinogenic effect.Citation75 The Phase I enzymes catalyze the conversion of a toxic or insoluble compound into a polar, water-soluble metabolite through oxidation. The resulting metabolites contain functional groups such as OH, NH2 and SH. These groups are sites for reactions catalyzed by the Phase II enzymes.Citation75 The Phase II enzymes give rise to compounds that are more hydrophilic and thus easily excreted in the urine.Citation75 Although the Phase I and Phase II enzymes act in the detoxification pathway, they usually generate unstable and more reactive intermediate compounds that, if not quickly removed, can bind covalently to DNA and generate DNA adducts with mutagenic and/or carcinogenic properties.Citation76 The presence of xenobiotics and polymorphisms in xenobiotic-metabolizing enzymes (XMEs) can increase the risks of sensitivity to ESCC.Citation77 These XMEs include Phase I enzymes such as CYP1A1, CYP1B1, CYP2A6 and CYP2E1 and Phase II enzymes such as GSTM1, GSTT1 and GSTP1 ().Citation31 Variations at 10q23 in PLCE1 were linked with ESCC ().Citation78 Alteration in the riboflavin transporter C20 ORF54 on chromosome 20p13 is a risk factor for ESCC.Citation43 ESCC is related to polymorphisms of the ADH enzymes ALDH2 and ADH1B1. These enzymes are engaged in alcohol metabolism, and with CYP1A1, are involved in xenobiotics’ detoxification.Citation79 NQO1 is one of the enzymes belonging to the NAD(P)H dehydrogenase (quinone) family that are involved in protecting cells from oxidative damage.Citation80 A significant polymorphism dealing with a single C to T replacement at nucleotide 609 of exon 6 in the NQO1 cDNA affects the NQO1 enzyme activity that induces a Pro187Ser amino acid substitution.Citation81 The NQO1 C609T polymorphism has been related to ECs.Citation82–Citation84 NQO1 is involved in cellular antioxidant defense systems by detoxifying quinines.Citation85 A meta-analysis showed that the NQO1 C609T polymorphism considerably increases the risk of developing EC.Citation85
Table 3 Genetic polymorphisms affecting the genes related to changing the risk of developing esophageal cancer in South Africa and China
The role of polymorphisms in alcohol metabolising enzymes: ADH and ALDH in the risk of developing EC
The WHO classifies alcohol as a Group 1 carcinogen. In the body, alcohol is initially metabolized by the ADHs to acetaldehyde. This is then further metabolized to acetic acid by ALDH2. Mutations and polymorphisms that affect the functioning of these enzymes may affect the ability of the individual to detoxify ethanol, leading to increased exposure of cells to carcinogens such as acetyl aldehyde which is dissolved in the saliva following consumption of alcohol or smoking tobacco ().Citation86 There is a guanine to adenine SNP within ALDH2 at position 1510, which leads to a lysine at codon position 487. This leads to a catalytically inactive subunit, and the allele containing this polymorphism is termed ALDH2*two (wild-type ALDH*1). Individuals homozygous for this mutation (ALDH2 *1/*2) have only 6.25% of normal ALDH2 *1/*one activity.Citation87 The ADH 1B *two mutant allele results from an SNP in Exon 3 of ADH1B, resulting in a His to Arg substitution at codon 48. This results in a protein with a much higher activity than the wild type encoded by the ADH1B *one allele. The homozygous ADH1B*2/*two polymorphism has a Vmax 40 times higher than the wild-type ADH1B*1/*1. This results in greater amounts of alcohol being oxidized by the more active enzyme.Citation88 Both these polymorphisms are frequently found in individuals originating from East Asia. There is a strong association between the ALDH2 *2/*two genotype and EC (OR =4.42). The lower activity of ADH1B *one allele is also associated with an increase in the risk of developing EC. This occurs in a manner associated with the number of ADH 1B *one alleles present, with *1/*one homozygous having a greater risk for developing EC than the *1/*two allele. In South Africa, the role played by mutations in the genes coding for these enzymes has not been studied intensively. The ALDH2*two allele is present in black and mixed ancestry South African populations, where it is significantly associated with an increased risk for EC (OR =2.35; ).Citation89,Citation90 The ADH1B *two allele was also detected in the mixed ancestry population in South Africa. As in other population groups, this allele is associated with a decreased ESCC risk.Citation90 Mutations affecting the function of ALDH2 are so prevalent among East Asian population groups that it is common for individuals in these population groups to have a flushed skin or red blotches on their face, neck and shoulders following consumption of alcohol. This is known as the alcohol flush reaction. People displaying this reaction have an increased risk of developing EC. This rash results from excess acetyl aldehyde, with the polymorphism most commonly associated with the flush response being the rs671 allele of ALDH2. This mutation in ALDH2 and the flush reaction occur in 30%–50% of South East Asians, but it is extremely rare in Europeans and sub-Saharan Africans (Tanzania =0.0025%). In South Africa, it is, therefore, of no use as a diagnostic or predictive tool in assessing the susceptibility of an individual to alcohol-related risks of developing ESCC.Citation91
Table 4 Genetic polymorphisms in alcohol metabolizing enzymes associated with EC in South Africa and China
The role of genetic polymorphisms in the androgen receptor (AR) gene in the risk of developing EC
The AR is a nuclear receptor that translocates to the nucleus and acts as a transcription factor once it has bound its ligand. This ligand is generally any of the androgenic hormones. As a transcription factor, it controls pathways associated with cellular proliferation and differentiation.Citation92 The receptor is expressed in multiple cancer tissues and cell lines. These include neoplastic colon tissues,Citation93 breast tumors,Citation94 hepatocellular cancerCitation95 and ESCC.Citation96 AR is a part of the steroid receptor family and the AR gene is situated at q11-12 on the X chromosome.Citation97 The AR gene is 75,000–90,000 nucleotides in length, consisting of eight exons.Citation98 The various protein isoforms range in size from 910 to 919 amino acids and consist of four domains. The first is an NH2-terminal transactivation domain. A central C4 zinc finger DNA-binding domain and a nuclear-localizing short hinge region follow this. Finally, the C terminal consists of a steroid hormone ligand-binding domain.Citation99 The gene for the AR is highly polymorphic in human populations due to the fact that the first exon contains two polymorphic poly amino acid tracts, a glutamine (CAG) 7–31 repeatCitation96,Citation100,Citation101 and a glycine (GGC)Citation102–Citation105 8–20 repeatCitation106,Citation107 ~1.1 kb apart. Examination of data concerning the (GGC) n allele indicates a genetic sensitivity element for EC in black males. A short (GGC) n allele was involved in the disease in this population, with a GGC shorter than 16 being associated with the disease with an OR of 2.7, 95% CI, and a GGC shorter than 14 being associated with EC with an OR of 3.3, 95% CI.Citation108 Among the Chinese population in Beijing, shorter CAG repeats were observed in male EC patients, implicating this polymorphism in predisposing individuals to a greater risk of developing EC.Citation109
The role of genetic polymorphisms in glutathione S-transferase (GST) genes in increased EC risk
The GSTs are Phase I enzymes that introduce reactive or polar groups onto a compound as the initial step to detoxify or remove a reactive or dangerous compound. GSTs accomplish this by catalyzing the conjugation of glutathione (GSH) to electrophilic compounds.Citation110 These compounds include carcinogens that may lead to the development of cancers such as ESCC.
The risk of developing ESCC in South African and Chinese populations is a result of genetic polymorphisms in an individual’s GST-coding genes and their exposure to environmental factors such as tobacco smoke and alcohol consumption ().Citation71 There are four major subfamilies of human GST genes, which are divided into the categories GST A, M, T and P.Citation111 Deletion polymorphisms have been observed in two of these families, GSTM1 and GSTT1.Citation112 Polymorphisms of GSTM 1, GSTT1 and GSTP1 have been demonstrated to be linked to susceptibility to various forms of cancer. This is especially true concerning cancer triggered by exposure to carcinogens such as cigarette smoke,Citation113 and they also play a role in the resistance to chemotherapy treatmentCitation114 and in disease outcomes.Citation115
Table 5 GST polymorphisms involved in esophageal cancer
GSTP1 is expressed in hepatic tissues, lungs and the esophagus and at very low levels in the liver.Citation111 Moreover, it has been shown to be overexpressed in several malignant tissues compared to normal tissues. Polymorphisms of GSTP1 take part in EC within a pathway of malfunctions in the p53 malignant tumor suppressor gene, which is prevalent in ESCC.Citation116 The human GSTM1 gene is composed of seven exons, is 5.9 kb in length and is found on chromosome 1p13.Citation117 The human GSTT2 gene, located on chromosome 22q11.2, is made up of five exons spanning 3.8 kb in length.Citation118
miRNA polymorphisms in EC development
Altered miRNA transcription changes the expression of oncogenes and tumor suppressor genes. These genes affect processes such as proliferation, apoptosis, as well as the motility and invasiveness of cancer cells. This has been found to apply to ESCC as well.Citation119–Citation121 Different studies have identified different numbers of miRNA whose transcription levels change in ESCC. Ogawa et al reported that 22 miR-NAs were upregulated in ESCC tissue, while 4 miRNAs were downregulated.Citation122 Yao et al found 27 downregulated and 16 upregulated miRNAs in ESCC tissue, while Ren et al detected 51 upregulated and 17 downregulated miRNAs.Citation123 Next-generation sequencing has identified 78 diversely expressed miRNAs in ESCC.Citation124 Silencing of cancer genes mainly occurs through DNA methylation. DNA methylation can, therefore, affect a range of cellular processes. These include the regulation of the cell cycle checkpoint, apoptosis, signal transduction, cell adhesion and angiogenesis. Hence, DNA methylation has a role in the deregulation of miRNAs in cancer, and miR-145, miR-30a-3p, miR133a and miR-133b are the possible tumor promoters.Citation125
In South Africa, the polymorphisms in miRNA genes have been connected to many cancer types along with ESCC.Citation126,Citation127 In black South Africans, a polymorphism in miRNA 3184, known as rs6505162, results in associated risks for ESCC. The location of the rs6505162 SNP allows it to influence two different miRNAs and a single protein coding gene. These include miR-423, miR-3184 and NSRP1. The two miRNAs, miR-423 and miR-3184, are oppositely oriented and overlap at rs6505162. The SNP is situated downstream of miR-423 and upstream of miR-3184.Citation128 Therefore, it is more likely that rs6505162 influences the transcription of miR-3184 rather than that of miR-423.Citation129 The increased risk of ESCC related to this SNP is also linked to environment. It was found that the rs6505162 polymorphism was related to increased cancer risk in black African patients who lived in an environment where they were subjected to high levels of smoke inhalation.Citation128 The risk of developing ESCC was greater in those individuals who possessed the SNP and used solid fuels for cooking than in those individuals with the SNP and who used electricity or gas for cooking. Due to population heterogeneity, this relationship does not seem to be present in the mixed ancestry group.Citation128 Two other SNPs previously identified as playing a role in EC, rs213210 (miR219-1) and rs7372209 (miR26a-1), were found to interact in both the black and mixed ancestry populations to reduce the risk of cancer development. Individuals with the genotype AArs213210-CTrs7372209 had a reduced risk of developing ESCC.Citation128
Polymorphisms in miRNA genes related to ESCC risk have also been identified in Chinese populations.Citation130 The SNP rs2910164 C > G in miR-146a increases the risk of individuals developing ESCC within the Han Chinese population, and the increased risk posed by the rs2910164 GG genotype was more notable in cigarette smokers.Citation131 The rs11614913 TC polymorphism in miR-196a2 is predicted to reduce ESCC risk among females who have never smoked or consumed alcohol. However, in males or those who smoke and drink, the miR-196a2 r s11614913 TC, CC or TC/CC genotype may play a role in increasing the risk of developing ESCC.Citation132 Similarly, individuals who did not smoke or drink alcohol and possessed the hsa-miR-34b/c rs4938723 CC genotype had a reduced threat of ESCC.Citation130,Citation133 The risk of developing ESCC is increased by the SNPs rs6505162 C.A in Hsa-miR-423Citation133 and rs531564 CG SNP in pri-miR-124-1.Citation130,Citation134
The role of polymorphisms in the DNA mismatch repair (MMR) genes and tobacco smoking in increasing EC risk
Environmental toxins are able to cause cancer due to their ability to damage DNA. An important safeguard against this is the DNA MMR enzyme system. The MMR system restores mistakes within DNA. These mistakes take place during normal DNA metabolism; however, they are also related to cancer caused by exposure to environmental carcinogens.Citation73 Studies suggest that the MMR gene polymorphisms control the etiology of EC. These genes are connected to the processes of DNA preservation and repair, alcohol, folate and carcinogen metabolism, cell cycle control and apoptosis.Citation73 Furthermore, MMR genes and their polymorphisms account for the increased risk in the development of lung or head and neck cancer, including EC.Citation36,Citation135–Citation138 A list of some of the polymorphisms in MMR genes that may contribute to the risk of EC is given in . The DNA MMR process is made up of MLH1, MSH2, MSH3, MSH6 and PMS2 proteins.Citation139 During DNA synthesis, the MMR corrects the microsatellite instabilities (MSIs) in order to sustain genomic integrity.Citation140 MSI is characterized by increased mutations in microsatellites that arise due to germline MMR gene mutations causing Lynch syndrome. MSI can also be caused through the epigenetic inactivation of the MLH1 gene and the CpG island methylator phenotype as a consequence of MLH1 hyper-methylation.Citation141 This may eventually lead to carcinogenesis due to replication errors that remain uncorrected in essential cancer regulating genes.Citation142
Table 6 Genetic polymorphisms in DNA mismatch repair genes that influence the risk of developing esophageal carcinoma in South Africa and China
Studies searching for extensive microsatellite modification in EC have discovered low-level MSI in 16%–67% of adenocarcinomas, while 2%–60% of squamous cell carcinoma tumors were MSI-L positive. These high-incidence populations possessed the maximum MSI abundances, signifying that MMR might be included in the pathogenesis of the esophagus.Citation143,Citation144 Genetic conversions in microsatellite regions, a property of a faulty DNA MMR procedure, have been found in ECs.Citation73
In black South Africans, no association between MMR polymorphisms and cancer risk was observed at the SNP level. It was suggested that different linkage disequilibrium patterns are the reason for the lack of significant associations in these individuals.Citation73 On the other hand, three regular polymorphisms were allied with ESCC in mixed ancestry South Africans.Citation73 Epithelial cells of the esophagus are regularly exposed to DNA destructive compounds and should have the capacity to fix the DNA damage provoked by numerous carcinogens in food, tobacco and solid fuel smoke.Citation132 A polymorphism within the DNA repair associated gene MSH3 () may interact with cigarette in some cases of carcinogenesis. A model was developed to explain the role played by the interaction between the MSH3 protein and tobacco smoke in EC,Citation145 as MSH3 is implicated in playing a significant role in DNA double-strand break (DSB) repair.Citation146–Citation148 Cigarette smoke has been shown to damage DNA by causing DSBs.Citation149 Therefore, any mutation or polymorphism decreasing MSH3 activity may increase the risk of developing EC.
Promoter CpG island methylation appears to be a frequent event in ESCC carcinogenesis, with methylated sequences of hMLH1, hMSH2 and MGMT being identified in a large proportion of Chinese ESCC patients.Citation150 Promoter methylation in the ML1 promoter of male Han Chinese ESCC patients is associated with a poor prognosis.Citation151
Genetic polymorphisms of CYP genes increase the risk of developing EC
CYPs play a considerable role in detoxifying chemicals that the esophagus is exposed to.Citation152,Citation153 The CYP enzymes are expressed in the esophageal mucosa, suggesting that this tissue can detoxify DNA-binding chemical carcinogens.Citation154–Citation156 CYP2E1 is an 11.4 kb gene containing nine exons positioned on chromosome 10q26.345.Citation157 The CYP2E1*six heterozygous genotype is linked to ESCC in South AfricaCitation158 and the c1/c1 genotype is linked to ESCC in China (). CYP3A is mostly regulated by genetic polymorphisms in the CYP3A4 and CYP3A5 genes, which exist together in a 231 kb area on chromosome 7q21.1.Citation159 CYP3A5 is an important enzyme in the esophagus and metabolizes carcinogenic compounds,Citation152 playing a role in both metabolic catalysis of xenobiotics to produce reactive intermediates found in the pathogenesis of EC and in the detoxification of carcinogens that the esophagus is exposed to. Functional polymorphisms in the CYP3A5 gene are mainly represented by CYP3A5*three and CYP3A5*six alleles.Citation160 CYP3A5*six appears to be African specific.Citation161 In South African populations, the CYP3A5*three allele was found to be present in 14%–16% of the black subjects and 48%–59% of the mixed ancestry population ().Citation162
Table 7 Genetic polymorphisms in CYP/CYP450 genes relating to ESCC in South Africa and China
As mentioned previously, South African maize is contaminated with high levels of aflatoxins and fumonisin.Citation163 Fumonisins stimulate xenobiotic metabolizing enzymes along with the CYPs.Citation164 CYP3A5 could be slightly decreased. CYP3A enzymes metabolize several steroids such as progesterone, estradiol, testosterone and corticosterone.Citation160 Similar associations between environmental factors and the CYP3A5*three allele have been found in a high-risk Chinese population from Shanxi Province.Citation158
Genetic polymorphisms in genes encoding sex hormone metabolizing enzymes increase the risk for EC
Males are two to four times more likely to develop EC than females. Also, while men are more commonly exposed to risk factors, one study seemed to indicate that these factors had an even greater harmful effect on females than they did in males ().Citation165 Evidence from epidemiological and experimental studies suggests that sex hormones may play a significant role in the development of ESCC.Citation15 Since hormone therapy is able to lower the risk of ESCC,Citation78,Citation166,Citation167 it has been suggested that low estrogen plays a role in increased risk of ESCC.Citation168 One line of evidence that suggests this is the increased levels of estradiol, which is an estrogen receptor agonist, in patients with ESCC, further suggesting that low levels of estrogen are associated with an increased risk of ESCC.Citation168 This implies that mutations in genes that play a role in metabolizing sex hormone may be important in the etiology of ESCC. Genetic variation in at least six of these genes that play a role in the metabolism of sex hormones have been associated with an increase in the risk of developing EC. These include cytochrome P450s (CYP1B1, CYP3A7, CYP3A5 and CYP11A1), SULT2B1 and SHBG. These genes regulate sex hormone activity by catalyzing their breakdown or regulating their bioavailability.Citation15
Table 8 The extent to which different risk factors influence the odds of developing ESCC in Swedish males and femalesCitation165
Earlier reports showed that ESCC cells grown in vitro express both estrogen and ARs and the addition of these hormones to the growth media promoted the growth of ESCC cells in vitro.Citation96,Citation169
Estrogen is metabolized into 4-hydroxy estrogen by the enzyme CYP/CYP450 1B1 (CYP1B1). This enzyme has a high catalytic activity for this reaction. The resulting 4-hydroxy estrogen is able to induce DNA damage.Citation170 Mutations in Cyp1B1 that result in a GG substitution rather than the GA in normal copies decrease the efficacy of the enzyme. South African individuals homozygous for the mutant allele (GG), and therefore possessing a less-efficient CYP1B1, had a lower risk of developing ESCC. These heterozygous individuals (GA) had an increased risk of developing ESCC.Citation158
SULT2B1 catalyzes the sulfate conjugation of many hormones, and is therefore required for the formation of these hormones. Two SNPs, SULT2B1a and SULT2B1b, in this gene are connected with the risk of ESCC in the Chinese population.Citation160
The role of dietary selenium in contributing to EC
Selenium is an essential factor in several metabolic activities. This is due to the use of selenium by selenoproteins, proteins containing a selenocysteine amino acid residue. These proteins function as enzymes in many vital processes. These include protecting the cell membranes from lipid peroxidation, defending cells from oxidative damage and regulation of the immune system.Citation171 Selenium has anticarcinogenic and chemoprotective effects, and selenium-containing proteins perform a significant function in the metabolism and detoxification of polycyclic aromatic hydrocarbons.Citation172 Selenium also protects the DNA from being damaged by oxygen free radicals by scavenging these oxygen molecules. Selenium also promotes the removal of damaged cells by inducing apoptosis. If they are not removed, these damaged cells could potentially develop into cancerous cells.Citation173
Selenium compounds stimulate apoptotic death of tumor cells,Citation174 and selenium regulates p53 in the role it plays in DNA repair or apoptosis.Citation175 Primarily, selenium is found as selenomethionine. Selenomethionine stimulates the repair of DNA damage through the p53 pathway. An essential component of this signaling pathway is the GSTP1 protein. GSTP1 activates p53 through a redox mechanism.Citation176 The function and activity of a mutant GSTP1 protein that has an Ile-to-Val substitution (rs947894) are decreased. This mutant protein is the result of a polymorphism in the GSTP1 gene at codon 105.Citation177 Mutations in the p53 gene are prevalent in all cancers including EC.Citation178,Citation179 A single base modification from arginine (CGC) or proline (CCC) is initiated on codon 72 (rs1042522).Citation180 This polymorphism is connected to tumorigenesis in a variety of cancers. It is also a risk factor for HPV-associated cervical neoplasia and ESCC.Citation181,Citation182 The GSTP1 and p53 gene polymorphisms modify selenium–ESCC relation.Citation183 Studies showed that p53 Pro/Pro was related to ESCC risk as compared with p53 Arg/Arg homozygotes.Citation183 Individuals with both the GSTP1 Ile/Ile and p53 Pro/Pro genotypes have an increased risk of ESCC.Citation183 Therefore, dietary selenium intake can alter an individual’s risk of developing EC. This risk can then be further increased if the polymorphisms in the GSTP1 and the p53 genes are also present.Citation183
Conclusion
This review highlights the contribution that genetic polymorphisms make to the incidence of EC in a South African population, how these genetic polymorphisms are found in other population groups, especially Chinese populations, and the extent to which they contribute to the incidence of EC in these population groups. The characterization of these genetic polymorphisms may allow us to identify the molecules that can serve as lead drug targets as well as the new diagnostic and prognostic markers. These polymorphisms can best be studied through the use of large cohort studies that take into account the role played by environmental and lifestyle factors that may contribute to or protect an individual from EC. These studies would not only enable us to identify new genetic polymorphisms that are involved in EC, but also allow us to more accurately establish the role played by environmental hazards, such as drinking alcohol, smoking tobacco and consumption of Fusarium-contaminated maize. However, the future challenges include combining the results of these multiple studies in such a way so as to obtain a set of genetic signatures that can be used as population-specific prognostic or diagnostic markers, or even as targets for the development of new drugs. Furthermore, simultaneous analysis of multiple polymorphic genes would allow us to obtain a complete picture of their contribution in the development of EC.
Acknowledgments
We would like to thank the National Research Foundation and the Medical Research Council of South Africa for funding this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- StricklandNJMatshaTErasmusRTZaahlMGMolecular analysis of ceruloplasmin in a South African cohort presenting with oesophageal cancerInt J Cancer2012131362363221901748
- World Health OrganizationGlobal Status Report on Alcohol and HealthGenevaWorld Health Organization2014 Available from: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/Accessed May 12, 2017
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- PennathurAGibsonMKJobeBALuketichJDOesophageal carcinomaLancet2013381986440041223374478
- HendricksDParkerMIOesophageal cancer in AfricaIUBMB Life2002534–526326812121007
- CooperSCDayRBrooksCLivingsCThomsonCSTrudgillNJThe influence of deprivation and ethnicity on the incidence of esophageal cancer in EnglandCancer Causes Control20092081459146719533393
- SomdyalaNIBradshawDGelderblomWCParkinDMCancer incidence in a rural population of South Africa, 1998–2002Int J Cancer2010127102420242920162610
- KuwanoHKatoHMiyazakiTGenetic alterations in esophageal cancerSurg Today200535171815622457
- WuXChenVWRuizBAndrewsPSuLJCorreaPIncidence of esophageal and gastric carcinomas among American Asians/Pacific Islanders, whites, and blacks: subsite and histology differencesCancer2006106368369216388522
- van BlankensteinMLoomanCWHopWCBytzerPThe incidence of adenocarcinoma and squamous cell carcinoma of the esophagus: Barrett’s esophagus makes a differenceAm J Gastroenterol2005100476677415784017
- KollarovaHMachovaLHorakovaDJanoutovaGJanoutVEpidemiology of esophageal cancer – an overview articleBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub20071511172817690734
- KamangarFDiawLWeiWQSerum pepsinogens and risk of esophageal squamous dysplasiaInt J Cancer2009124245646018844222
- CaoBTianXLiYLMP7/TAP2 gene polymorphisms and HPV infection in esophageal carcinoma patients from a high incidence area in ChinaCarcinogenesis20052671280128415774487
- HylandPLFreedmanNDHuNGenetic variants in sex hormone metabolic pathway genes and risk of esophageal squamous cell carcinomaCarcinogenesis20133451062106823358850
- SegalIReinachSGde BeerMFactors associated with oesophageal cancer in Soweto, South AfricaBr J Cancer19885856816863219281
- HigginsonJOettléAGCancer incidence in the Bantu and “Cape Colored” races of South Africa: report of a cancer survey in the Transvaal (1953–55)J Natl Cancer Inst196024358967114401758
- OettlAGAn epidemic of oesophageal carcinoma in AfricaS Afr Med J196337435
- RoseEFEsophageal cancer in the Transkei: 1955–69J Natl Cancer Inst19735117164720888
- JemalABrayFFormanDCancer burden in Africa and opportunities for preventionCancer2012118184372438422252462
- BlotWJEsophageal cancer trends and risk factorsSemin Oncol19942144034108042037
- LagergrenJBergströmRLindgrenANyrénOSymptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinomaN Engl J Med19993401182583110080844
- SammonAMCarcinogens and endemic squamous cancer of the oesophagus in Transkei, South Africa. Environmental initiation is the dominant factor; tobacco or other carcinogens of low potency or concentration are sufficient for carcinogenesis in the predisposed mucosaMed Hypotheses200769112513117258402
- MakaulaANMarasasWFVenterFSBadenhorstCJBradshawDSwanevelderSOesophageal and other cancer patterns in four selected districts of the Transkei, southern Africa:1985–1990Afr J Health Sci199631111517451288
- SumerukRSegalITe WinkelWvan der MerweCFOesophageal cancer in three regions of South AfricaS Afr Med J199281291931733032
- DlaminiZBhoolaKEsophageal cancer in African blacks of Kwazulu natal, South Africa: an epidemiological briefEthn Dis200515478678916259509
- ChenMHuangJZhuZZhangJLiKSystematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancerBMC Cancer201313153924206575
- ParkinDMStjernswärdJMuirCSEstimates of the worldwide frequency of twelve major cancersBull World Health Organ19846221631826610488
- GuoWBlotWJLiJYA nested case–control study of oesophageal and stomach cancers in the Linxian Nutrition Intervention TrialInt J Epidemiol19942334444507960367
- LinYTotsukaYHeYEpidemiology of esophageal cancer in Japan and ChinaJ Epidemiol201323423324223629646
- WangAHSunCSLiLSHuangJYChenQSRelationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi’anWorld J Gastroenterol200281495311833070
- LongNMooreMAChenWCancer epidemiology and control in north-East Asia – past, present and futureAsian Pac J Cancer Prev201011Suppl 2107148
- BarberJPSouth Africa in The Twentieth Century: a Political History – in Search of a Nation StateOxford, UKBlackwell Publishers1999
- VaughanTLDavisSKristalAThomasDBObesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinomaCancer Epidemiol Biomarkers Prev19954285927742727
- Pacella-NormanRUrbanMISitasFRisk factors for oesophageal, lung, oral and laryngeal cancers in black South AfricansBr J Cancer200286111751175612087462
- AnYJinGWangHPolymorphisms in hMLH1 and risk of early-onset lung cancer in a southeast Chinese populationLung Cancer200859216417017870204
- LinYTotsukaYShanBEsophageal cancer in high-risk areas of China: research progress and challengesAnn Epidemiol201727321522128007352
- SchandlLMalfertheinerPEbertMPPrevention of gastric cancer by Helicobacter pylori eradication? Results from clinical intervention studiesDig Dis2002201182212145416
- SewramVSitasFO’ConnellDMyersJTobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South AfricaCancer Epidemiol20164111312126900781
- ReddyPZumaKShisanaOKimJSewpaulRPrevalence of tobacco use among adults in South Africa: results from the first South African National Health and Nutrition Examination SurveyS Afr Med J2015105864865526449697
- ZenzesMTSmoking and reproduction: gene damage to human gametes and embryosHum Reprod Update20006212213110782570
- LiQHsiaJYangGPrevalence of smoking in China in 2010N Engl J Med2011364252469247021696322
- WangLDZhouFYLiXMGenome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54Nat Genet201042975976320729853
- LiangHWangJXiaoHEstimation of cancer incidence and mortality attributable to alcohol drinking in ChinaBMC Public Health201010173021108783
- SewramVSitasFO’ConnellDMyersJDiet and esophageal cancer risk in the Eastern Cape Province of South AfricaNutr Cancer201466579179924877989
- JaskiewiczKOesophageal carcinoma: cytopathology and nutritional aspects in aetiologyAnticancer Res198996184718522627130
- TaylorPRLiBDawseySMPrevention of esophageal cancer: the nutrition intervention trials in Linxian, China. Linxian Nutrition Intervention Trials Study GroupCancer Res1994547 Suppl2029s20318137333
- IsaacsonCThe change of the staple diet of black South Africans from sorghum to maize (corn) is the cause of the epidemic of squamous carcinoma of the oesophagusMed Hypotheses200564365866015617883
- PillayVIsaacsonCMothobiPCarcinogenic nitrosamines in traditional beer as the cause of oesophageal squamous cell carcinoma in black South AfricansS Afr Med J2015105865665826449698
- PinkRCBaileyTAIputoJESammonAMWoodmanACCarterDRMolecular basis for maize as a risk factor for esophageal cancer in a South African population via a prostaglandin E2 positive feedback mechanismNutr Cancer201163571472121667399
- SammonAMMorganADietary fat and salivary prostaglandin E2Prostaglandins Other Lipid Mediat200267213714111936619
- SammonAMMguniMMapeleLAwoteduKOIputoJEBimodal distribution of fasting gastric acidity in a rural African populationS Afr Med J2003931078678814652973
- SammonAMProtease inhibitors and carcinoma of the esophagusCancer19988334054089690530
- MorganGDeleterious effects of prostaglandin E2 in oesophageal carcinogenesisMed Hypotheses19974821771819076700
- GuptaRATejadaLVTongBJCyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancerCancer Res200363590691112615701
- AkbariMRMalekzadehRShakeriRCandidate gene association study of esophageal squamous cell carcinoma in a high-risk region in IranCancer Res200969207994800019826048
- YuLWuWKLiZJLiHTWuYCChoCHProstaglandin E(2) promotes cell proliferation via protein kinase C/extracellular signal regulated kinase pathway-dependent induction of c-Myc expression in human esophageal squamous cell carcinoma cellsInt J Cancer2009125112540254619623651
- YangCSResearch on esophageal cancer in China: a reviewCancer Res1980408 Pt 1263326446992989
- KgomoMElnagarAAMokoenaTJeskeCNagelGJPrevalence of Helicobacter pylori infection in patients with squamous cell carcinoma of the oesophagus. A descriptive case series studyJ Gastrointest Cancer201647439639827237135
- KamangarFQiaoYLBlaserMJHelicobacter pylori and oesophageal and gastric cancers in a prospective study in ChinaBr J Cancer200796117217617179990
- SchäferGKabandaSvan RooyenBMarušičMBBanksLParkerMIThe role of inflammation in HPV infection of the oesophagusBMC Cancer201313118523570247
- KoshiolJWeiWQKreimerARNo role for human papilloma-virus in esophageal squamous cell carcinoma in ChinaInt J Cancer201012719310019918949
- WuMLiuAMKampmanEGreen tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case–control studyInt J Cancer200912481907191319123468
- WillemPBrownJSchoutenJA novel approach to simultaneously scan genes at fragile sitesBMC Cancer20066120516895604
- SteinCKGloverTWPalmerJLGlissonBSDirect correlation between FRA3B expression and cigarette smokingGenes Chromosomes Cancer200234333334012007194
- ThorlandECMyersSLGostoutBSSmithDICommon fragile sites are preferential targets for HPV16 integrations in cervical tumorsOncogene20032281225123712606949
- CapaccioPOttavianiFCuccariniVCenzualesSCesanaBMPignataroLAssociation between methylenetetrahydrofolate reductase polymorphisms, alcohol intake and oropharyngolaryngeal carcinoma in northern ItalyJ Laryngol Otol2005119537137615949101
- YokoyamaTSaitoKLwinHEpidemiological evidence that acetaldehyde plays a significant role in the development of decreased serum folate concentration and elevated mean corpuscular volume in alcohol drinkersAlcohol Clin Exp Res200529462263015834228
- KnasmüllerSBresgenNKassieFGenotoxic effects of three Fusarium mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and in primary cultures of rat hepatocytesMutat Res19973911–239489219547
- OderaJOOderaEGithang’aJEsophageal cancer in KenyaAm J Dig Dis (Madison)201743233329082268
- CheungWYLiuGGenetic variations in esophageal cancer risk and prognosisGastroenterol Clin North Am2009381759119327568
- HiyamaTYoshiharaMTanakaSChayamaKGenetic polymorphisms and esophageal cancer riskInt J Cancer200712181643165817674367
- VogelsangMWangYVeberNMwapaghaLMParkerMIThe cumulative effects of polymorphisms in the DNA mismatch repair genes and tobacco smoking in oesophageal cancer riskPLoS One201275e3696222623965
- CargillMAltshulerDIrelandJCharacterization of single-nucleotide polymorphisms in coding regions of human genesNat Genet199922323123810391209
- SinghMSMichaelMRole of xenobiotic metabolic enzymes in cancer epidemiologyMethods Mol Biol200947224326419107436
- LiskaDJThe detoxification enzyme systemsAltern Med Rev1998331871989630736
- WangLDZhengSLiuBZhouJXLiYJLiJXCYP1A1, GSTs and mEH polymorphisms and susceptibility to esophageal carcinoma: study of population from a high-incidence area in North ChinaWorld J Gastroenterol2003971394139712854128
- AbnetCCFreedmanNDHuNA shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinomaNat Genet201042976476720729852
- Lao-SirieixPCaldasCFitzgeraldRCGenetic predisposition to gas-tro-oesophageal cancerCurr Opin Genet Dev201020321021720347291
- NebertDWRoeALVandaleSEBinghamEOakleyGGNAD (P) H: quinone oxidoreductase (NQO1) polymorphism, exposure to benzene, and predisposition to disease: a huge reviewGenet Med200242627011882782
- KuehlBLPatersonJWPeacockJWPatersonMCRauthAMPresence of a heterozygous substitution and its relationship to DT-diaphorase activityBr J Cancer19957235555617669561
- KlaidmanLKLeungACAdamsJDHigh-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regionsAnal Biochem199522823123178572312
- RadjendiraneVJosephPLeeYHDisruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicityJ Biol Chem199827313738273899516435
- LongDJGaikwadAMultaniADisruption of the NAD(P) H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasiaCancer Res200262113030303612036909
- YanlingHYuhongZWenwuHLeiXMingwuCNQO1 C609T polymorphism and esophageal cancer risk: a huge review and meta-analysisBMC Med Genet20131413123497461
- SalaspuroMAcetaldehyde and gastric cancerJ Dig Dis2011122515921401890
- YangSJWangHYLiXQGenetic polymorphisms of ADH2 and ALDH2 association with esophageal cancer risk in southwest ChinaWorld J Gastroenterol200713435760576417963305
- LiQDLiHWangMSMulti-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng CountyBMC Gastroenterol2011117421672255
- LiDPDandaraCWaltherGParkerMIGenetic polymorphisms of alcohol metabolising enzymes: their role in susceptibility to oesophageal cancerClin Chem Lab Med200846332332818254707
- ByeHPrescottNJMatejcicMPopulation-specific genetic associations with oesophageal squamous cell carcinoma in South AfricaCarcinogenesis201132121855186121926110
- LiHBorinskayaSYoshimuraKRefined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variantAnn Hum Genet200973Pt 333534519456322
- WildingGThe importance of steroid hormones in prostate cancerCancer Surv1992141131301423327
- FerroPCatalanoMGRaineriMSomatic alterations of the androgen receptor CAG repeat in human colon cancer delineate a novel mutation pathway independent of microsatellite instabilityCancer Genet Cytogenet20001231354011120331
- HackenbergRSchulzKDAndrogen receptor mediated growth control of breast cancer and endometrial cancer modulated by antiandrogen-and androgen-like steroidsJ Steroid Biochem Mol Biol1996561–6 Spec1131178603031
- NagasueNYuLYukayaHKohnoHNakamuraTAndrogen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resectionBr J Surg19958245425477613907
- YamashitaYHiraiTMukaidaHDetection of androgen receptors in human esophageal cancerJpn J Surg19891921952022724718
- LubahnDJosephDSullivanPWillardHFrenchFWilsonECloning of human androgen receptor complementary DNA and localization to the X chromosomeScience198824048503273303353727
- QuigleyCADe BellisAMarschkeKBEl-AwadyMKWilsonEMFrenchFSAndrogen receptor defects: historical, clinical, and molecular perspectivesEndocr Rev19951632713217671849
- DehmSMTindallDJAndrogen receptor structural and functional elements: role and regulation in prostate cancerMol Endocrinol200721122855286317636035
- ClaessensFDenayerSVan TilborghNKerkhofsSHelsenCHaelensADiverse roles of androgen receptor (AR) domains in AR-mediated signalingNucl Recept Signal20086e00818612376
- EdwardsAHammondHAJinLCaskeyCTChakrabortyRGenetic variation at five trimeric and tetrameric tandem repeat loci in four human population groupsGenomics19921222412531740333
- JiangWZhangYJKahnSMAltered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancerProc Natl Acad Sci U S A19939019902690308415648
- YinJSangYZhengLUracil-DNA glycosylase (UNG) rs246079 G/A polymorphism is associated with decreased risk of esophageal cancer in a Chinese populationMed Oncol2014311127225301111
- Du PlessisLDietzschEVan GeleMMapping of novel regions of DNA gain and loss by comparative genomic hybridization in esophageal carcinoma in the black and colored populations of South AfricaCancer Res19995981877188310213495
- HuNRothMJPolymeropolousMIdentification of novel regions of allelic loss from a genomewide scan of esophageal squamous-cell carcinoma in a high-risk Chinese populationGenes Chromosomes Cancer200027321722810679910
- SleddensHFOostraBABrinkmannAOTrapmanJTrinucleotide (GGN) repeat polymorphism in the human androgen receptor (AR) geneHum Mol Genet1993244938504320
- HsingAWGaoYTWuGPolymorphic CAG and Ggn repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case–control study in ChinaCancer Res200060185111511611016637
- DietzschELaubscherRParkerMIEsophageal cancer risk in relation to GGC and CAG trinucleotide repeat lengths in the androgen receptor geneInt J Cancer20031071384512925954
- FerroPCatalanoMGDell’EvaRFortunatiNPfefferUThe androgen receptor CAG repeat: a modifier of carcinogenesis?Mol Cell Endocrinol20021931–210912012161010
- MarchewkaZPiwowarARuzikSDługoszAGlutathione S-transferases class Pi and Mi and their significance in oncologyPostepy Hig Med Dosw (Online)201771054155028665283
- HayesJDFlanaganJUJowseyIRGlutathione transferasesAnnu Rev Pharmacol Toxicol200545518815822171
- NelsonHHWienckeJKChristianiDCEthnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase thetaCarcinogenesis1995165124312467767992
- FryerAAThe glutathione S-transferases: influence of polymorphism on cancer susceptibilityIARC Sci Publ1999148231249
- HayesJDPulfordDJThe glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistanceCrit Rev Biochem Mol Biol19953064455208770536
- DuttonMFFumonisinsDMFFumonisins, mycotoxins of increasing importance: their nature and their effectsPharmacol Ther19967021371618843466
- OhashiSMiyamotoSKikuchiOGotoTAmanumaYMutoMRecent advances from basic and clinical studies of esophageal squamous cell carcinomaGastroenterology201514971700171526376349
- PearsonWRVorachekWRXuSJBergerRHartIVannaisDPattersonDIdentification of class-mu glutathione transferase genes GSTM1–GSTM5 on human chromosome 1p13Am J Hum Genet19935312202338317488
- CogganMWhitbreadLWhittingtonABoardPStructure and organization of the human theta-class glutathione S-transferase and d-dopachrome tautomerase gene complexBiochem J1998334Pt 36176239729470
- HeBPanYChoWCThe association between four genetic variants in microRNAs (rs11614913, rs2910164, rs3746444, rs2292832) and cancer risk: evidence from published studiesPLoS One2012711e4903223155448
- GeHCaoYYChenLQPTEN polymorphisms and the risk of esophageal carcinoma and gastric cardiac carcinoma in a high incidence region of ChinaDis Esophagus200821540941519125794
- SongJHMeltzerSJMicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancersGastroenterology20121431354722580099
- OgawaRIshiguroHKuwabaraYExpression profiling of micro-RNAs in human esophageal squamous cell carcinoma using RT-PCRMed Mol Morphol200942210210919536617
- YaoLZhangYZhuQDownregulation of microRNA-1 in esophageal squamous cell carcinoma correlates with an advanced clinical stage and its overexpression inhibits cell migration and invasionInt J Mol Med20153541033104125672418
- LiuRGuJJiangPDNMT1–microRNA126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via ADAM9-EGFR-AKT signalingClin Cancer Res201521485486325512445
- SuzukiHMaruyamaRYamamotoEKaiMDNA methylation and microRNA dysregulation in cancerMol Oncol20126656757822902148
- BurgerHMLombardMJShephardGSRheederJRvan der WesthuizenLGelderblomWCDietary fumonisin exposure in a rural population of South AfricaFood Chem Toxicol2010488–92103210820488220
- GuledMLahtiLLindholmPMCDKN2A, NF2, and Jun are dysregulated among other genes by miRNAs in malignant mesothelioma – a miRNA microarray analysisGenes Chromosomes Cancer200948761562319396864
- WangYVogelsangMSchäferGMatejcicMParkerMIMicroRNA polymorphisms and environmental smoke exposure as risk factors for oesophageal squamous cell carcinomaPLoS One2013810e7852024205249
- LinJHuangSWuSMicroRNA-423 promotes cell growth and regulates G(1)/S transition by targeting p21Cip1/Waf1 in hepatocellular carcinomaCarcinogenesis201132111641164721890460
- YeYWangKKGuJGenetic variations in microRNA-related genes are novel susceptibility loci for esophageal cancer riskCancer Prev Res (Phila)20081646046919138993
- GuoHWangKXiongGA functional variant in microRNA-146a is associated with risk of esophageal squamous cell carcinoma in Chinese HanFam Cancer20109459960320680470
- WeiJZhengLLiuSmiR-196a2 rs11614913 T > C polymorphism and risk of esophageal cancer in a Chinese populationHum Immunol20137491199120523792053
- YinJWangXZhengLHsa-miR-34b/c rs4938723 T>C and hsa-miR-423 rs6505162 C>A polymorphisms are associated with the risk of esophageal cancer in a Chinese populationPLoS One2013811e8057024260422
- YangHDinneyCPYeYZhuYGrossmanHBWuXEvaluation of genetic variants in microRNA-related genes and risk of bladder cancerCancer Res20086872530253718381463
- WeiQEicherSAGuanYReduced expression of hMLH1 and hGTBP/hMSH6: a risk factor for head and neck cancerCancer Epidemiol Biomarkers Prev1998743093149568786
- HiraoTNelsonHHAshokTDTobacco smoke-induced DNA damage and an early age of smoking initiation induce chromosome loss at 3p21 in lung cancerCancer Res200161261261511212258
- BaviPPBuRUddinSAl-KurayaKSMMP7 polymorphisms – a new tool in molecular pathology to understand esophageal cancerSaudi J Gastroenterol201117529930021912054
- LinDXTangYMPengQLuSXAmbrosoneCBKadlubarFFSusceptibility to esophageal cancer and genetic polymorphisms in glutathione S-transferases T1, P1, and M1 and cytochrome P450 2E1Cancer Epidemiol Biomarkers Prev1998711101310189829710
- SchraderCEVardoJStavnezerJRole for mismatch repair proteins MSH2, MLH1, and pms2 in immunoglobulin class switching shown by sequence analysis of recombination junctionsJ Exp Med2002195336737311828012
- BolandCRGoelAMicrosatellite instability in colorectal cancerGastroenterology2010138620732087e20420947
- KawakamiHZaananASinicropeFAMicrosatellite instability testing and its role in the management of colorectal cancerCurr Treat Options Oncol20151673026031544
- LiGMMechanisms and functions of DNA mismatch repairCell Res2008181859818157157
- NaidooRRamburanAReddiAChettyRAberrations in the mismatch repair genes and the clinical impact on oesophageal squamous carcinomas from a high incidence area in South AfricaJ Clin Pathol200558328128415735161
- UeharaHMiyamotoMKatoKDeficiency of hMLH1 and hMSH2 expression is a poor prognostic factor in esophageal squamous cell carcinomaJ Surg Oncol200592210911516231369
- VogelsangMPaccezJDSchäferGDzoboKZerbiniLFParkerMIAberrant methylation of the MSH3 promoter and distal enhancer in esophageal cancer patients exposed to first-hand tobacco smokeJ Cancer Res Clin Oncol2014140111825183324934723
- CampregherCSchmidGFerkFMSH3-deficiency initiates EMAST without oncogenic transformation of human colon epithelial cellsPLoS One2012711e5054123209772
- ParkJMHuangSTougeronDSinicropeFAMSH3 mismatch repair protein regulates sensitivity to cytotoxic drugs and a histone deacetylase inhibitor in human colon carcinoma cellsPLoS One201385e6536923724141
- TakahashiMKoiMBalaguerFBolandCRGoelAMSH3 mediates sensitization of colorectal cancer cells to cisplatin, oxaliplatin, and a poly(ADP-ribose) polymerase inhibitorJ Biol Chem201128614121571216521285347
- HuangJOkukaMLuWTelomere shortening and DNA damage of embryonic stem cells induced by cigarette smokeReprod Toxicol201335899522824788
- LingZQLiPGeMHHuFJFangXHDongZMMaoWMAberrant methylation of different DNA repair genes demonstrates distinct prognostic value for esophageal cancerDig Dis Sci201156102992300421674174
- WuDChenXXuYPrognostic value of MLH1 promoter methylation in male patients with esophageal squamous cell carcinomaOncol Lett20171342745275028454461
- GuengerichFPCytochromes P450, drugs, and diseasesMol Interv20033419420414993447
- PereraFPEnvironment and cancer: who are susceptible?Science19972785340106810739353182
- StrassburgCPStrassburgANguyenNLiQMannsMPTukeyRHRegulation and function of family 1 and family 2 UDP-glucuronosyl-transferase genes (UGT1A, UGT2B) in human oesophagusBiochem J1999382Pt 2489498
- AdamsCHWerelyCJVictorTCHoalEGRossouwGvan HeldenPDAllele frequencies for glutathione S-transferase and N-acetyltransferase 2 differ in African population groups and may be associated with oesophageal cancer or tuberculosis incidenceClin Chem Lab Med200341460060512747608
- DingXKaminskyLSHuman extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tractsAnnu Rev Pharmacol Toxicol20034314917312171978
- UmenoMMcBrideOWYangCSGelboinHVGonzalezFJHuman ethanol-inducible P450IIE1: complete gene sequence, promoter characterization, chromosome mapping, and cDNA-directed expressionBiochemistry19882725900690133233219
- DandaraCBalloRParkerMICYP3A5 genotypes and risk of oesophageal cancer in two South African populationsCancer Lett2005225227528215978331
- GellnerKEiseltRHustertEGenomic organization of the human CYP3A locus: identification of a new, inducible CYP3A genePharmacogenetics200111211112111266076
- LambaJKLinYSSchuetzEGThummelKEGenetic contribution to variable human CYP3A-mediated metabolismAdv Drug Deliv Rev200254101271129412406645
- RoyJNLajoieJZijenahLSCYP3A5 genetic polymorphisms in different ethnic populationsDrug Metab Dispos200533788488715833928
- LechevrelMCassonAGWolfCRHardieLJFlintermanMBMontesanoRWildCPCharacterization of cytochrome P450 expression in human oesophageal mucosaCarcinogenesis199920224324810069460
- MarasasWFDiscovery and occurrence of the fumonisins: a historical perspectiveEnviron Health Perspect2001109Suppl 2239243
- SpottiMMaasRFde NijsCMFink-GremmelsJEffect of fumonisin B(1) on rat hepatic P450 systemEnviron Toxicol Pharmacol20008319720410925073
- LöfdahlHELuYLagergrenJSex-specific risk factor profile in oesophageal adenocarcinomaBr J Cancer20089991506151018841152
- BodelonCAndersonGLRossingMAChlebowskiRTOchs-BalcomHMVaughanTLHormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal womenCancer Prev Res (Phila)20114684085021505180
- GallusSBosettiCFranceschiSOesophageal cancer in women: tobacco, alcohol, nutritional and hormonal factorsBr J Cancer200185334134511487262
- WangQMQiYJJiangQRelevance of serum estradiol and estrogen receptor beta expression from a high-incidence area for esophageal squamous cell carcinoma in ChinaMed Oncol201128118819320195802
- TihanTHarmonJWWanXEvidence of androgen receptor expression in squamous and adenocarcinoma of the esophagusAnticancer Res2001214b3107311411712819
- HannaIHDawlingSRoodiNGuengerichFPParlFFCytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activityCancer Res200060133440344410910054
- Ryan-HarshmanMAldooriWThe relevance of selenium to immunity, cancer, and infectious/inflammatory diseasesCan J Diet Pract Res20056629810215975198
- KocdorHCehreliRKocdorMAToxicity induced by the chemical carcinogen 7,12-dimethylbenz[a]anthracene and the protective effects of selenium in Wistar ratsJ Toxicol Environ Health A200568969370116020197
- LongtinRSelenium for prevention: eating your way to better DNA repair?J Natl Cancer Inst20039529810012529339
- ZhouNXiaoHLiTKDNA damage-mediated apoptosis induced by selenium compoundsJ Biol Chem200327832295322953712766154
- SmithMLLanciaJKMercerTIIpCSelenium compounds regulate p53 by common and distinctive mechanismsAnticancer Res2004243a1401140815274301
- SeoYRKelleyMRSmithMLSelenomethionine regulation of p53 by a ref1-dependent redox mechanismProc Natl Acad Sci U S A20029922145481455312357032
- Ali-OsmanFAkandeOAntounGMaoJXBuolamwiniJMolecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase pi gene variants. Evidence for differential catalytic activity of the encoded proteinsJ Biol Chem19972721510004100129092542
- BennettWPHollsteinMCMetcalfRAP53 mutation and protein accumulation during multistage human esophageal carcinogenesisCancer Res19925221609260971394236
- LeeJMLeeYCYangSYGenetic polymorphisms of p53 and GSTP1, but not NAT2, are associated with susceptibility to squamous-cell carcinoma of the esophagusInt J Cancer200089545846411008209
- MatlashewskiGJTuckSPimDLambPSchneiderJCrawfordLVPrimary structure polymorphism at amino acid residue 72 of human p53Mol Cell Biol1987729619633547088
- LuXMZhangYMLinRYP53 polymorphism in human papillomavirus-associated Kazakh’s esophageal cancer in Xinjiang, ChinaWorld J Gastroenterol200410192775277815334668
- LiXDumontPdella PietraAShetlerCMurphyMEThe codon 47 polymorphism in p53 is functionally significantJ Biol Chem200528025242452425115851479
- CaiLMuLNLuHDietary selenium intake and genetic polymorphisms of the GSTP1 and p53 genes on the risk of esophageal squamous cell carcinomaCancer Epidemiol Biomarkers Prev200615229430016492918
- ChenWSunKZhengRCancer incidence and mortality in China, 2014Chin J Cancer Res201830111229545714
- South African National Cancer RegistryCancer in South Africa2014 Available from: www.ncr.ac.zaAccessed October 15, 2018
- World Health OrganizationPrevalence of Tobacco SmokingGenevaWorld Health Organization2015 Available from: www.who.int/gho/tobacco/use/en/Accessed August 12, 2017
- World Health OrganizationGlobal Status Report on Alcohol and HealthGenevaWorld Health Organization2014 Available from: https://www.who.int/gho/tobacco/use/en/Accessed May 12, 2017
- Torres-AguileraMRemes TrocheJMAchalasia and esophageal cancer: risks and linksClin Exp Gastroenterol20181130931630233226
- ColemanHGXieSHLagergrenJThe epidemiology of esophageal adenocarcinomaGastroenterology2018154239040528780073
- AbnetCCArnoldMWeiWQEpidemiology of esophageal squamous cell carcinomaGastroenterology2018154236037328823862
- SinghSDevannaSEdakkanambeth VarayilJMuradMHIyerPGPhysical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: a systematic review and meta-analysisBMC Gastroenterol20141410124886123
- ErőssBFarkasNVinczeÁHelicobacter pylori infection reduces the risk of Barrett’s esophagus: a meta-analysis and systematic reviewHelicobacter2018234e1250429938864
- XieFJZhangYPZhengQQHelicobacter pylori infection and esophageal cancer risk: an updated meta-analysisWorld J Gastroenterol201319366098610724106412
- BrusselaersNMaret-OudaJKoningsPEl-SeragHBLagergrenJMenopausal hormone therapy and the risk of esophageal and gastric cancerInt J Cancer201714071693169928006838
- LiPChengRZhangSAspirin and esophageal squamous cell carcinoma: bedside to benchChin Med J (Engl)201412771365136924709195
- QuYZhangSCuiLTwo novel polymorphisms in PLCE1 are associated with the susceptibility to esophageal squamous cell carcinoma in Chinese populationDis Esophagus201730117
- HuHYangJSunYPutatively functional PLCE1 variants and susceptibility to esophageal squamous cell carcinoma (EscC): a case–control study in eastern Chinese populationsAnn Surg Oncol20121972403241022203178
- ByeHPrescottNJLewisCMDistinct genetic association at the PLCE1 locus with oesophageal squamous cell carcinoma in the South African populationCarcinogenesis201233112155216122865593
- WangLTangWChenSN-acetyltransferase 2 polymorphisms and risk of esophageal cancer in a Chinese populationPLoS One201492e8778324586291
- MatejcicMVogelsangMWangYIqbal ParkerMParkerIMNAT1 and NAT2 genetic polymorphisms and environmental exposure as risk factors for oesophageal squamous cell carcinoma: a case–control studyBMC Cancer20151515025886288
- AbnetCCWangZSongXGenotypic variants at 2q33 and risk of esophageal squamous cell carcinoma in China: a meta-analysis of genome-wide association studiesHum Mol Genet20122192132214122323360
- HaoXDYangYSongXCorrelation of telomere length shortening with TP53 somatic mutations, polymorphisms and allelic loss in breast tumors and esophageal cancerOncol Rep201329122623623124483
- ZhouLYuanQYangMA functional germline variant in the p53 polyadenylation signal and risk of esophageal squamous cell carcinomaGene2012506229529722800615
- VosMAdamsCHVictorTCvan HeldenPDPolymorphisms and mutations found in the regions flanking exons 5 to 8 of the TP53 gene in a population at high risk for esophageal cancer in South AfricaCancer Genet Cytogenet20031401233012550754
- GaoYHeYXuJGenetic variants at 4q21, 4q23 and 12q24 are associated with esophageal squamous cell carcinoma risk in a Chinese populationHum Genet2013132664965623430454
- LiuPZhaoHRLiFCorrelations of ALDH2 rs671 and C12orf30 rs4767364 polymorphisms with increased risk and prognosis of esophageal squamous cell carcinoma in the Kazak and Han populations in Xinjiang ProvinceJ Clin Lab Anal2018322e22248
- GuoYMWangQLiuYZChenHMQiZGuoQHGenetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese malesWorld J Gastroenterol20081491444144918322963
- TownsendDMTewKDTapieroHThe importance of glutathione in human diseaseBiomed Pharmacother2003573–414515512818476
- LiDDandaraCParkerMIThe 341C/T polymorphism in the GSTP1 gene is associated with increased risk of oesophageal cancerBMC Genet20101114720540773
- MatejcicMLiDPrescottNJLewisCMMathewCGParkerMIAssociation of a deletion of GSTT2B with an altered risk of oesophageal squamous cell carcinoma in a South African population: a case–control studyPLoS One2011612e2936622216261
- GaoCMTakezakiTWuJZGlutathione-S-transferases M1 (GSTM1) and GSTT1 genotype, smoking, consumption of alcohol and tea and risk of esophageal and stomach cancers: a case–control study of a high-incidence area in Jiangsu Province, ChinaCancer Lett20021881–29510212406553
- DandaraCLiDPWaltherGParkerMIGene–environment interaction: the role of SULT1A1 and CYP3A5 polymorphisms as risk modifiers for squamous cell carcinoma of the oesophagusCarcinogenesis200627479179716272171
- BoltHMThierRRelevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicologyCurr Drug Metab20067661362816918316
- ZhaoYMarottaMEichlerEEEngCTanakaHLinkage disequilibrium between two high-frequency deletion polymorphisms: implications for association studies involving the glutathione-S transferase (GST) genesPLoS Genet200955e100047219424424
- LiTSuoQHeDEsophageal cancer risk is associated with polymorphisms of DNA repair genes MSH2 and WRN in Chinese populationJ Thorac Oncol20127244845222173703
- HayashiSWatanabeJKawajiriKGenetic polymorphisms in the 5’-flanking region change transcriptional regulation of the human cytochrome P450IIE1 geneJ Biochem199111045595651778977
- LuXMZhangYMLinRYRelationship between genetic polymorphisms of metabolizing enzymes CYP2E1, GSTM1 and Kazakh’s esophageal squamous cell cancer in Xinjiang, ChinaWorld J Gastroenterol200511243651365415968714
- ZhengHZhaoYAssociation of CYP1A1 MspI polymorphism in the esophageal cancer risk: a meta-analysis in the Chinese populationEur J Med Res20152014625886559
