Abstract
The health and performance of the gastrointestinal tract is influenced by the interaction of a variety of factors, including diet, nutritional status, genetics, environment, stress, the intestinal microbiota, immune status, and gut barrier. Disruptions in one or more of these factors can lead to enteropathy or intestinal disorders that are known to occur in concert with certain disease states or conditions such as irritable bowel syndrome or human immunodeficiency virus (HIV) infection. Nutritional support in the form of a medical food along with current therapies could help manage the adverse effects of enteropathy, which include effects on nutrient digestion, absorption, and metabolism, as well as utilization of nutrients from foodstuffs. Numerous studies have demonstrated that oral administration of plasma- or serum-derived protein concentrates containing high levels of immunoglobulins can improve weight management, normalize gut barrier function, and reduce the severity of enteropathy in animals. Recent trials in humans provide preliminary evidence that a serum-derived bovine immunoglobulin/protein isolate is safe and improves symptoms, nutritional status, and various biomarkers associated with enteropathy in patients with HIV infection or diarrhea-predominant irritable bowel syndrome. This review summarizes data from preclinical and clinical studies with immunoglobulin-containing plasma/serum protein concentrates, with a focus on the postulated mode of action of serum-derived bovine immunoglobulin/protein isolate for patients with enteropathy.
Introduction
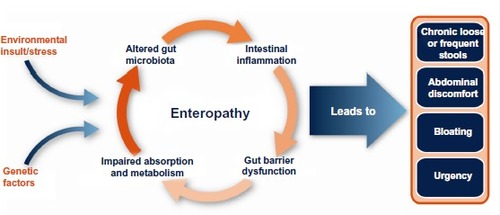
Enteropathy is frequently found in association with several human disease conditions, including irritable bowel syndrome (IBS) or human immunodeficiency virus (HIV) infection, and is caused by pathological changes in the lining of the intestinal tract. Such changes disrupt the homeostasis of the gastrointestinal (GI) tract and lead to symptoms of abdominal pain and discomfort, bloating, and abnormal bowel function (eg, diarrhea, urgency, constipation). While the pathophysiological mechanisms that lead to enteropathy are not well understood, there is a developing body of evidence to suggest the involvement of genetic predispositions, diet, stress, and exposure to external antigens, toxins, or environmental insults (including infection). Combinations of these trigger factors lead to a continuing cycle of altered gut microbiota, immune dysregulation, gut barrier dysfunction with permeability changes, and nutrient malabsorption, which serves to further amplify and prolong this cycle of events (). Oral administration of bovine immunoglobulin (Ig)-containing protein preparations has been shown to improve weight gainCitation1 and gut barrier function and permeabilityCitation2,Citation3 and to reduce the severity of enteropathy in animals.Citation4–Citation6 Serum-derived bovine protein isolate (SBI) is specially formulated to increase Ig levels and contains >90% protein, over 50% of which is IgG. Recent studies in humans demonstrate that SBI is safe and improves the nutritional status and GI symptoms (eg, chronic loose and frequent stools, abdominal discomfort, bloating, urgency) in patients with enteropathy associated with diarrhea-predominant IBS (IBS-D) or HIV infection.Citation7,Citation8 Other studies have provided evidence that SBI supports digestive and absorptive properties of the intestinal tract by: binding and neutralizing microbial components;Citation9–Citation11 helping to maintain a beneficial gut microbiota;Citation12 managing gut barrier function;Citation13–Citation15 and maintaining GI immune balance.Citation4,Citation5,Citation7,Citation13 While these effects have been observed in both nonclinical (in vitro, animal) and clinical studies, the significance of the observations from nonclinical studies to humans is not known.
Figure 1 Factors involved in the pathogenesis of enteropathy associated with various human disease states or conditions (eg, diarrhea-predominant irritable bowel syndrome or human immunodeficiency virus infection).
A commercial form of SBI (EnteraGam™; Entera Health, Inc., Cary, NC, USA) is available as a prescription medical food that is indicated for the clinical dietary management of enteropathy under physician supervision for patients who, because of therapeutic or chronic medical needs, have limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients. It is indicated in patients with chronic loose and frequent stools based on findings from clinical studies in patients with IBS-D or HIV-associated enteropathy.Citation7,Citation8 Although the exact mechanism(s) responsible for providing the benefits of SBI in patients with chronic loose and frequent stools (eg, IBS-D, HIV-associated enteropathy) are not well understood, findings from a number of nonclinical and clinical studiesCitation1,Citation7,Citation8,Citation33–Citation36 show that SBI provides the following nutritive benefits: improves the uptake and utilization of nutrients; increases lean body mass through increased utilization and decreased catabolism of protein; and decreases fecal fat and energy loss. The overall mechanisms that contribute to these nutritive benefits of SBI that help provide nutritional support for the management of enteropathy will be discussed in this review.
Etiology of enteropathy
The cycle of events that contribute to the prolonged nature of enteropathy (ie, altered gut microbiota, immune activation, gut barrier dysfunction, nutrient malabsorption) also occur in patients with IBS-D or HIV-associated enteropathy. While the understanding of the pathogenesis of IBS-D is still incomplete, a variety of factors have been implicated, including genetic susceptibility, exposure to environmental toxins or pathogens, deficiencies in tight junction proteins, intestinal abnormalities with bile acid metabolism, changes in GI motility, visceral hypersensitivity, and psychosocial factors.Citation16–Citation18 Recent studies into the pathogenesis of IBS have also focused on alterations of small bowel and colonic microflora, inflammation, changes in tryptophan metabolism, and dysregulation of the interaction between the central and enteric nervous system (brain–gut axis).Citation17,Citation19,Citation20 Enteropathy associated with HIV infection is likely related to direct infection of enterocytes by HIV, opportunistic infections or other intestinal dysbiosis, or host response to highly active antiretroviral therapy (HAART). HIV enteropathy has long been associated with inflammatory damage, decreased barrier function, increased permeability, and malabsorption of nutrients.Citation21,Citation22 Altered tryptophan catabolism to kynurenine and intestinal dysbiosis has also been demonstrated in HIV patients.Citation23 It is well known that inflammation or other aberrant immune responses can lead to changes in intestinal structure and functionCitation24 and may play a central role in enteropathy. The complex etiology of IBS and HIV enteropathy is one reason why no single therapy has proven effective in managing all symptoms associated with these conditions.
Impact of SBI on the nutritional requirements of enteropathy
The homeostasis of the GI tract, and occurrence of various intestinal disorders or enteropathies, is impacted by epigenetics, diet, stress, or exposure to external antigens or other environmental insults. There are dozens of mutated or dysregulated genes, for example, which have been implicated in IBS, a disorder that is present in 10%–20% of the population.Citation25 These mutations can affect intestinal permeability, metabolism of tryptophan,Citation19 and the synthesis and metabolism of bile acids,Citation26 which may result in imbalances of neurotransmitters and alterations in motility. Modifications in tryptophan, serotonin, and bile acid metabolism, as well as alterations in the host microbiome, have been implicated in causing or exacerbating many of the symptoms endured by patients with HIV or IBS-D.Citation16,Citation18–Citation20,Citation27 Besides the potential genetic contribution, alterations of the microbiota and gut permeability can limit or impair digestive and absorptive function, leading to changes in fluid balance, vitamin production and absorption, and maldigestion of carbohydrates and fats. Malabsorption of key micronutrients (eg, vitamins and minerals) and macronutrients (eg, protein, carbohydrate, fat) during chronic diarrhea can lead to malnutrition or chronic undernutrition and play a central role in patients with enteropathy.
It is widely recognized that colostrum and breast milk, the sole source of nutrition for the neonate, contain Igs and other proteins which, along with early exposure to external antigens and bacteria, play a critical role in establishing the intestinal microbiota, normal immune function and integrity of the gut barrier, and may provide anti-inflammatory effects.Citation28–Citation31 Breast milk is therefore considered to contain protective nutrients.Citation32 Studies evaluating specially formulated bovine Ig preparations have provided evidence of a similar role for Igs in being a protective nutrient by helping to restore gut homeostasis in enteropathy ().
Table 1 Effects of dietary administration with PPC or SBI on growth and measures of intestinal function from representative preclinical and clinical studies
Clinical studies
The impact of SBI on markers of intestinal absorption, GI symptom scores, and quality of life measures have been evaluated in two clinical studiesCitation7,Citation8 involving patients with HIV-associated enteropathy or IBS-D. In an open-label study by Asmuth et al,Citation7 eight subjects with HIV-associated enteropathy showed improvements in GI symptoms with reduced bowel movements per day (P<0.008) and improvements in stool consistency (P<0.008). Seven of the eight subjects also showed increased uptake of D-xylose, suggesting improved absorption of nutrients. Another randomized, double-blind, placebo-controlled study was conducted in subjects with IBS-DCitation8 to investigate the impact of SBI on improving GI symptom scores and quality of life. Study subjects consuming SBI reported a significant decrease in the number of days with GI symptoms (eg, abdominal pain, flatulence, bloating, urgency, loose stools), suggesting improved GI function with implications for nutritive benefits. The placebo, soy protein at the same daily dose, did not significantly decrease the number of days of any GI symptoms. These results demonstrate that SBI, a specially formulated bovine-IgG preparation, provides for a distinct nutritional requirement in enteropathy patients who have a limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, and those with fluid imbalance due to chronic loose and frequent stools (ie, those with IBS-D and HIV-associated enteropathy).
Earlier studies also evaluated the safety, acceptability, and digestibility of specially prepared bovine Ig preparations in children. In one randomized and controlled, community-based intervention study, the effects of dietary supplementation with bovine serum concentrate (22% IgG, weight/weight) and/or multiple micronutrients on growth, morbidity, and micronutrient status were evaluated in healthy children.Citation33 A total of 259 children were enrolled at 6 to 7 months of age and randomized to receive one of four study products daily for up to 8 months: whey protein concentrate (control group), bovine serum concentrate, whey protein concentrate plus multiple micronutrients, or bovine serum concentrate plus multiple micronutrients. One hundred and thirty-two children (51.0%) finished the full 8 months of observation during the study. There were no significant differences reported in the rate of dropouts between treatment groups during the study, and the rate of early dropouts did not correlate with which study product was consumed or prevalence/incidence of morbidity. While not significant, trends were observed for improvements in weight gain and lean body mass.Citation33
In another study, the acceptability, safety, and digestibility of spray-dried bovine plasma proteins was evaluated in young Peruvian children (9–25 months of age) recovering from severe protein-energy malnutrition.Citation34 A control diet prepared from rice, milk, vegetable oil, and sugar was compared to two study diets in which a bovine plasma protein mixture replaced either 25% or 50% of the milk protein in the control diet. Fractional absorption of dietary lipid and of total energy increased significantly in relation to the amount of SBI in the diet, as shown by decreased fecal fat and energy content. Diets containing greater amounts of plasma protein mixture led to progressive reductions in wet and dry stool weights and greater fat absorption compared to the control diet, leading the authors to suggest that the plasma protein mixtures were more digestible or enhanced recovery from malnutrition. There was also a trend toward superior nitrogen and carbohydrate absorption with increasing amounts of plasma protein. All children accepted each of the study diets and no product-related intolerances or side effects were noted in this trial.Citation34
Preclinical studies
An accumulating body of preclinical studies corroborates the clinical findings with regard to addressing a distinct dietary need for Igs in restoring homeostasis during states of enteropathy. A review of 75 preclinical studies in 43 different publications documents the nutritive benefits of the bovine Ig-containing protein preparations in terms of improving feed intake, growth, and sometimes feed conversion.Citation1 Replacement of several high-quality protein sources (eg, meat extracts, soy, pea and potato protein isolates, skimmed milk, whey protein, fishmeal) with Ig protein mixtures similar to the composition of SBI led to superior weight gain and feed intake in weaned piglets, suggesting that proteins unique to SBI were involved in stimulating beneficial digestive and metabolic effects.Citation1 Jiang et al,Citation35 for example, evaluated growth performance after pair-feeding early-weaned piglets a diet containing either soy protein or the Ig-containing plasma protein composition for 24 days. While protein intake was similar among groups, the rate of weight gain and protein conversion efficiency was significantly higher in the Ig/plasma protein group, particularly during the first 8 days of the early weaning period. Pigs fed the plasma protein mixture had a larger carcass weight and absolute mass of protein with no difference in fat mass, suggesting a higher efficiency of dietary protein utilization for lean tissue growth. In addition, pigs fed the Ig/plasma protein mixture showed reduced circulating levels of urea, arginine, citrulline, and ornithine, suggesting a reduction in the catabolism of amino acids to urea and increased availability of dietary amino acids for lean tissue mass.Citation35 Pierce et alCitation36 conducted several studies to evaluate the growth and feed intake of early-weaned piglets fed porcine plasma, bovine plasma, or an IgG protein fraction of the plasma preparations. The results showed that both porcine and bovine plasma protein preparations enhanced the growth rate and feed intake of early-weaned piglets, and that the IgG fraction from both plasma sources was the component responsible for the enhancement in growth performance.
A number of other studies have also evaluated the effects of orally administered bovine Ig preparations on improving intestinal digestive and absorptive capacity in piglets undergoing early weaning, a condition known to induce impairment in intestinal epithelial barrier function, as well as experimental models of intestinal inflammation. These studies are summarized below under sections describing the potential mechanisms involved in the benefits of SBI in enteropathy. Taken together, results from these clinical and nonclinical studies reveal a distinct dietary requirement for Igs for the purpose of nutritional support in maintaining homeostasis to the disrupted gut environment in enteropathy. This nutritional requirement parallels the role of Igs in neonatal nutrition for establishing the gut environment and homeostasis.
Mechanisms of action
The growing body of evidence from both preclinical and clinical studies suggests that Ig-containing protein preparations, such as that found in SBI, provide for a distinct nutritional requirement in patients with enteropathy that cannot be provided by other dietary protein sources. While the mechanisms that are involved are not completely known, evidence suggests that the specially formulated preparation of bovine serum Igs in SBI plays a key role in maintaining homeostasis in appropriate areas of the GI tract, which manages enteropathy in patients with IBS-D or HIV infection. Specifically, these Igs and other plasma proteins may provide nutritive benefits by supporting normal intestinal digestion, absorption, and metabolism of ordinary foodstuffs by:
binding and neutralizing endotoxins and other microbial components;
promoting a stable microbiota (which may aid in digestion and generation of specific nutrients);
managing gut barrier function (to improve the uptake and utilization of nutrients); and
maintaining a homeostatic immune balance in GI mucosa.
Digestibility
It is generally assumed that the Ig and protein content in SBI must first survive digestion in the upper GI tract to assert its effects in the management of enteropathy. Nonclinical studies have found that as much as 50% of IgG from SBI survives transit through the stomach, while 5%–10% survives during transit through the entire intestinal tract.Citation37,Citation38 In a human study of SBI tolerability and digestion, intact bovine IgG was detected in the feces of volunteers but not serum (n=12), providing evidence that bovine Ig is not absorbed from the intestinal lumen into the circulation (Hanning RM, Drew M [Bovine Immunoglobulin Feeding Trial, 1994], data on file). Elevated plasma levels of total amino acids and leucine were observed 1–2 hours after SBI administration, suggesting at least partial digestion of the protein mixture to the amino acid level during transit through the intestine. Survival of the bovine IgG fraction through the GI tract suggests that oral SBI remains biologically active to impart benefits in conditions such as IBS-D and HIV-associated enteropathy.
Binding endotoxin and other microbial factors
SBI is prepared from plasma obtained from hundreds of animal donors and, therefore, contains Igs directed against a wide array of pathogens and foreign antigens. This includes Igs capable of binding highly conserved antigens that may allow direct binding to microbial pathogens or their toxins,Citation9–Citation11 with possible downstream benefits. The fragment antigen-binding (FAb) regions of IgG recognize antigenic targets and provide diversity to antibodies, while the fragment crystalizable (Fc) region interacts with Fc gamma receptors on certain immune cells to enhance phagocytic activity by macrophages, monocytes, and polymorphonuclear neutrophils. Our hypothesis is that oral Igs provide benefits by binding highly conserved microbial antigens known as pathogen-associated molecular patterns (PAMPs), such as endotoxins or peptidoglycans, which may 1) influence the composition or metabolism of the intestinal microbiota, and/or 2) interfere with the ability of such microbial compounds to enter or damage epithelial cells or immune cells, thereby supporting intestinal homeostasis. For example, under normal conditions PAMP binding to pattern recognition receptors (PRRs) on cells of the innate immune system initiates downstream signaling cascades that culminate in the production of proinflammatory cytokines, inflammation, and subsequent antigen elimination. Continual PAMP binding can lead to a persistent state of inflammation associated with numerous chronic inflammatory disorders such as IBS, inflammatory bowel disease (IBD), and HIV enteropathy. Studies have shown that the IgG, IgA, and IgM contained in SBI bind to bacterial endotoxins and a wide array of other bacterial, viral, and fungal PAMPs.Citation9–Citation11 It is possible that Ig binding of endotoxins and other PAMPs may interfere with PRR interactions with PAMPS, thereby inhibiting PRR signaling of inflammation and reducing the manifestations of chronic inflammatory GI disorders.
Effects on gut microbiota
The microbial populations that reside in the human intestinal tract contribute nutrients and energy to the host via fermentation of nondigestible dietary components and influence many aspects of health, including intestinal cell proliferation and maturation, the maintenance of the immune system, and formation of metabolites with beneficial or adverse health effects. In contrast, certain components of the gut microbiota may have negative consequences, serving as the source of infection, inflammation, or involvement in GI or systemic disease. Intestinal bacteria also produce a variety of molecules or substances (eg, cell wall endotoxins, peptidoglycan, other toxins), which are known to affect the gut barrier and tight junction permeability, induce inflammation in the gut, and change nutrient absorption and fluid retention. Diet is one of the major determinants for the persistence of a given bacterium in the GI tract and, therefore, may influence the composition and activity of the human gut microbiota with implications for host GI function and health.
Igs may impart the nutritive benefits of SBI by impacting the growth and maintenance of the normal intestinal microbiota. Igs in SBI are directed against a wide array of foreign antigens and microbial organisms, due in part to genetic mechanisms that support broad diversity in Ig production and because SBI is prepared from plasma obtained from thousands of bovine donors. This would include Igs like IgG with activity directed against highly conserved microbial antigens that allows direct binding to microbial cell wall or other microbial components to interfere with their ability to enter or damage intestinal epithelial cells.
The effect of SBI on restoring imbalances of microbiota has also been studied in patients with HIV-associated enteropathy.Citation12,Citation39 In a study evaluating SBI in patients with HIV-associated enteropathy, Firmicutes and Bacteriodales were the dominant phyla found in stool samples from all eight patients.Citation12 When SBI was administered, proinflammatory Gammaproteobacteria tended to decrease from levels of 0.70% to 0.12%. Clostridium spp. tended to decrease from 6.5% to 3.4% in the stool and correlated with duodenal cluster of differentiation (CD)3+/CD4+ density (r=−0.63; P<0.01). Ruminococcus spp. and the Bacteroidetes/Firmicutes ratio also increased in six of eight subjects, which have been implicated in contributing to better calorie utilization from the diet.Citation40,Citation41 These results suggest that some component in SBI, perhaps the IgG fraction, may be effective in normalizing gut bacteria with potential implications for improving nutrient utilization. Clostridium and Ruminococcus decreased in seven of eight patients.Citation12
Barrier function effects
The intestinal barrier separates the antigen-rich lumen from the underlying lamina propria that contains immune and other host cells. The functionality of this barrier is attributed to a monolayer of epithelial cells linked by tight junctions forming an efficient polarized barrier. Damage to the intestinal barrier compromises its ability to prevent antigen-induced inflammation at the site of increased permeability. An aberrant immune response can lead to persistent inflammation and altered intestinal barrier function due to changes in the epithelial cells and the tight junction complexes. Nonclinical studiesCitation3,Citation13,Citation14,Citation15 have demonstrated that serum proteins, similar to those contained in SBI, can positively affect intestinal barrier function and nutrient absorption through changes in barrier permeability and tight junction protein expression.
The selective permeability of the intestinal barrier is paramount to prevention of luminal antigen-induced inflammation. Administration of Staphylococcus aureus enterotoxin B (SEB) in a weaned rat model resulted in significantly increased permeability of the intestinal barrier, as measured by increased flux of tracer molecules such as fluorescein isothiocyanate-dextran (molecular weight [MW] of 4 kD) or horseradish peroxidase ([HRP] MW of 40 kD), having similar MW to common antigenic food proteins.Citation14 The addition of bovine serum proteins or porcine Ig concentrate to the diet of weaned rats was shown to ameliorate the increased intestinal HRP and dextran fluxes associated with SEB challenge in the rat model. By measurement of two tight junction proteins, zonulin-1 and β-catenin, the authors concluded that the increased dextran and HRP flux across the intestinal barrier in SEB-treated animals was due to a relaxation of the tight junctions due to differential protein expression.Citation14 While the study diets did not alter tight junction expression, additional investigations (Detzel, unpublished data, 2013) suggests antigen binding by IgG provides steric barriers to translocation across damaged tight junctions.
Investigations by Detzel et al,Citation15 using an in vitro co-culture model of the gut epithelium and lamina propria, have shown that antigen flux across C2BBE1 epithelial cell monolayers is limited by incubation of antigen with bovine IgG. The co-culture model utilizes a C2BBE1 monolayer of cells cultured on the base of a permeable upper compartment that is separated from a lower compartment (HTS-Transwell® Culture System, Corning, Inc., Acton, MA, USA) seeded with THP-1 monocytes, designed to simulate the gut epithelium and immune-reactive cells of the lamina propria. C2BBE1 monolayers cultured for 21 days are impermeable to the antigen PAM3CSK4, a synthetic triacylated lipopeptide that mimics the amino terminus of bacterial lipopeptides. Damage of the C2BBE1 monolayer in the upper compartment allows the PAM3CSK4 to transverse the epithelial barrier and stimulate production of the proinflammatory cytokine interleukin (IL)-8 by THP-1 monocytes. Co-addition of SBI with PAM3CSK4 prevents antigen translocation (flux) across the C2BBE1 epithelial membrane. Preliminary results using this in vitro model demonstrate that IgG present in SBI binds antigen and prevents translocation across the epithelial membrane, suggesting that the overall size of the antigen/IgG complex may exceed the limits for passage through damaged tight junctions.Citation15
Another study using animal models and ex vivo test systems have evaluated tight junction protein expression in the epithelial barriers of early-weaned piglets fed diets containing porcine plasma with high levels of Igs.Citation13 Following the testing period of 7 or 14 days post-weaning, when piglets were fed a controlled diet containing Ig protein preparations, segments of the ileum and proximal colon were harvested and analyzed for permeability and expression of the tight junction protein claudin-1. Intestinal barrier function was shown to be improved in piglets fed diets containing porcine plasma as indicated by an increased transepithelial electrical resistance and significant reductions in colonic 14C-inulin permeability on day 7 post-weaning and reduced ileal permeability of 3H-mannitol and 14C-inulin on day 14.Citation13 Immunofluorescence staining demonstrated that claudin-1 was more highly expressed and localized to tight junctions in animals fed Ig-containing porcine plasma compared with the diffuse low-signal staining observed in control tissues at 7 days post-weaning.Citation13
Together, these studiesCitation3,Citation13–Citation15 demonstrate that serum proteins similar to those contained in SBI, including Igs, can manage the negative effects of antigen/toxin challenge on the intestinal epithelial barrier. The proteins contained in SBI have been shown to directly alter the permeability of the intestinal barrier, prevent antigen translocation across damaged tight junctions via direct binding and steric hindrance, and have an impact on tight junction protein expression.Citation13–Citation15 While increased permeability can be the direct result of environmental challenge of the intestinal epithelium, the protective effect of SBI proteins on immune cell migration, cytokine production, and the host microbiota may be equally important in determining if chronic immune activation and persistent increased intestinal barrier permeability result.
Effects on immune balance
The exact etiology of IBS-D is not entirely understood, but immune activation with inflammation is recognized as an important contributor to symptoms in these patients.Citation42–Citation44 For example, inflammation following GI infection is a well-recognized initiating factor in IBS.Citation45 Intestinal inflammation can alter the gut barrier and lead to increased epithelial permeability. Using immunohistochemistry staining, patients with IBS-D were shown to have an increased number and activation of mucosal mast cells in the lamina propria compared to control subjects without IBS-D.Citation42,Citation44 In addition, some studies showed dysregulation of tight junction proteins leading to increased intestinal permeability.Citation43,Citation44 Inflammatory responses are mediated by various cytokines such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α.Citation46 Other cytokines, such as interferon-γ, IL-12, and IL-18 affect the production and cellular response to IL-1β and TNF-α. In models of inflammation where several cytokines are produced, specific blockade of IL-1β and/or TNF-α results in a reduction in the severity of the inflammation.Citation46
Addition of SBI to diets has been shown to reduce the expression of proinflammatory cytokines (eg, TNF-α, IL-6) and alter the lymphocyte response of immune challenge in weaned piglets,Citation13 as well as in experimental models of intestinal inflammation in mice,Citation47,Citation48 rats,Citation4,Citation49 and pigs.Citation5 In an initial clinical trial in patients with HIV-associated enteropathy, SBI significantly increased mucosal CD4+ lymphocyte densities over 8 weeks, but had no effect on circulating CD4+ counts.Citation7 In addition, a marker for enterocyte damage, intestinal fatty acid protein, initially rose in seven of eight subjects after 8 weeks, but then fell below baseline in four of five subjects who continued taking SBI for 40 additional weeks, suggesting that inflammation-based destruction of enterocytes had been ameliorated. Monocyte chemoattractant protein-1 levels were also negatively correlated with lamina propria CD4+ density, suggesting a commonality of systemic inflammation and mucosal immunity. In addition, inflammation-induced tissue remodeling matrix metalloproteinases decreased over time, also suggesting a dampening of inflammation and tissue-specific remodeling in the intestine.Citation7 Collectively, data from these studies support the hypothesisCitation4,Citation5,Citation7,Citation13,Citation47–Citation49 that oral SBI can play a role in restoring GI immune balance.
Summary
The potential mechanisms of action for SBI can be summarized as follows:
SBI consists of >90% protein, over 50% of which is IgG that appears to survive digestion in the stomach and upper intestine.
SBI is a protective nutrient, which supports digestive and absorptive properties of the intestinal tract by binding and neutralizing microbial components, which helps to maintain a beneficial gut microbiota, manage gut barrier function, and maintain immune balance. While these effects have been observed in both nonclinical (in vitro, animal) and clinical studies, the significance of the observations from nonclinical studies to humans is not known.
SBI has been shown in preclinical studies to improve intestinal barrier function with associated decreases in epithelial permeability.
SBI has been shown to help maintain a GI immune balance in the lamina propria.
Nutritive effects of SBI include improved uptake and utilization of nutrients as shown in both preclinical and clinical studies that demonstrate: 1) increases in lean body mass; 2) increased utilization and decreased catabolism of protein; and 3) decreased fecal fat and energy loss.
Conclusion
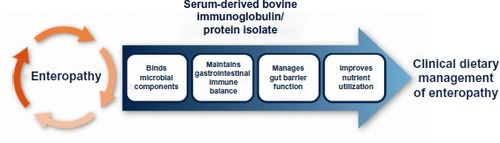
Most therapies that are currently used in the management of patients with enteropathy, such as dietary changes, probiotics, or use of medications to modify gut motility, are aimed at lessening symptoms rather than managing the underlying causes of the disorder. This reality may be due to the complexity of pathophysiological mechanisms involved in enteropathy, which may serve to explain why no one treatment has been shown to be effective in patients with enteropathy. There is a need for a safe nutritional therapy in addition to current treatments that can potentially address the underlying etiology involved in the various patient types. SBI is uniquely formulated and provides a distinct nutrient with a multifaceted mode of action that involves binding and neutralization of microbial components, which helps to maintain a beneficial gut microbiota, manage gut barrier function, and maintain immune balance (). These effects collectively serve to improve and maintain nutrient utilization to aid in the management of enteropathy in IBS-D and HIV-infected patients.
Figure 2 Proposed mechanism of action for serum-derived bovine immunoglobulin/protein isolates to aid in the management of enteropathy.
The multifaceted mechanisms described for SBI may help explain the results from current published clinical trials. In one randomized, placebo-controlled, 6-week clinical trial, for example, subjects who received SBI at 10 g/day had statistically significant, within-group reductions in abdominal pain, loose stools, bloating, flatulence, urgency, and any symptom reported in the daily diary.Citation8 Subjects receiving 5 g/day of SBI in the same study had statistically significant within-group reductions in days with flatulence, incomplete evacuation, and any symptom reported in the daily diary. Subjects who were administered an equivalent level of soy protein showed no statistically significant, within-group reductions in any symptom. Similar results were seen in HIV-associated enteropathy, a debilitating diarrheal condition, in which there was reduction of chronic loose and frequent stools over 8 weeks to normal consistency and frequency with sustained management out to 9 months.Citation7
These studies indicate that SBI provides for a distinct nutritional requirement in patients who, as a result of their condition, do not adequately ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, or experience excessive water loss due to chronic loose and frequent stools, in such conditions as IBS-D and HIV-associated enteropathy.
Disclosure
BWP, BB, ALS, EMW, and GLK are employees of Entera Health, Inc. The authors report no other conflicts of interest in this work.
References
- TorrallardonaDSpray dried animal plasma as an alternative to antibiotics in weanling pigs – a reviewAsian-Australasian Journal of Animal Sciences2010231131148
- MoretóMPérez-BosqueADietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosaJ Anim Sci200987Suppl 14E92E10018820151
- CampbellJMPoloJRussellLECrenshawJDReview of spray-dried plasma’s impact on intestinal barrier functionLivest Sci2010133239241
- Pérez-BosqueAMiróLPoloJDietary plasma protein supplements prevent the release of mucosal proinflammatory mediators in intestinal inflammation in ratsJ Nutr20101401253019923397
- BosiPCasiniLFinamoreASpray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88J Anim Sci20048261764177215217004
- CorlBAHarrellRJMoonHKEffect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirusJ Nutr Biochem2007181277878417475463
- AsmuthDMMaZMAlbaneseAOral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathyAIDS2013272207221723660579
- WilsonDEvansMDWeaverEShawALKleinGLEvaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndromeClin Med Insights Gastroenterol20136496024833942
- NavarroAEslavaCGarcía de la TorreGCommon epitopes in LPS of different Enterobacteriaceae are associated with an immune response against Escherichia coli O157 in bovine serum samplesJ Med Microbiol200756Pt 111447145417965343
- TomitaGMTodhunterDAHoganJSSmithKLAntigenic crossreactivity and lipopolysaccharide neutralization properties of bovine immunoglobulin GJ Dairy Sci19957812274527528675757
- WeaverEMKleinGLDeVriesBKMaasKShawALEndotoxin neutralization activity (ENA) of bovine plasma and bovine immunoglobulin (IgG)-rich fractions as compared to human plasma. Experimental Biology 2013; April 20–24, 2013; Boston, MA, USA.FASEB J2013271079.58
- AsmuthDMStombaughJMaZMChanges in stool microbiota, bacterial translocation and mucosal immunity after oral serum-derived bovine immunoglobulin (SBI) administration20th Conference on Retroviruses and Opportunistic Infections (CROI)March 3–6, 2013Atlanta, GA
- PeaceRMCampbellJPoloJCrenshawJRussellLMoeserASpray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigsJ Nutr201114171312131721613450
- Pérez-BosqueAAmatCPoloJSpray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned ratsThe J Nutr20061361128382843
- DetzelCJHorganAHendersonALMaasKJWeaverEMDevelopment of a co-culture model of the intestinal epithelium to study barrier function and immune exclusion by bovine IgG. Clinical Nutrition Week January 18–21, 2014; Savannah, GAParenter Enteral Nutr201438146.27
- GasbarriniALauritanoECGarcovichMSparanoLGasbarriniGNew insights into the pathophysiology of IBS: intestinal microflora, gas production and gut motilityEur Rev Med Pharmacol Sci200812Suppl 111111718924450
- CamilleriMLaschKZhouWIrritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndromeAm J Physiol Gastrointest Liver Physiol20123037G775G78522837345
- CamilleriMPeripheral mechanisms in irritable bowel syndromeN Engl J Med2012367171626163523094724
- KeszthelyiDTroostFJMascleeAAUnderstanding the role of tryptophan and serotonin metabolism in gastrointestinal functionNeurogastroenterol Motil200921121239124919650771
- ClarkeGFitzgeraldPCryanJFCassidyEMQuigleyEMDinanTGTryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohortBMC Gastroenterol20099619154614
- BjarnasonISharpstoneDRFrancisNIntestinal inflammation, ileal structure and function in HIVAIDS19961012138513918902068
- SharpstoneDNeildPCraneRSmall intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoeaGut1999451707610369707
- Vujkovic-CvijinIDunhamRMIwaiSDysbiosis of the gut microbiota is associated with hiv disease progression and tryptophan catabolismSci Transl Med20135193193r a191
- PeuhkuriKVapaataloHKorpelaREven low-grade inflammation impacts on small intestinal functionWorld J Gastroentero201016910571062
- SaitoYAThe role of genetics in IBSGastroenterol Clin North Am2011401456721333900
- WongBSCamilleriMCarlsonPIncreased bile acid biosynthesis is associated with irritable bowel syndrome with diarrheaClin Gastroenterol Hepatol201210910091015 e322610000
- SimrénMBarbaraGFlintHJRome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation reportGut201362115917622730468
- PetherickADevelopment: mother’s milk: a rich opportunityNature20104687327S5S721179083
- StockingerSHornefMWChassinCEstablishment of intestinal homeostasis during the neonatal periodCell Mol Life Sci201168223699371221952827
- MartinRJiménezEHeiligHIsolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCRAppl Environ Microbiol200975496596919088308
- AaltonenJOjalaTLaitinenKPoussaTOzanneSIsolauriEImpact of maternal diet during pregnancy and breastfeeding on infant metabolic programming: a prospective randomized controlled studyEur J Clin Nutr2011651101920948557
- WalkerABreast milk as the gold standard for protective nutrientsJ Pediatr2010156Suppl 2S3S720105662
- BéginFSantizoMCPeersonJMTorúnBBrownKHEffects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low-income, peri-urban Guatemalan communityEur J Clin Nutr2008621395017299460
- LembckeJLPeersonJMBrownKHAcceptability, safety, and digestibility of spray-dried bovine serum added to diets of recovering malnourished childrenJ Pediatr Gastroenterol Nutr19972543813849327366
- JiangRChangXStollBDietary plasma protein is used more efficiently than extruded soy protein for lean tissue growth in early-weaned pigsJ Nutr200013082016201910917918
- PierceJLCromwellGLLindemannMDRussellLEWeaverEMEffects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigsJ Anim Sci200583122876288516282627
- MorelPCHShollumLMBuwaldaTRPearsonGDigestibility of bovine immunoglobulin in the pigletHennessyDPCranwellPDManipulating Pig ProductionCanberraCSIRO Publishing1995181
- RodriguezCBlanchFRomanoVSaboridoNRodenasJPoloJPorcine immunoglobulins survival in the intestinal tract of adult dogs and cats fed dry food kibbles containing spray-dried porcine plasma (SDPP) or porcine immunoglobulin concentrate (PIC)Animal Feed Science and Technology2007139201211
- AsmuthDMUrsellLMaZMDuodenal lamina propria CD4+ T-lymphocyte (CD4+ LPL) increases following serum-derived bovine immunoglobulin administration leads to reduced enterocyte damage and improved collagen turnover in HIV-enteropathyPresented at Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC)September 10–13, 2013Denver, CO
- LeyRETurnbaughPJKleinSGordonJIMicrobial ecology: human gut microbes associated with obesityNature200644471221022102317183309
- ArumugamMRaesJPelletierEEnterotypes of the human gut microbiomeNature2011473734617418021508958
- GuilarteMSantosJde TorresIDiarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunumGut200756220320917005763
- MartínezCVicarioMRamosLThe jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestationsAm J Gastroenterol2012107573674622415197
- MartínezCLoboBPigrauMDiarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrierGut20136281160116822637702
- SpillerRGarsedKPostinfectious irritable bowel syndromeGastroenterology200913661979198819457422
- DinarelloCARole of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findingsJ Biol Regul Homeost Agents1997113911039498158
- MoretóMMiróLMaijóMDietary supplementation with porcine plasma proteins reduce lymphocyte recruitment and cytokine and chemokine expression in a mouse model of spontaneous colitisGastroenterology20101385S-743.W1801
- JiangHBeckerCPrzybyszewskiJMacDonaldRSDietary immunoglobulins affect colon cytokines in mouse model of inflammatory bowel disease Paper presented at:FASEB J201024720.1
- Pérez-BosqueAMiróLPoloJDietary plasma proteins modulate the immune response of diffuse gut-associated lymphoid tissue in rats challenged with Staphylococcus aureus enterotoxin BJ Nutr2008138353353718287362
