Abstract
Aim
This study assesses the cost-effectiveness of secukinumab vs currently licensed biologics for the treatment of ankylosing spondylitis (AS) from the Finnish health care system perspective.
Methods
A semi-Markov model compared secukinumab with adalimumab, adalimumab biosimilar, certolizumab pegol, etanercept, etanercept biosimilar, golimumab, and infliximab in a biologic-naïve population over a lifetime horizon. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) was used to assess the treatment response. Efficacy inputs were obtained from the network meta-analysis, and other model inputs were obtained from the published literature and Finnish sources. Main study outcomes included quality-adjusted life years (QALYs) gained and incremental cost-effectiveness ratio in terms of cost per QALY gained. Robustness of results was confirmed by sensitivity analyses and alternative scenario analyses.
Results
Secukinumab achieved highest QALYs (13.1) at lowest expected lifetime cost (€279,872) vs other comparators in biologic-naïve AS patients in the base case analysis, thus it dominated other biologics. Golimumab had a second highest QALYs (12.9) at the total cost of €309,551. Results were sensitive to variation in BASDAI 50 response for secukinumab, baseline Bath Ankylosing Spondylitis Functional Index (BASFI) score across all drugs, change in BASDAI and BASFI scores, and discount rates as observed in the one-way sensitivity analyses. Secukinumab was either dominant or cost-effective treatment in different alternative scenarios.
Conclusion
Secukinumab presented itself to be the dominant (ie, less costly and more effective) treatment vs other comparators for the biologic-naïve patients with AS in Finland.
Introduction
Ankylosing spondylitis (AS) is a chronic, systemic, inflammatory disease that affects primarily the sacroiliac joints and spine and leads to back pain, stiffness, discomfort, fatigue, impaired spinal mobility, and postural abnormalities.Citation1 AS can also inflame peripheral joints and enthesesCitation2 and has extra-articular manifestation.Citation3 The New York classification criteria for AS require radiographic sacroiliitis, which may take many years to develop, and hence the diagnosis as well as disease management is often delayed. The epidemiological data for AS are scarce in Finland. The annual incidence of AS or nonradiographic axial spondyloarthritis patients requiring advanced treatments beyond NSAIDs has been 17 per 100,000 adults during 2012–2014 in Finland.Citation4
AS manifests itself usually in early adulthood (particularly during the third decade of life) and impacts patients for most of their life. AS is associated with decreased quality of life (QoL), increased mortality, and substantial health care-related costs, making it a burden to the patient and society.Citation5 The AS-related costs were reported to vary across the European countries, and indirect costs contribute to the major component of the total costs ranging from 53.4% to 62%. Also, as the disease severity increases, direct costs increase two times while indirect costs increase almost four times.Citation6
As per the recently updated treatment recommendations (2016) from ASAS and the European League Against Rheumatism,Citation7 NSAIDs and physical therapy have been recommended as first-line treatment for AS; however, the disease becomes refractory to these agents over time.Citation8–Citation10 The use of anti-tumor necrosis factor (anti-TNF) biologics or IL-17A inhibitor is recommended in axial spondyloarthritis after the failure of NSAIDs. In Finland, however, biologic drugs are unfortunately not reimbursed until at least one traditional disease-modifying antirheumatic drug (DMARD) (sulfasalazine and methotrexate) has been tried or if DMARD is contraindicated.Citation11
Despite major improvement in treatment results with the adoption of anti-TNFs, up to 40% of the patients do not respond sufficiently to or tolerate anti-TNFs or efficacy may reduce over time,Citation12 indicating a significant unmet medical need in the treatment of AS patients. If patients are not responsive to initial biologic therapy, it is recommended to switch a second anti-TNF or secukinumab.Citation7 These updated recommendations also include an overarching principle that addresses the cost issues with AS for the very first time. It highlights the need for “best care” along with the use of cost-effectiveness analyses results while making treatment decisions.
Secukinumab is the first and fully human recombinant antihuman IL-17A IgG1 monoclonal antibody, which is licensed for use in AS.Citation8 Secukinumab was approved by European Medicines Agency (EMA) in 2015 for the treatment of adult patients with active AS who have responded inadequately to conventional therapy.Citation13 Secukinumab is a highly efficacious treatment for AS providing sustained improvement in AS signs and symptoms, a rapid onset of action, and a favorable safety profile compared with placebo according to the results of multiple phase 3 clinical trials.Citation14–Citation17 Additionally, secukinumab has demonstrated superior efficacy in indirect comparison methods (matching-adjusted indirect comparison [MAIC],Citation18 network meta-analysis [NMA]).Citation19
This analysis reports the results of a cost-effectiveness study of secukinumab in patients with AS who have not been previously treated with any biologic (biologic-naïve) in Finland. Additionally, cost-effectiveness of secukinumab was also analyzed in mixed population (a combination of both biologic-naïve and biologic-experienced patients [patients who had been previously treated with biologics]) in the alternative scenario analysis.
Methods
Patient population and interventions
Adult AS patients (18 years or older) fulfilling the modified New York criteria for AS and having inadequate response to NSAIDs were included in the analysis. The patients who were naïve to biologic therapy were considered for the base-case analysis. For an alternate analysis, secukinumab 150 mg results were also analyzed in mixed population (a combination of both biologic-naïve and biologic-experienced patients). Population data inputs were obtained as weighted average across all patients from the MEASURE 1 and MEASURE 2 pooled trial data (Table S1).
The model evaluated the cost-effectiveness of subcutaneous (s.c.) secukinumab 150 mg compared with the currently licensed biologics (s.c. treatments adalimumab and its bio-similar, certolizumab pegol, etanercept and its biosimilar, golimumab and intravenous [i.v.] infliximab) for the treatment of AS from the Finnish health care system perspective. The list of dose and dosage frequencies of the treatments for this analysis is listed in Table S2. These are based on the EMA authorization approval.Citation20
Model structure
A semi-Markov model was developed using Microsoft Excel (). The semi-Markov model structure was chosen due to time-dependent probabilities associated with mortality, unlike standard Markov model where transition probabilities are constant over time. In the model, patients start a given treatment and response to the treatment was assessed at the end of 3 months by considering a 50% improvement from baseline in the initial Bath Ankylosing Spondylitis Disease Activity Index (BASDAI 50) response. Previous economic evaluations of anti-TNFs in AS considered similar model to assess the treatment response.Citation21
Figure 1 Markov model structure with 3-month induction period.
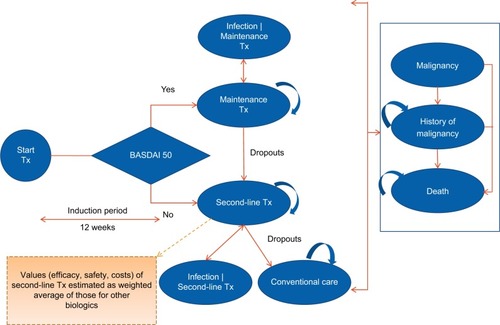
Patients transitioned to different health state based on the probabilities of BASDAI response rate, malignancy, chance of serious infection, dropout rate, and death (). Adverse events (AEs) such as serious infection (including tuberculosis) and malignancy were included as separate health states to better track the associated costs and QoL effects. Patients who experienced infections were allowed to continue biologic treatment or switch to conventional care. In contrast, patients discontinued biologic treatment upon entering the “malignancy” health state. Patients in “malignancy” state were assumed to be at a higher mortality risk for 5 years into that state.
Patients withdrawing from initial biologic treatment were treated long-term with the subsequent biologic therapy. Considering data on the effectiveness of subsequent biologics were limited; average values of efficacy, treatment withdrawal rates, cost, and AE rates were used for all biologics. Patients receiving subsequent-line biologics either stayed on the treatment for rest of time horizon or could drop out to conventional care. Since there was no discontinuation rate available for patients dropping out from subsequent-line biologic to conventional therapy, it was assumed minimum of annual dropout rate from year 2 onward of all biologics (ie, annual dropout rate of 1.6% was applied for transition from second-line treatment to conventional care, see Table S3).
Model inputs
Clinical inputs
Comparative effectiveness data for treatments were obtained from a Bayesian fixed effects NMA,Citation22 which included a total of seven trials with 1,361 biologic-naïve patients. The main clinical input was BASDAI 50 response at 3 months, which was used as primary response criteria (). Although the use of Ankylosing Spondylitis Disease Activity Score (ASDAS) as a primary response criteria measure was discussed when building the model, there are no sufficient data from trials available from different anti-TNFs to establish a sufficient data basis for a valid health economic model. Also, ASDAS is closely related to BASDAI, as three out of the six BASDAI items are used to calculate ASDAS and a study by Eder et al found that ASDAS was not superior to BASDAI in its ability to discriminate between high and low disease activity states in AS.Citation23 Moreover, the use of BASDAI 50 is consistent with existing British Society of Rheumatology guidelinesCitation24 and the National Institute for Health and Care Excellence appraisals for AS.Citation25,Citation26 Additional clinical inputs included short-term changes in BASDAI and Bath Ankylosing Spondylitis Functional Index (BASFI) scores for responders and nonresponders (), long-term changes in BASFI captured through modified Stoke Ankylosing Spondylitis Spinal Score (), and biologic withdrawal rates (which were treatment specific and obtained for year 1 as well as year 2 and beyond) (Table S3).
Table 1 BASDAI 50 response at 3 months in biologic-naïve patients
Table 2 Short-term changes in BASDAI and BASFI in biologic-naïve patients
Table 3 Long-term changes in BASFI
Resource utilization and cost data
Four types of direct medical costs were incorporated in the model: drug acquisition costs, disease-related costs, medical support costs, and AE costs. Local unit costs were valued using public health care cost index, and where needed, costs were inflated per the 2017 exchange rate for Euro (Tables S4 and S6). The drug acquisition costs for secukinumab 150 mg and for brand comparators () were obtained from the Finnish medicinal products and price database.Citation27 Biosimilar pricing for etanercept and adalimumab was assumed to be 30% less than brand; as they were not, at the time of analyses, available in the market and were only used for sensitivity analysis. Additional drug administration costs were considered for i.v. administration of infliximab compared with other s.c. treatments.
Table 4 Drug acquisition costs for biologics in Finland
Disease-related costs account for the AS disease management costs incurred. These costs are estimated based on an exponential BASFI regression model:Citation28 Cost (€)×1,508.99 € = EXP (0.213 BASFI). Costs were calculated for 3 months according to the model cycle length.
Costs associated with medical support resources are presented in Table S4. Incidence of AEs and cost per event are available in supplementary materials (Tables S5 and S6).
Other inputs
Utility weight inputs were used to calculate quality-adjusted life years (QALYs) to reflect the improvement in QoL experienced by patients who achieve various levels of BASDAI and BASFI response. A linear mixed model was used to fit EuroQol 5-dimensions questionnaire utility score as a response variable, and BASDAI and BASFI scores, age, and sex as predictors. The coefficients for the regression equation were taken from the MEASURE 1 and MEASURE 2 pooled trial data (Table S7).
Three types of mortality risks were included in the analysis. Patients with AS have higher mortality rates compared with the general population. Disease-specific mortality was included as relative risks to account for patients having mortality vs the general populationCitation29 (Table S8). In addition, the increased risk of mortality due to AEs, such as serious infection or tuberculosis and malignancy, was obtained from the literatureCitation30 (Table S8). All-cause age-related mortality was estimated using National Life Tables for Finland (Statistics Finland, 2015).Citation31
Base-case analysis
The primary effectiveness outcome was QALY. Total costs and QALYs were estimated for all treatments. First, it was checked if secukinumab is a dominant treatment option (having higher QALYs at a lower cost vs comparators). In case of nondominance, an incremental cost-effectiveness ratio was reported.Citation32
The primary analysis was conducted in biologic-naïve population for 60 years of time horizon (lifetime) to account for the chronic nature of the disease. BASFI rebound was assumed to occur according to “natural history” for patients who discontinue biologic therapy. Annual discount rates were applied at 3% for both costs and outcomes, from the second year onward. The base-case analysis was done from a Finnish payer perspective; hence, only direct costs were included.
Sensitivity analysis
Three different sensitivity analyses (probabilistic, deterministic, and alternative scenario analyses) assessed the impact of changes in the input parameters on outcomes.
In the one-way sensitivity analysis, one parameter is varied at a time using lower and upper bounds of 95% CI, and their effects on results were estimated. Inputs and distribution used for each parameter in base-case population are shown in Table S9.
A probabilistic sensitivity analysis evaluated the impact of simultaneous variation in clinical outcome and resource utilization parameters on the model results. The results of the probabilistic sensitivity analysis were presented using cost-effectiveness acceptability curves calculated from the net monetary benefit statistic across a wide range of willingness-to-pay (WTP) thresholds. Also, it allows for the comparison of all treatment regimens simultaneously for each scenario.
Various alternative scenarios were analyzed using following alternative settings or input values: mixed population (biologic-naïve and experienced), alternative BASFI rebound assumption, AE disutilities, utilities sourced from McLeod et al,Citation33 inclusion of indirect costs, and exclusion of disease-specific costs.
Results
Base-case results
Secukinumab 150 mg was compared with the other biologics to assess its cost-effectiveness in the biologic-naïve population. Patients treated with secukinumab 150 mg achieved highest expected QALYs of 13.1 at lowest expected total cost (€279,872) in comparison with all other comparators when considering 60 years of time horizon (). In addition, patients treated with secukinumab had better treatment outcomes; they spent more time (27.9 years) in the BASDAI 50 response state than patients in other biologics. Time spent in the BASDAI 50 response state corresponds to the time patients spend on maintenance treatment after exhibiting treatment response.
Table 5 Health outcomes, direct costs, and ICER for secukinumab 150 mg vs comparators over a lifetime horizon (60 years)
Apart from total costs, disaggregated cost components (drug costs, administration costs, disease-related costs, and AE-related costs) that contribute to the total costs for each treatment are also presented in supplementary materials (Table S10). Post-discontinuation costs associated with drug, disease, administration, and monitoring were much lower for patients who started on secukinumab vs other biologics, leading to the lower total costs for secukinumab when compared with other biologics.
Overall, secukinumab was less costly and more effective than the comparators and presented itself to be a dominant treatment for biologic-naïve patients with AS in Finland. The QALYs gained and corresponding total direct costs of other biologic treatment options considered are presented in .
Sensitivity analysis
One-way sensitivity analysis
In one-way sensitivity analysis, BASDAI 50 for secukinumab, baseline BASFI score for nonresponders across all drugs, change in BASDAI and BASFI scores in responders, and discount rates had the highest impact on incremental NMB across all biologics at WTP of €30,000 (Figure S1).
Probabilistic sensitivity analyses
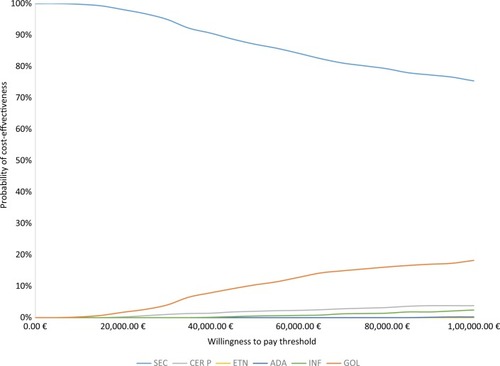
In the probabilistic sensitivity analysis, secukinumab 150 mg had the highest probability of being cost-effective vs other comparators at different WTP thresholds in the biologic-naïve population (). The probability of secukinumab being cost-effective was 98% at WTP levels of €20,000 per QALY gain. Additionally, secukinumab had 95%, 87%, and 75% probability of being cost-effective at other WTP thresholds of €30,000, €50,000, and €100,000 per QALY gained, respectively (). The means of the total QALYs, total cost, and NMB are summarized in supplementary materials (Table S11). Comparing the means of costs and QALYs from probabilistic sensitivity analysis with base-case results showed minimal difference, indicating the robustness of the results and lower uncertainty in the parameters.
Scenario analyses
Results for the following scenarios are presented: overall population including a mix of biologic-naïve and experienced AS patients, alternative BASFI rebound assumption, an analysis with AE disutilities, an analysis assuming utilities sourced from McLeod et al, indirect costs, and disease-specific costs ().
Table 6 Alternative scenario analyses: cost-effectiveness results for secukinumab vs other biologics by varying base-case assumptions
In one of the alternative scenario analysis done on mixed population (naïve and experienced biologic users), secukinumab 150 mg dominated all its comparators with higher QALYs and lower costs. Similarly, in other alternative scenarios, except for the scenario including indirect cost, it was also observed that secukinumab dominated all its comparators with higher QALYs and lower costs (). In the scenario when indirect cost was included, secukinumab dominated all branded biologics and was cost-effective against biosimilars. Detailed results regarding alternative scenarios are available in supplementary materials (Tables S12–S17).
Discussion
Secukinumab 150 mg dominated all its currently licensed comparators (s.c. treatments adalimumab and its biosimilar, certolizumab pegol, etanercept and its biosimilar, golimumab, and i.v. infliximab) in the treatment of biologic-naïve AS patients in Finland.
The analysis was carried out from the perspective of the Finnish health care system. Secukinumab 150 mg had better health outcomes (calculated in terms of QALYs) at lowest total cost, which could be beneficial to the patient and health care system as a whole. Model uses multiple parameters like BASDAI 50, change in BASDAI, change in BASFI, discontinuation rates, utility values, and safety, and these parameters have different impact on the final results. Particularly, factors such as AEs and discontinuation rates have a strong impact on the lifetime QALYs, leading to higher QALYs for secukinumab vs other comparators over 60 years of time horizon. The robustness of the analyses was confirmed by one-way sensitivity analyses, probabilistic analyses, and scenario analyses.
This is among the first full-length studies assessing cost-effectiveness of secukinumab 150 mg compared with other biologics. However, other modeling studies have been conducted in different countries such as Canada,Citation34 UK,Citation35,Citation36 Turkey,Citation37 Colombia,Citation38 Bulgaria,Citation39 and Russia;Citation40 which reported similar results. All these studies reported secukinumab to be dominant (more effective and less costly) over anti-TNFs in biologic-naïve as well as biologic-experienced AS population. Across multiple studies, secukinumab resulted in an additional 0.20–0.86 QALY gain at a total cost, which was €4,644 to €9,134 lower compared with other biologics.Citation34,Citation37,Citation38 The current study is also the first to assess cost-effectiveness of secukinumab in a Finnish context. Thus, these findings add important evidence to support the use of secukinumab while making a comparison of this new class of biologic to anti-TNF agents.
Strengths and limitations
The cost-effectiveness model structure and many of its assumptions were based on models used in previous assessments of biologics in the treatment of AS. In the absence of direct comparative evidence from clinical trials, the relative efficacy of licensed treatments for AS was evaluated through a mixed treatment comparison using NMA, which is considered as standard modeling methodology in the field. We used a lifetime horizon (60 years) for base-case analysis, which is more appropriate to use for chronic disease like AS. Moreover, analyses included detailed cost details (such as costs for drug acquisition, hospitalization, AEs) to estimate economic burden of disease. We also established the robustness of our results through probabilistic and deterministic sensitivity analysis.
The current analysis has several limitations. The major limitations of this analysis may be related to the scarcity of data and the complexity coupled with the long-term duration of the disease. There might be a potential underestimation of long-term efficacy of secukinumab 150 mg as the effectiveness data are derived from NMA (with data up to week 16). However, an extension of MEASURE trial has established the significant clinical efficacy of secukinumab for >3 years in AS patients.Citation41 In addition, secukinumab has shown statistically significant improvement in efficacy (in terms of ASAS20 and 40 responses) in comparison with adalimumab in MAIC for up to 52 weeks.Citation18
Another limitation of this study is the lack of data in all subpopulations for available comparators and the lack of efficacy data for subsequent biologic treatment after withdrawal from first-line biologic. Thus, data for comparators that did not report all necessary subpopulations or outcomes were set to those of the average of other bio-logics in the NMA. Although a country-specific (Finland) cost-effectiveness model was built, the globally conducted clinical trials were used as a basis for efficacy parameters, which could be considered as one of the limitations of the present analysis.
The subsequent-line biologic treatment was modeled using average values of the efficacy, costs, and AE incidence rates across all biologics in the model. Patients with AS are reported to have a significant impairment in work productivity.Citation42–Citation44 The base-case analysis did not take into account any indirect costs (mainly in the form of productivity loss) associated with AS; however, indirect cost was included as a part of the alternative scenario analysis. In the real-world clinical practice, patients having disease remission or low disease activity can be managed by tapering the dose of biologics (by reducing the dose or increasing the interval between dosing frequency) to reduce the cost of treatment.Citation45–Citation49 However, for secukinumab, such dose tapering is not recommended (not part of drug label). We would need robust long-term clinical effectiveness data for all biologic treatments (including secukinumab) from real-world settings to enable comparison based on dose tapering. Once such data become available for all biologics, it can be included in the cost-effectiveness analyses. Currently, analysis considered drug withdrawal rates from clinical trials. In future, this analysis can also be rerun using drug withdrawal rates from long-term registries once such data for secukinumab become available.
Conclusion
Secukinumab 150 mg dominated all other biologics (more QALYs at lower costs) in the biologic-naïve population in Finland for the treatment of AS over a lifetime horizon. This analysis implied that secukinumab is the most cost-effective biologic option for the treatment of AS patients unresponsive to conventional therapy in Finland.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Kavita Rodha and Niraj Modi (Novartis Healthcare Private Limited, Hyderabad, India) for editorial writing support. This study was funded by Novartis Pharma AG, Basel, Switzerland.
Disclosure
TP is a full-time employee of Novartis Finland Oy, Espoo, Finland. KP receives honoraria from Abbvie, Bristol-Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB; and congress trips from Abbvie and Pfizer outside the submitted work. DM and PG are full-time employees of Novartis Product Lifecycle Services-NBS, Novartis Health-care Private Limited, Hyderabad, India. JM is a founding partner of ESiOR Oy, which provides health economic, outcome research, and market access services for pharmaceutical and medical companies, as well as hospitals and other health and social care providers. The authors report no other conflicts of interest in this work.
References
- Spondylitis Association of AmericaOverview of Ankylosing Spondylitis Available from: https://www.spondylitis.org/Ankylosing-SpondylitisAccessed January 4, 2018
- SchettGLoriesRJD’AgostinoMAEnthesitis: from pathophysiology to treatmentNat Rev Rheumatol2017131273174129158573
- WenkerKJQuintJMAnkylosing SpondylitisTreasure Island (FL)StatPearls Publishing LLC2018
- RantalaihoVKautiainenHVirtaLPuolakkaKFRI0560 the increase in the nationwide incidence of inflammatory rheumatic diseases in Finland during this millenniumAnn Rheum Dis201675Suppl 2643644
- BoonenAvan der LindenSMThe burden of ankylosing spondylitisJ Rheumatol Suppl20067841117042055
- BlanchCComellasMPradaCLizanLEconomic burden of ankylosing spondylitis in Europe. A systematic review of the literatureValue Health2016197A541A542
- van der HeijdeDRamiroSLandewéR2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritisAnn Rheum Dis201776697899128087505
- BraunJBaraliakosXKiltzUSecukinumab (AIN457) in the treatment of ankylosing spondylitisExpert Opin Biol Ther201616571172226982813
- Busquets-PerezNMarzo-OrtegaHEmeryPEmerging drugs for axial spondyloarthritis including ankylosing spondylitisExpert Opin Emerg Drugs2013181718623253176
- PalazziCD’AngeloSGilioMLeccesePPadulaAOlivieriIPharmacological therapy of spondyloarthritisExpert Opin Pharmacother201516101495150426073668
- The Social Insurance Institution of FinlandSpecial reimbursement (281) Available from: https://www.kela.fi/laake281Accessed April 24, 2018
- DougadosMBaetenDSpondyloarthritisLancet201137797832127213721684383
- NOVARTISNovartis receives two landmark European approvals for Cosentyx to treat patients with ankylosing spondylitis and psoriatic arthritis 2015 Available from: https://www.myscience.org/news/wire/novartis_receives_two_landmark_european_approvals_for_cosen-tyx_treat_patients_with_ankylosing_spond-2015-novartisAccessed January 31, 2019
- BraunJBaraliakosXDeodharAEffect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III measure 1 studyAnn Rheum Dis20177661070107727965257
- SieperJDeodharAMarzo-OrtegaHSecukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the measure 2 studyAnn Rheum Dis201776357159227582421
- Marzo-OrtegaHSieperJKivitzASecukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase III studyArthritis Care Res (Hoboken)20176971020102928235249
- DeodharAABaetenDSieperJPorterBRichardsHWidmerASafety and tolerability of secukinumab in patients with active anky-losing spondylitis: pooled safety analysis of two phase 3, randomized, controlled trialsArthritis Rheum20156734783479
- MaksymowychWPStrandVNashPComparative effectiveness of secukinumab and adalimumab in ankylosing spondylitis as assessed by matching-adjusted indirect comparison: an analysis based on all pivotal phase 3 clinical trial dataArthritis Rheum201668Suppl 10
- LeeYHSongGGComparative efficacy and safety of secukinumab and adalimumab in patients with active ankylosing spondylitis: a Bayesian network meta-analysis of randomized controlled trialsJ Rheum Dis2017244211219
- European Medicines Agency Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyxAccessed January 31, 2019
- CorbettMSoaresMJhutiGTumour necrosis factor-α inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluationHealth Technol Assess20162091334
- BaetenDMeasePStrandVSAT0390 secukinumab for the treatment of ankylosing spondylitis: comparative effectiveness results versus currently licensed biologics from a network meta-analysisAnn Rheum Dis201675Suppl 2809.280810
- EderLChandranVShenHCookRJGladmanDDIs ASDAS better than BASDAI as a measure of disease activity in axial psoriatic arthritis?Ann Rheum Dis201069122160216420627946
- KeatABarkhamNBhallaABSR guidelines for prescribing TNF-alpha blockers in adults with ankylosing spondylitis. Report of a working Party of the British Society for RheumatologyRheumatology (Oxford)200544793994715901904
- NICEGolimumab for the treatment of ankylosing spondylitisNICE Technology Appraisal 2332011 Available from: https://www.nice.org.uk/guidance/ta233Accessed July 2017
- NICETNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: NICE technology appraisal2016 Available from: https://www.nice.org.uk/guidance/TA383Accessed July 2017
- Kustannus Oy DuodecimMedicinal products and prices database Available from: https://www.duodecim.fi/duodecim/Accessed February 1, 2018
- CorbettMSoaresMJhuntiGTNF-alpha inhibitors for ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis (including a review of technology appraisal 143 and technology appraisal 233)CRD/CHE Technology Assessment Group (Centre for Review and Dissemination/Centre for Health Economics) University of York122014 Available from: https://www.nice.org.uk/guidance/ta383/documents/ankylosing-spondylitis-and-axial-spondylo-arthritis-nonradiographic-adalimumab-etanercept-infliximab-and-golim-umab-inc-rev-ta143-and-ta233-id694-appraisal-consultation-document2Accessed February 7, 2018
- BaklandGGranJTNossentJCIncreased mortality in ankylosing spondylitis is related to disease activityAnn Rheum Dis201170111921192521784726
- AbuabaraKAzfarRSShinDBNeimannALTroxelABGelfandJMCause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UKBr J Dermatol2010163358659220633008
- Statistics FinlandRisk of dying during a year by age and sex (year 2015) Available from: http://www.stat.fi/index_en.htmlAccessed August 15, 2017
- Garrido-CastroJLMedina-CarnicerRSchiottisRGalisteoAMCollantes-EstevezEGonzalez-NavasCAssessment of spinal mobility in ankylosing spondylitis using a video-based motion capture systemMan Ther201217542242622560166
- McLeodCBagustABolandAAdalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluationHealth Technol Assess200711281158iiiiv
- GoereeRChiva-RazaviSGundaPJainMJuglSMCost-effectiveness analysis of secukinumab in ankylosing spondylitis from the Canadian perspectiveJ Med Econ2019221455230346844
- Marzo-OrtegaHHallidayAJuglSThe cost-effectiveness of secukinumab versus tumour necrosis factor α inhibitor biosimilars FOR ankylosing spondylitis in the UKRheumatology201756suppl_2kex062.108
- EmeryPvan KeepMBeardSCost effectiveness of secukinumab for the treatment of active ankylosing spondylitis in the UKPharmacoeconomics20183681015102729797186
- SariozFOzdemirODirekSCavusoglu SezenSBarutcugilMSecukinumab is dominant vs. TNF-inhibitors in the treatment of active ankylosing spondylitis: results from a Turkish cost-effectiveness modelValue Health2017209A534
- Romero PradaMERoa CardenasNCSerranoGYHuerfanoLMCostutility analysis of secukinumab use versus TNF-α inhibitors, in patients with ankylosing spondilytisValue Health2017209A938A939
- DjambazovSVekovTIncremental cost-effectiveness analysis of biological drug therapies for the treatment of ankylosing spondylitis in Bulgaria, 2016ISPOR 22nd Annual International MeetingMay 2017Boston, MA, USA
- FedyaevDDerkachEVCost-effectiveness analysis of different biologic agents for ankylosing spondylitis treatment in RussiaValue Health2016197A539
- BaraliakosXKivitzAJDeodharAALong-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the phase 3 measure 1 trialClin Exp Rheumatol20183615055
- HaglundEBremanderABergmanSJacobssonLTPeterssonIFWork productivity in a population-based cohort of patients with spondyloarthritisRheumatology (Oxford)20135291708171423804223
- RamondaRMarchesoniACarlettoAPatient-reported impact of spondyloarthritis on work disability and working life: the Atlantis surveyArthritis Res Ther20161817827037139
- de HoogeMRamondaRLorenzinMWork productivity is associated with disease activity and functional ability in Italian patients with early axial spondyloarthritis: an observational study from the space cohortArthritis Res Ther201618126527852321
- EdwardsCJFautrelBSchulze-KoopsHHuizingaTWJKrugerKDosing down with biologic therapies: a systematic review and clinicians’ perspectiveRheumatology (Oxford)201756111847185628339632
- LorenzinMOrtolanAde HoogeMLengthening the time intervals between doses of biological agents in psoriatic arthritis patients: a single-center retrospective studyInt J Immunopathol Pharmacol201528447948726384393
- ArendsSvan der VeerEKampsFBPatient-tailored dose reduction of TNF-alpha blocking agents in ankylosing spondylitis patients with stable low disease activity in daily clinical practiceClin Exp Rheumatol201533217418025797228
- ZávadaJUherMSisolKA tailored approach to reduce dose of anti-TNF drugs may be equally effective, but substantially less costly than standard dosing in patients with ankylosing spondylitis over 1 year: a propensity score-matched cohort studyAnn Rheum Dis20167519610225165033
- De StefanoRFratiEde QuattroDMenzaLManganelliSLow doses of etanercept can be effective to maintain remission in ankylosing spondylitis patientsClin Rhfeumatol2014335707711
- RamiroSStolwijkCvan TubergenAEvolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS studyAnn Rheum Dis2015741525923956249
- SoiniEJLeussuMHallinenTAdministration costs of intravenous biologic drugs for rheumatoid arthritisSpringerplus20132153124255834