Abstract
Aim
The objectives of this study were to: 1) analyze the drug utilization pattern among adult psoriasis patients who were newly prescribed with topical medication; and 2) assess their adherence to topical therapy and the possibility of switching to other strategies in the treatment process.
Methods
An observational retrospective analysis was conducted based on administrative databases of two Italian local health units. All adult subjects who were diagnosed with psoriasis or who were newly prescribed for topical medication with at least one prescription between January 1, 2010, and December 31, 2014, were screened. Only patients who were “non-occasional users of topical drugs” (if they had at least two prescriptions of topical drugs in a time space of 2 years) were considered for the first and second objectives in the analysis. The date of the first prescription of topical agents was identified as the index date (ID), which was then followed for all time available from ID (follow-up period). The adherence to therapy was assessed on the basis of cycles of treatment covered in the 6 months before the end of the follow-up period. The mean health care costs in patients who switched to disease-modifying antirheumatic drugs (DMARDs) or biologics after the ID were evaluated.
Results
A total of 17,860 patients with psoriasis who were newly prescribed for topical medication were identified. A total of 2,477 were identified as “non-occasional users of topical drugs”, of whom 70.2% had a prescription for a topical fixed combination regimen at ID. Around 19% adhered to their medication, whereas 6% switched to other options of psoriasis treatment. Multivariable logistic regression model shows that patients on fixed combination treatment were less likely to be non-adherent to treatment and less likely to switch to other treatments. The annual mean pharmaceutical costs were €567.70 and €10,606.10 for patients who switched to DMARDs and biologics, respectively.
Conclusion
Our findings show that the use of fixed combination topical treatment can lead to improve the likelihood of patients being adherent to treatment and can decrease the likelihood of switching the treatment to DMARDs or biologics.
Introduction
Psoriasis is a chronic inflammatory skin disorder that impacts negatively on patients’ physical and psychological well-being, reduces quality of life, and causes significant economic burden in terms of loss of productivity and increased utilization of health care resources.Citation1 Psoriasis is associated with higher risk of developing cardiovascular disease, psychiatric/psychologic issues, and psoriatic arthritis.Citation2 The accurate etiology of psoriasis is still not completely understood, but a number of possible risk factors are identified such as family history and environmental risk factors.Citation3
Psoriasis affects ~2%–3% of the Caucasian population;Citation4,Citation5 recent data indicate that the incidence of the disease among the adult population in Italy is 230/100,000 person-years.Citation6 In Italy, it has been estimated that about 1.5 million of people are affected by this inflammatory disease.Citation7
This chronic disorder has no cure; however, there are many treatment options available whose goal is to reduce disease severity and symptoms. Treatment options include traditional topical therapies such as vitamin D analogs, corticosteroids, dithranol, and tar preparations as first-line therapy. Second-line therapy includes treatment with phototherapy and non-biological systemic agents. Third-line therapy includes systemic biological therapies including treatment with anti-tumor necrosis factor-alpha agents and anti-IL12-23 monoclonal antibody.Citation7–Citation9
The choices of treatments depend on many important factors that influence decision making, such as disease severity, location and characteristics of the lesion, and patients’ preference.Citation9,Citation10 Lack of adherence to topical therapy regimens or incorrect use can cause poor treatment outcomes.Citation11 Because the course and symptom of psoriasis vary from person to person, physician must experiment or combine different treatments to ensure that every patient receives best therapy. Recent guidelines have recommended the use of current antipsoriatic therapies in daily practice.Citation8–Citation10,Citation12 Topical therapy plays an important role in the treatment of psoriasis and it is considered the most appropriate therapy option for patients with mild-to-moderate disease.Citation10,Citation13 However, there is still limited evidence on the management of psoriasis with topical medication in routine clinical practice in Italy.
The objectives of this study were to: 1) analyze the drug utilization pattern among adult psoriasis patients who were newly prescribed with topical medication; and 2) assess their adherence to topical therapy and the possibility of switching to other strategies in the treatment process.
Methods
Data sources
The study was based on administrative databases (DBs) of two Italian local health units (LHUs), located in Lombardy and Campania, covering a total population of more than 1.5 million health-assisted individuals. The structure of this database has been described in detail elsewhere.Citation14,Citation15
Briefly, the following DBs were used: 1) the health-assisted subjects’ DB, which contains patient data such as sex and data of birth; 2) outpatients and inpatients pharmaceutical drugs DB, which contains information regarding prescription records such as the Anatomical-Therapeutic-Chemical (ATC) code of the drug purchased, the number of packages, the number of units per package, and the dosages; 3) hospital discharge DB, which contains all hospitalization data with discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM); and 4) finally, the medical exemption register, which contains the records of all disease exemptions, including the exemption code (identifying the disease for which the exemption was granted). The anonymous data file is routinely used by the regional health authorities for epidemiological and administrative purposes. Informed consent is not required for using encrypted retrospective information, according to Italian regulations. As per Italian regulations regarding the conduct of the observational analysis,Citation16 this study has been notified to the local Ethics Committee in of the each participating LHU (Pavia, Lombardy; Caserta, Campania) and each LHU Ethics Committee has approved the study.
Study population
An observational retrospective analysis was conducted. All adult subjects aged ≥18 years with at least one hospitalization with a main or secondary diagnosis of psoriasis (ICD-9-CM code: 696.1) or newly prescribed for topical medications with at least one prescription of topical antipsoriatic drugs (ATC code: D05A) between January 1, 2010, and December 31, 2014, were screened for eligibility. In agreement with recent published evidence,Citation17,Citation18 only naïve psoriasis patients who received at least two prescriptions of topical antipsoriatic drugs (ATC code: D05A) in a time space of 2 years were recruited in the analysis. We considered patients who were newly prescribed for topical medications as “occasional users of topical drugs” if they had only one prescription of topical drugs and as “non-occasional users of topical drugs” if they had at least two prescriptions of topical drugs in a time space of 2 years, respectively. Only the cohort of patients who were “non-occasional users of topical drugs” was considered for the first and second objectives in the analysis. Patients were also stratified according to prescription of single component formulations of topical drugs or combined treatment (fixed combination vitamin D analog [calcipotriol] and corticosteroid [betamethasone dipropionate]).
The index date (ID) was defined as the data of the first prescription of topical agents in patients with psoriasis in the study period, which represents the enrollment day of each patient, who was then followed for all time available from ID to the last prescription of topical antipsoriatic drugs or to the first switch to other treatment strategies (follow-up period). The number of prescriptions filled for the index drug was recorded. The median length of time that patients continued their index therapy was also evaluated. Patients who were transferred to another LHU and/or patients with concomitant pathologies (rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease) were excluded. Additionally, all subjects with unconventional switching between treatment strategies were also excluded (e.g., patients who switched from disease-modifying antirheumatic drugs [DMARDs] to topical agents or from biological drugs to topical agents).
Definitions and study outcomes
Data on baseline characteristics, including demographics, risk factors, and medical history, were collected. The clinical characteristics of the patients enrolled in this study were also investigated in the 1-year period before the ID. Naïve patients were defined as those who had no prior topical agents prescription filled during the 1 year preceding the enrollment date. Comorbidities were measured using the Charlson comorbidity index.Citation19
The adherence to topical therapy was assessed on the basis of cycles of treatment covered during the 6 months before the end of the follow-up period (last topical prescription or switch to DMARDs or biologic therapy). A cycle of treatment with gel formulation and ointment or other formulation was defined as 56 and 28 days, respectively.Citation20 Current recommendations on the management of psoriasisCitation7 suggest that topical agents should be used for almost two treatment cycles before switching to another treatment options such as DMARDs or biological agents. For this reason, we defined that a patient was adherent if there was at least one cycle of treatment in the past 6 months available during the follow-up period.
A switch was defined to occur when a new prescription (say medication B) filled by the patient was for a different product than the previous prescription (say, medication A). The treatment to which the switch occurred could be a systemic or biologic treatment. Switching was evaluated in all the patients included in the study and in a subgroup of patients treated with a fixed combination of calcipotriol and betamethasone dipropionate given as a gel or topical suspension formulation at ID without taking into consideration the last prescription the patient had in the past 6 months. This analysis allowed to evaluate the switch more accurately.
Cost analysis
The mean health care costs were evaluated in patients with switches to DMARD or biologic agents after the index therapy. Costs are reported in Euros (€). The consumption of health care resources was classified as related or not related to the disease analysis. Drug costs were evaluated using the Italian National Health Service (NHS) purchase price. Hospitalization costs were determined using the diagnosis-related group tariff. The cost of instrumental and laboratory tests was defined according to the tariffs applied by the regions. The cost analysis was conducted from the perspective of the Italian NHS.
Statistical analysis
We summarized data as mean and standard deviation (median and range as appropriate) for continuous variables and as numbers and percentages for categorical variables. When comparisons were made, groups were assessed and compared using the Students’ t-test. Qualitative variables were expressed as absolute and relative frequencies.
To identify factors potentially associated with therapeutic switch, a multivariable Cox regression model was used. To assess the proportional hazards assumption, Schoenfeld residuals were analyzed.
Covariates considered in the models were age, sex, adherence to therapy, disease exemption (exemption code: 045.696.1), and/or hospitalization (ICD-9-CM code: 696.1) records, with diagnosis of psoriasis, during the follow-up period. Effect sizes were reported as hazard ratios (HRs) and 95% confidence intervals (CIs).
Multivariable logistic regression analyses were performed to analyze the effect of various factors on adherence to therapy and to determine the odds ratios (ORs) and 95% CIs. We used age, sex, and variables that were clinically relevant and significantly associated with adherence in univariate analyses. p-values of ≤0.05 were considered to be statistically significant, and all analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX, USA).
Results
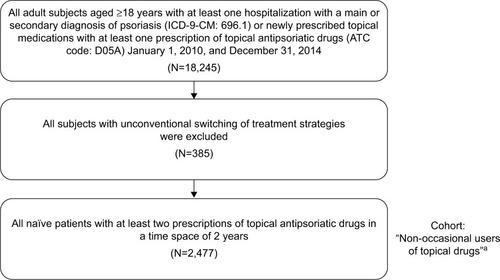
A total of 18,245 study participants were identified in the database with at least one hospitalization with a diagnosis of psoriasis or newly prescribed for topical medications with at least one prescription of topical antipsoriatic drugs between January 1, 2010, and December 31, 2014. All subjects with unconventional switching treatment strategies were excluded from analysis (N=385). Of these, 15,383 patients were identified as patients newly prescribed for topical medications who had only one prescription and were grouped as “occasional users of topical drugs”. A total of 2,477 patients were identified as patients newly prescribed for topical medications with at least two prescriptions of topical agents in a time space of 2 years and were grouped as “non-occasional users of topical drugs”. Most relevant determinant of exclusion was single prescription of topical antipsoriatic drugs in the 2-year time spaces. shows the details of the inclusion and exclusion criteria of this study.
Figure 1 Sequential sample selection of subjects.
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ATC, anatomical therapeutic chemical.
summarizes the baseline characteristics of patients (non-occasional users of topical drugs), stratified according to prescription of topical drugs alone or in fixed combination, adherence to treatment, and switching of therapy. Of the 2,477 patients included in the analysis, the mean (SD) age at the ID was 56.3±17 years, of whom 1,359 (55%) were males. Of these 2,477 patients, 29.8% (n=739) had a prescription for monocomponent of topical drugs and 70.2% (n=1,738) had a prescription for fixed combination therapy at ID (baseline). Among patients who received fixed combination topical treatment, 1,607 had a prescription of a gel formulation and 131 had a prescription of ointment or topical suspension formulations at baseline. Of the 2,477 patients, the average number of prescriptions filled was 2.6 per year and 2.3 per year for those who received index therapies with a fixed combination and monocomponent, respectively.
Table 1 Baseline of patients, stratified according to topical antipsoriatic drug prescription and level of adherence
The percentage of adherent patients to topical drugs was 18.7% (n=464) of a total sample (); of these patients, 74.6% (n=346) and 25.4% (n=118) received index therapies with a fixed combination and monocomponent, respectively. Considering the multivariate predictors of adherence to therapy, a lower 25% probability of non-adherence was observed among fixed combination therapy users (OR =0.755, 95% CI: 0.599–0.950, p=0.017; ).
Table 2 Predictors of non-adherence: multivariable logistic regression model
Among the non-occasional users of topical drugs, approximately 6% (n=156) of patients switched to other treatment options for psoriasis during the follow-up period (); of these, 50.6% (n=79) patients received fixed combination topical treatment, while 49.4% (n=77) received monocomponent treatment. With regard to switchers, about 0.5% of patients switched to a DMARDs and then to a biologic, while ~5% and 1% switched to DMARDs and biologics, respectively. The median length of time that switched patients continued their index therapy was 38.2 months.
Among patients treated with fixed combination gel at ID without prescriptions in the past 6 months (n=695), ~10% (n=70) switched to other treatment options for psoriasis during the follow-up period. About 1% of this cohort of patients switched to a DMARD and then to a biologic, while 8.2% and 1% switched to DMARDs and biologics, respectively. The median length of time that switched patients continued their index therapy was 40.6 months. However, among patients who were treated with ointment or topical suspension formulations of fixed combination at baseline without prescriptions in the past 6 months (n=1,782), 5% (n=86) switched to other treatment options for psoriasis during the follow-up period. Less than 0.5% of this cohort of patients switched to a DMARD and then to a biologic, while 3.6% and about 1% switched to DMARDs and biologics, respectively. The median length of time that switched patients continued their index therapy was 37.5 months.
A multivariable logistic model showed that the use of fixed combination therapy was associated with a lower probability of switching to another treatment (HR =0.568, p=0.001) (). Annual health care costs for the management of psoriasis patients based on resource consumption from ID are as follows: €687.6 for patients who switched to DMARDs, of which about 83% was spent on drugs (€427.5 specifically related to DMARDs), and €10,776.8 for patients who switched to biologics, of which about 98% was spent on drugs (€10,411.7 specifically related to biologics).
Table 3 Predictors of probability of therapy substitution: multivariable proportional hazards Cox regression model
Discussion
This retrospective analysis in an unselected Italian population under clinical practice setting showed that only about 14% of all patients with psoriasis who were newly prescribed for topical medications had at least two prescriptions of topical agents in a time space of 2 years.
In other words, a significant proportion of psoriasis patients did not receive more than one prescription of topical drugs in the period of analysis. This is consistent with previous findings,Citation21 and since there was no direct measure of disease severity, the patients on topical treatments may have had limited disease or they may have used topical agents as adjunctive treatment and, therefore, may not have needed continuous treatment. Nevertheless, according to recently published evidence,Citation22,Citation23 psoriasis is a chronic disease that requires continuous treatment to achieve optimal control of inflammatory activity and to minimize skin involvement, despite the fact that disease severity may wax and wane.
Currently, several topical therapies are available and there are both positive and negative aspects to each, but the preferences of patients and clinicians affect the choice of treatment.Citation10 The majority of patients enrolled in this study had a prescription for fixed combination vitamin D analog/corticosteroid therapy at ID. In recent years, several studies have reported the efficacy and safety of fixed combination regimens and its advantages compared with individual components.Citation10,Citation24–Citation27 Indeed, a recent Cochrane review of this therapy area has shown that use of fixed combination of vitamin D/corticosteroid could be better compared with individual active components administered alone in patients with mild–moderate psoriasis.Citation13 At the same time, real-world evidence suggest that combined treatment with vitamin D analog/corticosteroid therapy is preferred to single components used as monotherapies by most patients with psoriasis.Citation25,Citation28
The success of psoriasis treatment depends not only on the efficacy of the medication but also on the patient, for example, patients should follow doctor’s advice and the medications prescribed. In patients with psoriasis indicated for antipsoriatic drug treatment, optimal use of psoriasis therapy can limit the physical manifestations of psoriasis and help improve quality of life, but non-adherence is common.Citation29,Citation30 Considering that patients with chronic disorders are less likely to be compliant with treatment regimen compared with those with acute conditions;Citation31 non-adherence is one of the key factors in the real-life effectiveness of treatments for chronic disorders, such as psoriasis.Citation32 Medication non-adherence can have harmful effects on optimal management of psoriasis: non-adherence leads to poor outcomes, more hospitalizations, and significantly higher health care costs.Citation30,Citation33–Citation35 Our findings are consistent with previous investigationsCitation36,Citation37 that demonstrated a suboptimal adherence to topical treatments, likely reflecting the intermittent use of most topical products. For example, in one study, 46% of patients, who were prescribed topical therapies, reported low levels of adherence and about half of these reported that they use treatment only when they think it is necessary.Citation38 Nevertheless, we identified notable variations in the definitions and methodologies of how adherence was measured in previous research, making it difficult to compare the results of the studies assessing this issue.
One of the most relevant aspects of the present study is that the use of combined treatment with vitamin D and corticosteroid for the management of psoriasis could not only reduce the probability of non-adherence to therapy, and our data also showed that use of fixed combination is significantly associated with a lower probability of switching to other antipsoriatic treatments. In accordance with evidence from both randomized controlled trials and real-life data observed in previous analyses,Citation26,Citation28,Citation39,Citation40 the complementary mechanisms of action and potentially synergistic effects could lead to an improved tolerability and eventually to a better adherence of the patients. Also, the results of this study are in line with the findings of a published analysis showing that the use of fixed combination topical treatment may offer a good possibility for patients to maintain compliance with their therapy.Citation25,Citation41
Psoriasis treatments can be very expensive.Citation11,Citation42–Citation44 The costs of antipsoriatic medications vary considerably from relatively cheap topical treatments to highly expensive biological therapies.Citation45 Indeed, our study showed that the health care costs in the patients group who switched from topical drugs to DMARDs or biologic agents were considerable. Better treatment strategies could lead to faster clinical improvement and reduction in treatment cost, which would lower the overall expenditure of the health care system and society, allowing increased health investment in other areas.
The findings of this study should be contextualized in light of some limitations. Though the strength of our study is that this approach reflects real-life clinical practice situations, the results must be interpreted in the context of some limitations inherent to any observational study based on administrative DBs. Limitations include 1) lack of important clinical information in the data setting (such as severity of disease and outcome of treatment); 2) no lifestyle information was available; 3) adherence was estimated using pharmacy data on filled prescriptions, but no data on actual medication consumption were available; 4) the reasons for non-adherence to prescribed treatment among included patients are not retrievable from DBs; and 5) only a relatively limited sample size was analyzed, and the study was performed using the DBs of two LHUs. In addition, as we have focused our analysis on naïve patients who received at least two prescriptions of topical antipsoriatic drugs in a time space of 2 years, classified as “non-occasional users of topical drugs”, further studies are necessary to confirm and enhance the generalizability of the findings.
Conclusion
This retrospective analysis shows that the use of fixed-combination topical treatment can lead to improve the likelihood of patients being adherent to treatment and can decrease the likelihood of switching the treatment to DMARDs or biologics. Our findings are potentially helpful in assessing and improving the quality of care for psoriasis patients in clinical practice. Further research using a larger sample of patients are needed to confirm and contextualize our results.
Acknowledgments
Manuscript development was supported by unconditional funding from LEO Pharma.
Disclosure
The authors report no conflicts of interest in this work.
References
- de KorteJSprangersMAMombersFMBosJDQuality of life in patients with psoriasis: a systematic literature reviewJ Investig Dermatol Symp Proc200492140147
- GriffithsCEBarkerJNPathogenesis and clinical features of psoriasisLancet2007370958326327117658397
- HuertaCRiveroERodríguezLAIncidence and risk factors for psoriasis in the general populationArch Dermatol2007143121559156518087008
- Ragnarson TennvallGHjortsbergCBjarnasonATreatment patterns, treatment satisfaction, severity of disease problems, and quality of life in patients with psoriasis in three Nordic countriesActa Derm Venereol201393444244523138500
- NestleFOKaplanDHBarkerJPsoriasisN Engl J Med2009361549650919641206
- ParisiRSymmonsDPGriffithsCEMAshcroftDMIdentification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project TeamGlobal epidemiology of psoriasis: a systematic review of incidence and prevalenceJ Invest Dermatol2013133237738523014338
- Il trattamento della psoriasi nell’adulto. Linee guida SNLG-SS [The treatment of psoriasis in adults. Guidelines SNLG-SS] Available from: http://www.snlg-iss.it/cms/files/LG_Psoriasi.pdfAccessed January 16, 2017
- National Clinical Guideline Centre (UK)Psoriasis: Assessment and Management of PsoriasisLondonRoyal College of Physicians (UK)2012 [cited 2015 Aug 24]. (National Institute for Health and Clinical Excellence: Guidance). Available from: http://www.ncbi.nlm.nih.gov/books/NBK247829/
- MenterAGottliebAFeldmanSRGuidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologicsJ Am Acad Dermatol200858582685018423260
- MurphyGReichKIn touch with psoriasis: topical treatments and current guidelinesJ Eur Acad Dermatol Venereol201125Suppl 43821507077
- ColomboGLDi MatteoSBrunoGGirolomoniGVenaGACalcipotriol and betamethasone dipropionate in the treatment of mild-to-moderate psoriasis: a cost-effectiveness analysis of the ointment versus gel formulationClinicoecon Outcomes Res201226126823028233
- MenterAKormanNJElmetsCAAmerican Academy of Dermatology Work GroupGuidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusionsJ Am Acad Dermatol201165113717421306785
- MasonARMasonJCorkMDooleyGHancockHTopical treatments for chronic plaque psoriasisCochrane Database Syst Rev20133283CD00502823543539
- PerroneVSangiorgiDBudaSDegli EspostiLDisease progression and health care resource consumption in patients affected by hepatitis C virus in real practice settingClinicoecon Outcomes Res2016859159727789966
- Degli EspostiLSangiorgiDBudaSDegli EspostiEScaglioneFTherapy discontinuation or substitution in patients with cardiovascular disease, switching among different products of the same off-patent active substance: a ‘real-world’ retrospective cohort studyBMJ Open2016611e012003
- Agenzia Italiana del Farmaco (AIFA)Guideline for the classification and conduction of the observational studies on medicines2010 Available from: https://www.agenziafarmaco.gov.it/ricclin/sites/default/files/files_wysiwyg/files/CIRCULARS/Circular%2031st%20May%202010.pdfAccessed January 16, 2017
- ChaptiniCQuinnSMarshmanGDurable dermatology life quality index improvements in patients on biologics associated with psoriasis areas and severity index: a longitudinal studyAustralas J Dermatol2016573e72e7526010650
- HernánzJMSánchez-RegañaMIzuRMendiolaVGarcía-CalvoCClinical and therapeutic evaluation of patients with moderate to severe psoriasis in Spain: the secuence studyActas Dermosifiliogr201210310897904
- GonnellaJSLouisDZGozumMVCallahanCABarnesCADisease staging clinical and coded criteria. Version 5.26Ann Arbor, MIThomson Medstat2010
- European Medicine Agency (EMA)Calcipotriol/Betamethasone – Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Daivobet_30/WC500094997.pdfAccessed January 16, 2017
- SvedbomADalénJMamoloCCappelleriJCPeterssonIFStåhleMTreatment patterns with topicals, traditional systemics and biologics in psoriasis – a Swedish database analysisJ Eur Acad Dermatol Venereol201529221522324813476
- Ramirez-FortMKLevinAAAuSCGottliebABContinuous versus intermittent therapy for moderate-to-severe psoriasisClin Exp Rheumatol2013314 Suppl 78S63S7024129141
- EissingLRadtkeMAZanderNAugustinMBarriers to guideline-compliant psoriasis care: analyses and conceptsJ Eur Acad Dermatol Venereol201630456957526538533
- SticherlingMEickeCAngerTPracticability of combined treatment with calcipotriol/betamethasone gel (Daivobet® Gel) and improvement of quality of life in patients with psoriasisJ Dtsch Dermatol Ges201311542042723437972
- HendriksAGKeijsersRRde JongEMSeygerMMvan de KerkhofPCEfficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature reviewJ Eur Acad Dermatol Venereol201327893195123279069
- KoyamaGLiuJScaffidiAKhazraeeMEpsteinBNovel Approaches to topical psoriasis therapyInt J Pharm Compd201519535736526775441
- RogalskiCCalcipotriol/betamethasone for the treatment of psoriasis: efficacy, safety, and patient acceptabilityPsoriasis Targets Ther2015597107
- NeriLMiracapilloATreatment adherence and real-life effectiveness of topical therapy in patients with mild or moderate psoriasis: uptake of scientific evidence in clinical practice and dermatologists’ preferences for alternative treatment optionsG Ital Dermatol Venereol20151501192625521808
- MorrillJAShresthaMGrantRWBarriers to the treatment of hepatitis C. Patient, provider, and system factorsJ Gen Intern Med200520875475816050887
- VangeliEBakhshiSBakerAA systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseasesAdv Ther20153211983102826547912
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- BewleyAPageBMaximizing patient adherence for optimal outcomes in psoriasisJ Eur Acad Dermatol Venereol201125Suppl 491421507078
- McDonaldHPGargAXHaynesRBInterventions to enhance patient adherence to medication prescriptions: scientific reviewJAMA2002288222868287912472329
- DevauxSCastelaAArchierEAdherence to topical treatment in psoriasis: a systematic literature reviewJ Eur Acad Dermatol Venereol201226Suppl 3616722512682
- FeldmanSRHornEJBalkrishnanRInternational Psoriasis CouncilPsoriasis: improving adherence to topical therapyJ Am Acad Dermatol20085961009101618835062
- NelsonPABarkerZGriffithsCECordingleyLChew-GrahamCAIMPACT Team‘On the surface’: a qualitative study of GPs’ and patients’ perspectives on psoriasisBMC Fam Pract20131415824138455
- ThorneloeRJBundyCGriffithsCEAshcroftDMCordingleyLAdherence to medication in patients with psoriasis: a systematic literature reviewBr J Dermatol20131681203122963128
- BewleyABurrageDMErsserSJHansenMWardCIdentifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative studyJ Eur Acad Dermatol Venereol201428676377023663069
- DaudénEBewleyALambertJGirolomoniGCambazardFReichKExpert recommendations: the use of the fixed combination calcipotriol and betamethasone dipropionate gel for the topical treatment of psoriasisJ Eur Acad Dermatol Venereol201428Suppl 2223224684740
- GirolomoniGVenaGAAyalaFConsensus on the use of the fixed combination calcipotriol/betamethasone dipropionate in the treatment of plaque psoriasisG Ital Dermatol Venereol2012147660962423149707
- VakirlisEKastanisAIoannidesDCalcipotriol/betamethasone dipropionate in the treatment of psoriasis vulgarisTher Clin Risk Manag20084114114818728704
- FeldmanSRBurudpakdeeCGalaSNanavatyMMallyaUGThe economic burden of psoriasis: a systematic literature reviewExpert Rev Pharmacoecon Outcomes Res201414568570525052261
- SvedbomADahlénJMamoloCEconomic burden of psoriasis and potential cost offsets with biologic treatment: a Swedish register analysisActa Derm Venereol201696565165726716136
- Burgos-PolRMartínez-SesmeroJMVentura-CerdáJMElíasICalotoMTCasadoMÁThe cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic reviewActas Dermosifiliogr2016107757759027316590
- PearceDJThomasCGFleischerABJrFeldmanSRThe cost of psoriasis therapies: considerations for therapy selectionDermatol Nurs200416542142843215624706
