Abstract
Purpose:
To assess change in hospitalization and cost of care from 6 months pre- to 6 months post-initiation on any depot antipsychotic among schizophrenia patients.
Patients and methods:
Using a large United States commercial claims and encounters database, patients younger than 65 years diagnosed with schizophrenia were identified. Patients initiated on a depot antipsychotic were studied in a mirror-image design to assess change in hospitalization rates, mean duration hospitalized, and hospitalization cost. McNemar’s test and paired t-tests compared the proportions of patients hospitalized and the mean duration. Paired t-test and bootstrapping methods compared costs.
Results:
In these patients (n = 147), psychiatric hospitalizations declined from 49.7% pre-initiation to 22.4% post-initiation (P < 0.001), and the mean hospitalized duration for psychiatric purposes numerically declined from 7.3 to 4.7 days (P = 0.05). Total health care costs declined from $11,111 to $7884 (P < 0.05) driven by reduction in costs for psychiatric hospitalizations from $5384 to $2538 (P < 0.05).
Conclusion:
Initiation of depot antipsychotic therapy appeared to be associated with a decline in hospitalization rates and costs. Current findings suggest that treatment with depot antipsychotics may be a cost-effective option for a subgroup of patients with schizophrenia who are at high risk of nonadherence with their oral antipsychotic medication regimen.
Introduction
Longer treatment duration with antipsychotics is associated with better clinical and functional outcomes.Citation1,Citation2 Despite the benefits, many patients have difficulty sustaining maintenance treatment because of difficulties adhering to daily regimens of oral medications. More than 35% of patients have adherence issues during their first 4 to 6 weeks of treatmentCitation3 and by 2 years, 75% are considered only partially adherent.Citation4 A 1998 study reported that patients receiving antipsychotics took an average of only 58% of the recommended amount of the medications.Citation5
Antipsychotics in long-acting injection form (“depot”) were developed in the 1960s to improve long-term schizophrenia treatment.Citation6 Depot antipsychotics are often used to treat schizophrenia patients who are at high risk of nonadherence with their oral antipsychotics and, thus, also at a possible high risk of relapse and hospitalization.Citation7,Citation8 Treatment with a depot antipsychotic requires the patient to visit the clinic every 1 to 6 weeks to receive an intramuscular injection, which eliminates the patient’s need to take the oral antipsychotic medication daily.
Although the efficacy of oral antipsychotic medications has been compared with depot medications by using randomized clinical trials (RCTs), there are difficulties using RCTs in this patient group. For example, the patients who are most appropriate for depot treatment tend to have additional problems such as substance abuse and legal issues,Citation9 making them less likely to enroll in RCTs. Patients who are switched to depot antipsychotics generally have a history of poor adherence to oral antipsychotics and are frequently coaxed into depot treatment via legal commitments (“compulsory treatment”) and thus unable to give a valid informed consent for a clinical trial. It has also been suggested that RCTs are likely to recruit adherent patients selectively by excluding patients with characteristics that are associated with poor adherence (eg, comorbid substance abuse).Citation10 Yet these patients with poor adherence are the best candidates for depot treatment.
In contrast with RCTs, retrospective mirror-image studies do not require the patients to enroll in a study, as their medication and use of services in usual care are routinely captured in claims databases. Importantly, the mirror-image study design does not require a parallel active control group, as each patient serves as his or her own control. In these studies, patients maintained on oral medication are switched to depot medications and the outcome before and after the switch is compared. While some researchers reported a decline in the number of hospital admissions after initiation on depot antipsychotics,Citation11,Citation12 other researchers reported an increase in hospitalization days and resource utilization.Citation13–Citation15 Prior mirror-image research publications have been confined to patients treated in the United Kingdom with one exception: one United States-based publicationCitation16 reported change in hospitalization and resource utilization after initiation of depot antipsychotics. In that study, from the Ohio Veterans Affairs (VA) Healthcare System, 75% of patients experienced a psychiatric-related hospitalization before depot initiation but only 42% were hospitalized during an equal amount of time after initiation. In addition to fewer psychiatric-related hospitalizations, these investigators reported shorter length of stay, fewer inpatient days per month, and one additional outpatient visit per month post-initiation. These researchers expressed a need for further United States studies in a non-VA population.
To fill this information gap and expand on the sparse United States-based research findings, the present mirror-image study aimed to assess change in hospitalization risk from 6 months pre- to 6 months post-initiation on any depot antipsychotic among patients treated for schizophrenia in the United States. Hospitalization risk was defined as the proportion of patients hospitalized and the number of psychiatric hospital admissions. Secondary objectives included assessment of the change in patients’ adherence level, assessment of the change in utilization of outpatient services (emergency room, day treatment, and other outpatient visits), and assessment of the change in total direct cost and cost components (any inpatient hospitalization, psychiatric hospitalization, outpatient visits, and medication cost).
Material and methods
Data source
The data source for this study was the Thomson Medstat MarketScan commercial claims and encounters databases (January 1, 2004 to March 31, 2008; MarketScan® Databases, Thomson Healthcare, Inc., Ann Arbor, MI). The databases capture person-specific clinical utilization, expenditures, and enrollment across inpatient, outpatient, prescription drug, and carve-out services from a selection of about 100 payers, including large employers, health plans, and government and public organizations. The MarketScan Databases link paid claims and encounter data over time and to detailed patient information across sites and types of providers, and over time.Citation17
Study sample selection
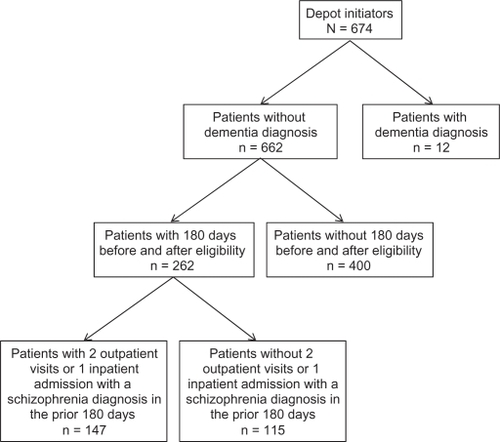
The sample selection consisted of patients (<65 years of age) who were diagnosed with schizophrenia (International Classification of Diseases [ICD]-9-CM codes 295.XX) between January 1, 2004 and March 31, 2008 and who had at least 2 outpatient visits or 1 inpatient hospitalization associated with the schizophrenia diagnosis. Patients diagnosed with dementia type disorder were excluded. Patients who were initiated on any depot antipsychotic, who had no depot injection in the 6 months before this injection, and who had continuous enrollment for the 6 months before and 6 months after the depot initiation date (“index date”) were included if the 2 outpatient visits or 1 inpatient hospitalization occurred within 180 days before the depot initiation. The index date was the date of the first depot injection.
Study measures
Patient demographics, including age and gender, were assessed for all patients. The specific antipsychotic depot medication, schizophrenia patients’ related medical comorbidities, and substance use were determined based on ICD-9-CM diagnosis codes during the 6-month pre-index period. During the 6-month pre-index period and 6-month post-index period, data were collected on the proportion of patients hospitalized at least once for any reason, hospitalized at least once with a psychiatric diagnosis, and hospitalized at least once with a schizophrenia diagnosis, the number of psychiatric hospital admissions, total number of days hospitalized for psychiatric purposes, adherence with antip-sychotic medication (defined as the Medication Possession Ratio [MPR] – the proportion of days the patient is in possession of any antipsychotic during each 180-day observation period), outpatient service use (emergency room, day treatment, and other office visits), total direct cost, and cost components (any hospitalization, psychiatric hospitalizations, outpatient services, and medication).
Statistical methods
Patients’ baseline characteristics, comorbidities, and drug use disorders in the 6-month pre-index period were summarized. Analyses comparing the pre- vs post-initiation data employed McNemar’s test for categorical variables, including the proportion of patients hospitalized for psychiatric reasons and the proportion of patients who used outpatient services, and paired t-tests to assess the continuous variables including the mean number of admissions, mean of total hospitalized duration, and MPR. Mean cost comparisons were conducted with paired t-tests and bootstrapping methods. Because cost data frequently do not exemplify a typical random distribution (ie, they are usually right-skewed and truncated at zero due to a small number of patients with high costs, a large number of patients with no costs, and the impossibility of costs less than zeroCitation18), bootstrapping methods were used. Nonparametric bootstrapping is a technique where an empirical distribution of the mean cost difference between groups is constructed through resampling with replacement from the observed cost data. Bootstrapping is an alternative for analysis of cost data because it uses a nonparametric approach, which can directly address arithmetic means without making assumptions about the shape of the distribution.Citation19 Bootstrap resampling (5000 iterations) was used to provide a nonparametric comparison of total cost as well as component costs (eg, any hospitalization cost, psychiatric hospitalization cost, outpatient cost, and medication cost) in the 6-month pre- vs post-index periods. No statistical adjustments were made for the multiple comparisons. Sensitivity analyses include examining the impact of acute care costs occurring just after the medication change, which may be incurred due to the failure on the prior treatment.Citation20
Results
From a total of 674 patients with schizophrenia who were initiated on depot antipsychotics, data from 147 patients met inclusion criteria and were included in the analyses (). Their baseline characteristics are presented in , showing that the mean age was the early 40s and slightly more than half of patients were male.
Table 1 Patient baseline characteristics
After initiation of depot antipsychotics, patients improved their medication adherence. Mean antipsychotic MPR increased from 36.8% in the 6 months preceding depot initiation to 60.0% in the 6 months after initiation (P < 0.001). After depot initiation, patients were less likely to be hospitalized for any reason, for any psychiatric reason, and for schizophrenia specifically (). During the 6 months preceding initiation, 79 patients (53.7%) were hospitalized for any reason compared with 44 patients (29.9%) in the 6 months after initiation (P < 0.001). Hospitalization for any psychiatric reason decreased from 73 patients (49.7%) to 33 patients (22.4%; P < 0.001). Hospitalization for schizophrenia decreased from 63 patients (42.9%) to 30 patients (20.4%; P < 0.001).
Table 2 Change in hospitalization parameters in the 6 months pre- vs 6 months post-depot initiation
The mean number of hospitalizations for any reason decreased from 0.78 to 0.41 (P < 0.001). The mean number of inpatient psychiatric admissions decreased from 0.67 to 0.31 (P < 0.001). The mean number of hospitalizations for schizophrenia decreased from 0.53 to 0.29 (P = 0.002). There were nonsignificant trends for decreases in the mean number of days of hospitalization for any reason (from 8.0 to 5.3; P = 0.067) and for psychiatric hospitalization (from 7.3 to 4.7; P = 0.054). The mean number of days of hospitalization for schizophrenia decreased from 5.7 to 4.0 but this change was not significant (P = 0.190).
During the 6 months preceding initiation, 36.1% of patients had 1 hospitalization for any reason, 12.2% had 2, and 5.5% had 3 or more. In the 6 months after initiation, 21.8% had 1 hospitalization for any reason, 5.4% had 2, and 2.7% had 3 or more. During the 6 months preceding initiation, 35.4% of patients had 1 hospitalization for psychiatric reasons, 12.2% had 2, and 2.1% had 3 or more. In the 6 months after initiation, 16.3% had 1 hospitalization for psychiatric reasons, 4.1% had 2, and 2.0% had 3 or more. During the 6 months preceding initiation, 34.0% of patients had 1 hospitalization for schizophrenia, 7.5% had 2, and 1.4% had 3 or more. In the 6 months after initiation, 14.3% had 1 hospitalization for schizophrenia, 4.1% had 2, and 2.0% had 3 or more.
Change in outpatient service use was not significant. From the 6 months before depot initiation to the 6 months after initiation, the percent of patients who used emergency room services changed from 25.2% to 19.1%, the percentage of patients who used day treatment changed from 56.5% to 53.7%, and the percentage of patients having office visits changed from 88.4% to 86.4%.
Mean total direct costs of treatment decreased from $11,111.30 in the 6 months before depot initiation to $7883.80 in the 6 months after initiation (P < 0.05; ). Median total direct costs decreased from $7089.40 to $4051.93. Mean total inpatient costs decreased from $6696.40 to $3593.20 (P < 0.05) and psychiatric-related inpatient costs decreased from $5384.20 to $2537.70 (P < 0.05). Total outpatient costs and total medication costs did not change significantly. Median total outpatient costs decreased from $1591.05 to $1297.62. Median total medication costs were slightly changed ($853.04 before depot initiation and $851.46 after initiation). The sensitivity analyses indicated that mean total direct costs of treatment decreased from $10,615.60 in the 6 months before depot initiation to $8379.50 in the 6 months after initiation (P < 0.05).
Table 3 Change in total cost (US$) and cost component in the 6 months pre- vs 6 months post-depot initiation
Discussion
This study used a mirror-image design to assess and compare hospitalization risk and health care costs during the 6 months before and 6 months after initiation of depot antipsychotics for the treatment of patients with schizophrenia in the United States. This study found an improvement in medication adherence, a decrease in the rate of psychiatric hospitalization for any reason, for any psychiatric reason, and for schizophrenia specifically, and a decrease in health care costs after patients initiated depot antipsychotics. These results suggest that depot antipsychotic therapy may be a cost-effective option for a subgroup of patients typically at high risk of nonadherence with their oral antipsychotic regimen.Citation9
Current findings are consistent with prior research in and outside the United States. A study in a VA population in the United States by Fuller et alCitation16 found decreases in psychiatric-related hospitalization from 75% to 42% after initiation of depot antipsychotics. In addition to fewer psychiatric-related hospitalizations, these researchers reported shorter length of stay, fewer inpatient days/month, and one additional outpatient visit/month post-initiation. Our results are also consistent with two United Kingdom studies that reported a decline in the proportion of patients requiring hospital admissions after initiation of depot antipsychotics.Citation11,Citation12 The Taylor et alCitation11 study reported a decline in hospital admission rate from 62% before to 22% after initiation of depot. Similarly, the study by Niaz and HaddadCitation12 found a reduction in hospital admissions, compulsory admissions, and total inpatient days.
Our study expanded on prior research by demonstrating an increase in medication adherence after initiating depot antipsychotics. This is important because patients being treated for schizophrenia often have problems with adherence to medications, and stopping medication often has serious consequences.Citation21 Increased adherence with depot antipsychotics has the additional benefit of allowing clinicians to differentiate compliance failure from efficacy failure which can reduce the use of rescue medications and the need for switching to a second-choice antipsychotic.Citation22 Our study also demonstrated potential cost savings following depot initiation. Cost analyses found a significant decline in total cost of treatment, driven by decline in hospitalization cost from pre- to-post-depot initiation.
Results need to be considered in the context of the study limitations. The sample size was rather small (n = 147). Also, the study design is devoid of a control group. In this mirror-image study, each patient served as his or her own control. As such, observed changes from pre- to post-depot initiation may reflect regression to the mean. Thus, we cannot determine if similar or even better results would have occurred with a different intervention. In addition, we used data from a large United States commercial claims and encounters database. Findings may not be generalizable to patients with schizophrenia who lack commercial insurance, which is a large segment of the schizophrenia population in the United States.
Conclusion
In summary, results from this study suggest that initiating depot antipsychotic therapy is associated with declines in hospitalization rates and related costs, compared with the prior treatment periods. These findings also suggest that treatment with depot antipsychotics may be a cost-effective option for a subgroup of patients with schizophrenia who are at high risk of nonadherence with their oral antipsychotic medication regimen.
Acknowledgments/disclosure
This work was supported by Eli Lilly and Company. All authors are full-time employees and minor shareholders of Eli Lilly and Company.
References
- Liu-SeifertHAdamsDHKinonBJDiscontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugsBMC Med200532116375765
- DunayevichEAscher-SvanumHZhaoFLonger time to antipsychotic treatment discontinuation for any cause is associated with better functional outcomes for patients with schizophrenia, schizophreniform disorder, or schizoaffective disorderJ Clin Psychiatry2007681163117117854239
- ChuePLong-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychoticNeuropsychiatr Dis Treat20073133919300536
- WeidenPZygmuntAMedication noncompliance in schizophrenia. Part I. AssessmentJ Pract Psychiatry Behav Health19973106110
- CramerJARosenheckRCompliance with medication regimens for mental and physical disordersPsychiatr Serv1998491962019575004
- SimpsonGMA brief history of depot neurolepticsJ Clin Psychiatry199845346143745
- DavisJMMatalonLWatanabeMDBlakeLDepot antipsychotic drugs. Place in therapyDrugs1994477417737520856
- KaneJMAgugliaEAltamuraACGuidelines for depot antipsychotic treatment in schizophreniaEur Neuropsychopharmacol1998855669452941
- ShiLAscher-SvanumHZhuBFariesDMontgomeryWMarderSRCharacteristics and use patterns of patients taking first-generation depot antipsychotics or oral antipsychotics for schizophreniaPsychiatr Serv20075848248817412849
- HaddadPMTaylorMNiazOSFirst-generation antipsychotic long-acting injections vs oral antipsychotics in schizophrenia: systematic review of randomised controlled trials and observational studiesBr J Psychiatry Suppl200952S20S2819880913
- TaylorMCurrieALloydKPriceMPeperellKImpact of risperidone long acting injection on resource utilization in psychiatric secondary careJ Psychopharmacol20082212813118308820
- NiazOSHaddadPMThirty-five months experience of risperidone long-acting injection in a UK psychiatric service including a mirror-image analysis of in-patient careActa Psychiatr Scand2007116364617559599
- TaylorDMYoungCLMaceSPatelMXEarly clinical experience with risperidone long-acting injection: a prospective, 6-month follow-up of 100 patientsJ Clin Psychiatry2004651076108315323592
- YoungCLTaylorDMHealth resource utilization associated with switching to risperidone long-acting injectionActa Psychiatr Scand2006114142016774656
- TaylorDFischettiCSparshattAThomasABisharaDCorneliusVRisperidone long-acting injection: a 6-year mirror-image study of healthcare resource useActa Psychiatr Scand20091209710119207128
- FullerMShermockKRussoPHospitalisation and resource utilisation in patients with schizophrenia following initiation of risperidone long-acting therapy in the Veterans Affairs Healthcare SystemJ Med Econ20091231732419817665
- Thomson HealthcareMarketScan® research databases user guide and database dictionaryAnn Arbor, MIThomson Healthcare, Inc2007
- RamseySWillkeRBriggsAGood research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force reportValue Health2005852153316176491
- FariesDEPengXObenchainRLCosts and cost effectiveness analysis using propensity score bin bootstrappingFariesDELeonACHaroJMObenchainRLAnalysis of Observational Health Care Data Using SASCary, NCSAS Institute2010
- FariesDENyhuisAWAscher-SvanumHMethodological issues in assessing changes in costs pre- and post-medication switch: a schizophrenia study exampleCost Eff Resour Alloc200971119473545
- ByerlyMJNakoneznyPALescouflairEAntipsychotic medication adherence in schizophreniaPsychiatr Clin North Am20073043745217720031
- KeithSJKaneJMPartial compliance and patient consequences in schizophrenia: our patients can do betterJ Clin Psychiatry2003641308131514658944