Abstract
Spinal cord injury (SCI) is a traumatic, life-disrupting event with an annual incidence of 17,000 cases in the US. SCI is characterized by progressive physical deconditioning due to limited mobility and lack of modalities to allow safe physical activity that may partially offset these deleterious physical changes. Approximately, 50% of patients with SCI report no leisure-time physical activity and 15% report leisure-time physical activity below the threshold where meaningful health benefits could be realized. Collectively, about 363,000 patients with SCI, or 65% of the entire spinal cord injured population in the US, engages in insufficient physical activity and represents a target population that could derive considerable health benefits from even modest physical activity levels. Currently, the annual direct costs related to SCI exceed US$45 billion in the US. Rehabilitation protocols and technologies aimed to improve functional mobility have potential to significantly reduce the risk of medical complications and cost associated with SCI. Patients who commence routine physical activity in the first post-injury year and experience typical motor function improvements would realize US$290,000 to US$435,000 in lifetime cost savings, primarily due to fewer hospitalizations and less reliance on assistive care. New assistive technologies that allow patients with SCI to safely engage in routine physical activity are desperately needed.
Introduction
Spinal cord injury (SCI) is a traumatic, life-disrupting event with an annual incidence of 17,000 cases in the US.Citation1 Despite concerted efforts to develop medical and surgical interventions intended to improve perioperative survival and minimize chronic neurological deficit associated with acute SCI, prognosis for full recovery is poor in most of the cases. In-hospital mortality rates following traumatic SCI range from 3% to 13%, most commonly due to polytrauma.Citation2 Further, less than 1% of patients experience complete neurological recovery by hospital discharge.Citation3 Once patients complete acute rehabilitation and are discharged home, most will begin a chronic period of progressive physical deconditioning due to limited mobility and lack of modalities to allow safe physical activity that may partially offset these deleterious physical changes. This period is characterized by lifelong physical deterioration in the functioning of major body systems such that the life span of spinal cord-injured patients is 18 years shorter than age- and sex-matched healthy individuals.Citation3,Citation4 Further, these patients will endure severe long-term medical, functional, and psychological complications following the injury, thus increasing their risk for loss of employment or employability, decreasing quality of life, and resulting in tremendous societal cost.Citation5,Citation6 Overall, the clinical and economic burden of SCI is substantial and, given that the condition is currently irreversible, it will likely remain a major societal issue for decades to come.
Chronic deconditioning in SCI patients
Emerging therapies intended to reverse SCI and restore neural and motor pathways are on the horizon, although a definite cure remains elusive. Experimental studies have shown that stem cell injections may regrow glial cells and restore partial function in some patients, but exorbitant costs and inconsistent clinical benefit limit the utility of this therapy.Citation7,Citation8 Thus, except in rare cases, a patient will live with the physical, psychological, and economic burdens imposed by SCI for the remainder of life. SCI is responsible for a cascade of physiological decline, which collectively contributes to secondary complications, frequent hospitalizations, high cost, and shorter life expectancy. While SCI itself cannot be currently reversed, the associated physical deterioration may be partially offset by engaging in even modest levels of physical activity on a routine basis. However, for a variety of different reasons, exercise participation levels are extremely low in patients with SCI.Citation9 Thus, from the time of injury through the remainder of life, the typical patient experiences declining physical health, reduced quality of life, and a significant financial burden. The reasons for the treatment gap for patients with SCI are several, including fear of causing additional bodily harm, inability to access exercise facilities, and lack of modalities to accommodate patient needs. There is a clear need to develop new and accessible technologies that may safely foster independent physical activity in spinal cord-injured patients.
How many spinal cord-injured patients are affected by chronic deconditioning?
The number of chronically sedentary patients with SCI has not been well characterized, but may be reasonably estimated from previous studies. The annual incidence of SCI is reported to be 54 per million.Citation3 Given the current US population of 323 million and a median life expectancy of 32 years post-injury, the most recent estimate of SCI prevalence is 558,000 cases.Citation1 Approximately, 50% of patients with SCI report no leisure-time physical activity and 15% report leisure-time physical activity below the threshold required for meaningful health benefits (<1 hour/week).Citation9 This suggests that there are approximately 279,000 completely sedentary patients with SCI in the US and 84,000 more patients who participate in leisure-time activities inadequate to positively impact health. Collectively, about 363,000 patients with SCI, or 65% of the entire spinal cord-injured population, engage in insufficient volumes of physical activity and represent a target population that could derive considerable health benefits from even modest activity levels.
What is the duration of chronic deconditioning after SCI?
The median age at time of SCI is 30 years.Citation3 While life expectancy following SCI is dependent on factors such as age, sex, cause of injury, and injury level, the median life expectancy following injury across all patients with SCI is 32 years, about 18 years less than age- and sex-matched non-disabled individuals.Citation3,Citation4 Annual mortality rates for those who survive the first post-injury year average 2.5% and increase as patients age.Citation3 Therefore, unlike common chronic diseases that predominantly affect the elderly, such as coronary heart disease or chronic obstructive pulmonary disease, the typical SCI patient is faced with significant disability throughout the majority of their adult life.
Physical consequences of SCI
As individuals with SCI age, they experience the same chronic health risks that arise in the general population. The goals of an aging SCI patient remain largely the same as those of a nondisabled person – to maintain overall health, independence, and life satisfaction. However, aging with the additional challenge of a significant disability makes it exponentially more difficult for the SCI patient to maintain health status. At the time of injury, there is immediate and severe loss of sensory and motor function that triggers an extreme catabolic reaction due to loss of normal physiological stresses to tissueCitation10 and neurohumoral responses.Citation11 Protein degradation by-products lead to chronic increases in renal demand. Bones undergo extreme demineralization due to reduction or loss in muscle contraction forces. Spinal cord-injured patients have increased risks of osteoporosis, cardiovascular disease, respiratory problems, and muscular spasticity and contractures compared to the general population.Citation12,Citation13 Obesity risk increases in patients with SCI due to hypercaloric diet, lack of physical activity, and a reduction in metabolic rate, the latter a result of declines in fat-free mass and sympathetic nervous system activity. Severe immunosuppression leads to systemic inflammation and weakened immunity.Citation14 Associated secondary complications such as fractures, falls, pressure ulcers, and systemic infections result in costly hospitalizations. Most secondary complications resulting from SCI are thought to be the direct result of immobility.
The typical SCI patient engages in only 40% of the activity levels of nondisabled peers.Citation15 Given that the typical nondisabled adult is largely inactive highlights the extremely sedentary existence of patients with SCI. The percentage of patients with SCI able to walk at least 1 street block with or without assistive devices is only 32% at 1 year post-injury and declines as patients age, with 25% at 10 years and 15–20% between 20 and 30 years post-injury able to ambulate 1 block. Concomitantly, wheelchair dependence rises, from 59% at 1 year, to 70% at 10 years post-injury, and between 75% and 80% thereafter.Citation3 The combination of sedentary lifestyle combined with increased secondary complication risk exponentially increases the risk of expensive hospitalizations. In the year following SCI, 36% of patients will be hospitalized at least once and 13% will be hospitalized at least twice, with an average length of stay of 16 to 24 days per episode.Citation3,Citation16 Even beyond the first year of injury, 30% of patients will be hospitalized in any given year, with average stays of 22 days.Citation3 Primary causes of hospitalization are genitourinary complications and, to a lesser extent, complications related to skin, respiratory, digestive, circulatory, and musculoskeletal diseases.Citation3,Citation16 Notably, pressure sores accounted for 7% of readmissions, but 28% of total bed-days, with a median length of stay per episode of 7 weeks.Citation17
Economic burden of SCI
Starting at the time of initial injury, there is tremendous lifelong utilization of health care resources. The typical life span following SCI is 32 years, with considerable variation depending on the level of injury.Citation18 Costs are highest during the first year following injury, but remain substantial each subsequent year. Average direct costs are US$523,000 in the first year and US$80,000 in each subsequent year, accounting for over US$3 million in costs over a typical patient life span.Citation19
In the first year after injury, private payers are responsible for the greatest percentage of SCI-related costs. Thereafter and throughout the typical post-injury life span of a spinal cord-injured patient, the Centers for Medicare & Medicaid Services (CMS) pays the greatest portion of costs (). Over the life span of a typical patient, the cumulative direct costs to primary payers will total US$1.6 million for CMS, US$1.1 million for private payers, and US$400,000 for other payers (e.g. worker’s compensation, Veteran’s Administration) (). Currently, the annual direct costs related to SCI exceed US$45 billion annually in the US. Assuming that the incidence of SCI stays constant at 54 cases per million, median post-injury life expectancy remains 32 years, and given the US population projections,Citation4 the cumulative direct costs to the health care system will exceed US$1.4 trillion over the next 30 years, with CMS responsible for over US$750 million (). Moreover, given that the median survival rates following SCI have been increasing over timeCitation3 and with increasing health care costs, the costs of care for an SCI may be expected to increase beyond these conservative assumptions.
Figure 1 Percentage of lifetime medical costs by primary payer in a typical spinal cord-injured patient in the US.
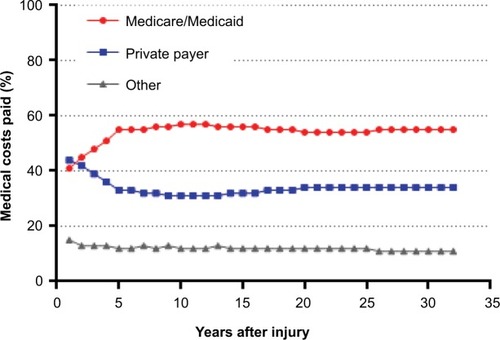
Figure 2 Cumulative lifetime primary payer costs in a typical spinal cord-injured patient in the US.
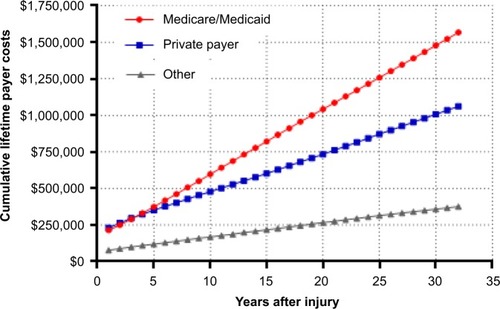
Figure 3 Cumulative 30-year primary payer costs for spinal cord-injured patients in the US.
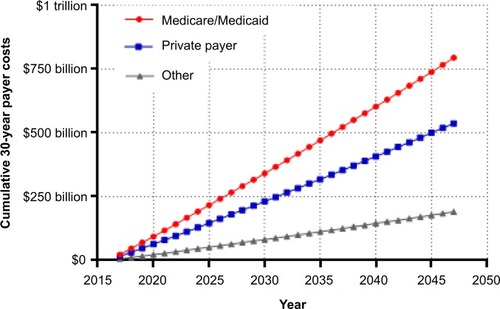
In addition to the direct costs of SCI, indirect costs related to lost wages, productivity, and fringe benefits are responsible for an additional burden of US$72,000 per year.Citation3 The health-related burden of disability due to SCI is among the highest of any known disease or disability.Citation20 Prior to injury, 83% of individuals were employed whereas only 25% were employed at 4 years.Citation18 When considering the lifetime direct costs of US$3 million and US$2.3 million in indirect costs, the total economic burden attributable to SCI is US$5.3 million per patient.
Health benefits of physical activity in spinal cord-injured patients
Recovery of locomotion is one of the main priorities for spinal cord-injured patients.Citation21,Citation22 In addition to overcoming the obvious mobility and social issues related to the inability to stand or walk, regular ambulation may profoundly combat secondary medical problems associated with lack of weight-bearing activity.Citation23 There are a number of well-documented health benefits from even modest physical activity, such as improved lipid profile, lower diabetes risk, greater lean muscle mass, and greater quality of life. Physical activity at an intensity equivalent to a nondisabled person walking at 3 mph maintained for 1 hour per day, 3 days per week, is associated with preventive health benefits including cardiovascular and all-cause mortality risk reduction of 20% in the general adult population.Citation23 For patients with SCI, those same health benefits should be a reasonable expectation. In addition, intermittent standing and habitual ambulation are known to improve upper body muscular fitness,Citation24 slow decline in bone mineral by exposure to gravitational and muscular loading forces,Citation25 improve circulatory responses,Citation26 and reverse health risks associated with prolonged sitting.Citation27
As with nondisabled individuals, the American College of Sports Medicine’sCitation28 exercise recommendations for patients with SCI include moderate-intensity exercise three to five times per week for 20 to 60 minutes per session. Appropriate modalities of cardiopulmonary exercise include arm crank ergometry, wheelchair propulsion, swimming, seated aerobics, functional electrical stimulation, and ambulation with assistive devices. Appropriately prescribed exercise programs are needed to improve health status and overall quality of life in patients with SCI. Although moderate physical activity is well tolerated by most patients, many patients are discouraged from participation due to incorrect perceptions about their functional capacity and exaggerated concern about causing harm. Many perceived barriers to participation in an exercise program have since been refuted, such as the notion of exercise leading to exacerbated contractures and increased risk of falling.Citation29–Citation31 The far greater risk to the spinal cord-injured patient is maintaining a chronically sedentary lifestyle, which leads to a progressive and predictable decline in physical deconditioning. At 1-year post-injury, 48% of patients with SCI are ambulatory, most with the assistance of a mobility aid.Citation3 However, as patients age, the ability declines to 37% at 10 years, 26% at 20 years, and 22% at 30 years post-injury.Citation3 Clearly, there is great need for novel modalities to maintain or improve ambulatory ability and to facilitate safe exercise at sufficient intensity known to elicit health benefits.
Cost benefits of physical activity in spinal cord-injured patients
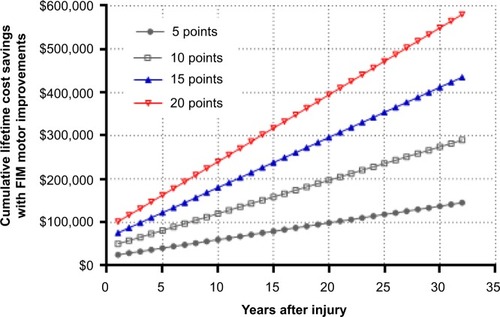
Rehabilitation protocols and technologies aimed to improve functional mobility have potential to significantly reduce the risk of medical complications and cost associated with SCI. Following discharge from inpatient rehabilitation, physical activity levels dramatically declineCitation32 such that the great majority of patients remain sedentary for the remainder of life. However, patients who are able to participate in physical activity enjoy tremendous health benefits and health care cost savings. Patients who exercise at least twice per week have a 50% lower risk of hospitalization in the first year versus sedentary patients.Citation16 Furthermore, for every 5-point increase in Functional Independence Measure (FIM) motor score, the number of hospitalizations each year is reduced by 0.022 and percentage of patients requiring assistive care decreases by 3.6%.Citation33 Given that hospitalizations and assistive care represent almost 90% of the direct costs attributable to SCI,Citation19 the potential cost benefits of improved motor function can be approximated.
For every 5-point increase in FIM motor score, annual direct costs decrease by about US$25,000 in the first year and US$4,000 annually thereafter. Based on previous studies of outpatient physical activity programs, FIM motor score improvements of 5 to 20 points can be reasonably expected with chronic exercise.Citation34,Citation35 Therefore, in a patient who commences routine physical activity in the first post-injury year and given a FIM motor score improvement of 10 to 15 points, cumulative direct cost savings would total US$81,000 to US$122,000 at 5 years and US$290,000 to US$435,000 over a lifetime, primarily due to fewer hospitalizations and less reliance on assistive care ().
Figure 4 Cumulative lifetime cost savings with motor function improvements in a typical spinal cord-injured patient in the US.
Abbreviation: FIM, Functional Independence Measure.
Further, it is reasonable to assume that mobility improvement may comparably reduce indirect costs. Riggins et al reported that patients who transitioned from walking at rehabilitation discharge to wheelchair use at 1 year had the lowest quality of life, whereas those who transitioned from wheelchair to walking reported the highest quality of life.Citation36 Also, higher motor function 5 years after injury is associated with higher employment rates.Citation37 Although no known data are available to accurately estimate the reduction in indirect costs due to improved functional ability, given that direct costs of SCI approximate the indirect costs, a parallel reduction in indirect costs over a patient’s lifetime is plausible.
Discussion
Despite the fact that SCI affects only 1 in 18,000 people,Citation1 this disability is responsible for a disproportionately excessive physical, psychological, and economic burden. Rehabilitative therapies intended to facilitate physical activity in patients with SCI suffer from poor adoption due to high expense, safety concerns, lack of availability, or inability to tailor exercise intensity to current fitness levels. This paper highlights a treatment gap for patients with SCI whereby new technologies that facilitate physical activity should be developed and incorporated into treatment protocols for patients with SCI. Availability of such modalities and given modest improvements in motor function ability has the potential to reduce the lifetime economic burden of SCI by 10–15%. This is in stark contrast to the current paradigm whereby the typical SCI patient endures chronic deconditioning such that medical complications greatly exceed that of age-matched healthy individuals, life expectancy is greatly reduced, and substantial economic resources are depleted, totaling over US$3 million in direct costs over a lifetime.
Orthotic technologies such as hip-knee-ankle-foot orthoses and reciprocating gait orthoses have been employed with mixed success as a means of addressing this critical unmet health care need in the SCI population. The main limitation of these devices is a high metabolic demand such that most patients eventually discontinue use.Citation38–Citation41 Since the health benefits of physical activity are largely dependent on exercise frequency and duration,Citation42 these traditional orthotic technologies likely have limited utility in ameliorating the chronic effects of inactivity due to SCI. Functional electric stimulation-assisted exercise is a treatment modality in which electrical impulses are applied to intact peripheral nerves supplying paralyzed muscles in order to produce functional movement and stimulate contractions of those muscles to promote recovery of motor function. Although improvements in cardiovascular function may be realized with routine use, these devices are primarily limited to arm cranking and cycle ergometry and do not facilitate weight-bearing ambulation. Despite the significant technical progress achieved in the last 10 to 15 years in this field, there is a general consensus that these systems are not sufficiently advanced and that they need further development.Citation43,Citation44 Powered exoskeletons are prescription devices composed of an external, powered, motorized orthosis that is placed over a person’s paralyzed or weakened limbs for the purpose of facilitating standing, walking, climbing stairs, and performing activities of daily living. A recent meta-analysis concluded that powered exoskeletons allow patients with SCI to safely ambulate in real-world settings at a physical activity intensity conducive to prolonged use and known to yield health benefits.Citation45 Specifically, the physiologic demand of exoskeleton-assisted ambulation was comparable to that of a nondisabled person walking at 3 mph. Further, 76% of patients were able to ambulate with no physical assistance. Improvements in bowel regularity and spasticity were reported in 61% and 38% of patients, respectively. Importantly, in this review, most patients presented with complete SCI. Generally, less than 5% of such patients have the ability to ambulate without physical assistance. The fact that 67% of patients in this review were able to engage in exoskeleton-assisted ambulation without physical assistance is very promising.
Limitations
There are several limitations of the methodology used in the current review. For simplicity, cost projections were predicted on assumptions applicable to a “typical” spinal cord-injured patient. This profile included median age of 30 years, median post-injury survival of 32 years, and averaged direct costs with no discount applied. Factors such as actual age of injury, neurological level of injury, sex, and baseline motor function scores will introduce variability when estimating costs for a single patient. Cost savings estimates with motor function improvement may be impacted by factors unrelated to physical activity, such as patient education and increased proficiency in activities of daily living. Additionally, we did not consider factors such as potential increases in longevity after SCI, increases in SCI incidence, or future increases in health care costs. To the extent that these factors might increase in the future, our cost projections may be underestimated. However, when considering all patients with SCI (as a group) within the population, we believe that the cost estimates are reasonable and should be considered as hypothesis generating and a basis to argue for more rigorous prospective studies.
Conclusion
SCI is responsible for significant lifelong loss of functional ability, frequent secondary complications, and tremendous costs to the health care system. Routine physical activity reduces the physical and economic burden of SCI. Therefore, widespread clinical adoption of new technologies that safely facilitate routine physical activity for patients with SCI in the home setting would result in significant cost savings.
Disclosure
This study was supported by ReWalk Robotics (Marlborough, MA, USA). The authors report no other conflicts of interest in this work.
References
- JainNBAyersGDPetersonENTraumatic spinal cord injury in the United States, 1993–2012JAMA2015313222236224326057284
- ChamberlainJDMeierSMaderLvon GrootePMBrinkhofMWMortality and longevity after a spinal cord injury: systematic review and meta-analysisNeuroepidemiology201544318219825997873
- National Spinal Cord Injury Statistical CenterSpinal Cord Injury Model Systems: 2015 Annual Report2016 Available from: https://www.nscisc.uab.edu/reports.aspxAccessed May 15, 2016
- U.S. Census BureauProjections of the Size and Composition of the US Population: 2014 to 20602015 Available from: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdfAccessed May 25, 2016
- CadotteDWFehlingsMGSpinal cord injury: a systematic review of current treatment optionsClin Orthop Relat Res2011469373274121080129
- McKinleyWOJacksonABCardenasDDDeVivoMJLong-term medical complications after traumatic spinal cord injury: a regional model systems analysisArch Phys Med Rehabil199980111402141010569434
- LuPKadoyaKTuszynskiMHAxonal growth and connectivity from neural stem cell grafts in models of spinal cord injuryCurr Opin Neurobiol20142710310924709371
- ReevesAKeirsteadHSStem cell based strategies for spinal cord injury repairAdv Exp Med Biol2012760162423281511
- GinisKALatimerAEArbour-NicitopoulosKPLeisure time physical activity in a population-based sample of people with spinal cord injury part I: demographic and injury-related correlatesArch Phys Med Rehabil201091572272820434609
- Dudley-JavoroskiSShieldsRKMuscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulationJ Rehabil Res Dev200845228329618566946
- FredricksonMDAcute spinal cord injury managementJ Trauma2007626 SupplS917557005
- JensenMPTruittARSchomerKGYorkstonKMBaylorCMoltonIRFrequency and age effects of secondary health conditions in individuals with spinal cord injury: a scoping reviewSpinal Cord2013511288289224126851
- SezerNAkkusSUgurluFGChronic complications of spinal cord injuryWorld J Orthop201561243325621208
- EserPSchiesslHWillneckerJBone loss and steady state after spinal cord injury: a cross-sectional study using pQCTJ Musculoskelet Neuronal Interact20044219719815615125
- van den Berg-EmonsRJBussmannJBStamHJAccelerometry-based activity spectrum in persons with chronic physical conditionsArch Phys Med Rehabil201091121856186121112426
- DeJongGTianWHsiehCHRehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitationArch Phys Med Rehabil2013944 SupplS87S9723527776
- MiddletonJWLimKTaylorLSodenRRutkowskiSPatterns of morbidity and rehospitalisation following spinal cord injurySpinal Cord200442635936715007376
- KrauseJSEdlesPCharlifueSChanges in employment status and earnings after spinal cord injury: a pilot comparison from pre to post injuryTop Spinal Cord Inj Rehabil20111647479
- DeVivoMJChenYMennemeyerSTDeutschACosts of care following spinal cord injuryTop Spinal Cord Inj Rehabil201116419
- SalomonJAHaagsmaJADavisADisability weights for the Global Burden of Disease 2013 studyLancet Glob Health2015311e712e72326475018
- DitunnoPLPatrickMStinemanMDitunnoJFWho wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional studySpinal Cord200846750050618209742
- LoCTranYAndersonKCraigAMiddletonJFunctional priorities in persons with spinal cord injury: using discrete choice experiments to determine preferencesJ Neurotrauma Epub201659
- AremHMooreSCPatelALeisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationshipJAMA Intern Med2015175695996725844730
- HicksALMartin GinisKAPelletierCADitorDSFoulonBWolfeDLThe effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic reviewSpinal Cord201149111103112721647163
- AstorinoTAHarnessETWitzkeKAEffect of chronic activity-based therapy on bone mineral density and bone turnover in persons with spinal cord injuryEur J Appl Physiol2013113123027303724097172
- MiliaRRobertoSMarongiuEImprovement in hemodynamic responses to metaboreflex activation after one year of training in spinal cord injured humansBiomed Res Int2014201489346824809060
- StamatakisERogersKDingDAll-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adultsInt J Behav Nutr Phys Act20151212126419654
- MooreGLarry DurstineJPainterPACSM’s Exercise Management for Persons With Chronic Diseases and Disabilities-4th Ed, By American College of Sports MedicineChampaign, ILHuman Kinetics2016
- TurbanskiSSchmidtbleicherDEffects of heavy resistance training on strength and power in upper extremities in wheelchair athletesJ Strength Cond Res201024181619996772
- JacobsPLEffects of resistance and endurance training in persons with paraplegiaMed Sci Sports Exerc200941599299719346989
- JacobsPLNashMSExercise recommendations for individuals with spinal cord injurySports Med2004341172775115456347
- van den Berg-EmonsRJBussmannJBHaismaJAA prospective study on physical activity levels after spinal cord injury during inpatient rehabilitation and the year after dischargeArch Phys Med Rehabil200889112094210118996237
- CohenJTMarinoRJSaccoPTerrinNAssociation between the functional independence measure following spinal cord injury and long-term outcomesSpinal Cord2012501072873322641254
- LugoLHSalinasFGarciaHIOut-patient rehabilitation programme for spinal cord injured patients: evaluation of the results on motor FIM scoreDisabil Rehabil20072911–1287388117577722
- DuranFSLugoLRamirezLEusseEEffects of an exercise program on the rehabilitation of patients with spinal cord injuryArch Phys Med Rehabil200182101349135411588736
- RigginsMSKankipatiPOysterMLCooperRABoningerMLThe relationship between quality of life and change in mobility 1 year postinjury in individuals with spinal cord injuryArch Phys Med Rehabil20119271027103321704781
- FerdianaAPostMWHoekstraTvan der WoudeLHvan der KlinkJJBultmannUEmployment trajectories after spinal cord injury: results from a 5-year prospective cohort studyArch Phys Med Rehabil201495112040204624832572
- BernardiMCanaleICastellanoVDi FilippoLFeliciFMarchettiMThe efficiency of walking of paraplegic patients using a reciprocating gait orthosisParaplegia19953374094157478731
- ScivolettoGPetrelliALucenteLDOne year follow up of spinal cord injury patients using a reciprocating gait orthosis: preliminary reportSpinal Cord200038955555811035478
- FranceschiniMBarattaSZampoliniMLoriaDLottaSReciprocating gait orthoses: a multicenter study of their use by spinal cord injured patientsArch Phys Med Rehabil19977865825869196464
- SykesLEdwardsJPowellESRossERThe reciprocating gait orthosis: long-term usage patternsArch Phys Med Rehabil19957687797837632135
- FouldsHJBredinSSCharlesworthSAIveyACWarburtonDEExercise volume and intensity: a dose-response relationship with health benefitsEur J Appl Physiol201411481563157124770699
- RagnarssonKTFunctional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directionsSpinal Cord200846425527417846639
- BrazGPRussoldMDavisGMFunctional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristicsNeuromodulation200912318019022151359
- MillerLEZimmermannAKHerbertWGClinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysisMed Devices (Auckl)2016945546627042146
