Abstract
Background
Compared with basal-bolus insulin therapy (insulin glargine U100 plus insulin aspart), IDegLira has been shown to be associated with similar improvements in HbA1c, with superior weight loss and reduced hypoglycemia in patients with type 2 diabetes. The present analysis evaluated the cost per patient with type 2 diabetes achieving HbA1c-focused and composite treatment targets with IDegLira and insulin glargine U100 plus insulin aspart (≤4 times daily).
Methods
The proportions of patients achieving treatment targets were obtained from the treat-to-target, non-inferiority DUAL VII study (NCT02420262). The annual cost per patient achieving target (cost of control) was analyzed from a US healthcare payer perspective. The annual cost of control was assessed for eight prespecified endpoints and four post-hoc endpoints.
Results
The number needed to treat to bring one patient to targets of HbA1c <7.0% and HbA1c ≤6.5% was similar with IDegLira and insulin glargine U100 plus insulin aspart. However, when weight gain and/or hypoglycemia were included, the number needed to treat was lower with IDegLira. IDegLira and insulin glargine U100 plus insulin aspart had similar costs of control for HbA1c <7.0%. However, cost of control values were substantially lower with IDegLira when the more stringent target of HbA1c ≤6.5% was used, and when patient-centered outcomes of hypoglycemia risk and impact on weight were included.
Conclusion
IDegLira was shown to be a cost-effective treatment vs insulin glargine U100 plus insulin aspart for patients with type 2 diabetes not achieving glycemic targets on basal insulin in the USA.
Plain language summary
Modern treatment decisions for patients with type 2 diabetes aim not only to achieve glycemic control (measured in terms of HbA1c), but also to avoid increasing body weight and to reduce the risk of hypoglycemic events. As the prevalence and costs of type 2 diabetes continue to rise, choosing therapies that achieve clinical goals in a cost-effective manner is becoming increasingly important. In the DUAL VII clinical trial, IDegLira was shown to be associated with equivalent efficacy to insulin glargine U100 plus insulin aspart in terms of bringing patients with type 2 diabetes to HbA1c-focused targets, but was more efficacious when composite treatment targets (also capturing weight gain and/or hypoglycemia) were considered. The present analysis assessed the cost per patient achieving HbA1c-focused and composite treatment targets with IDegLira and insulin glargine U100 plus insulin aspart, putting the cost of the two treatments into perspective relative to achievement of relevant clinical outcomes. IDegLira and insulin glargine U100 plus insulin aspart had similar costs of control for HbA1c <7.0%. However, cost of control values were substantially lower with IDegLira when the more stringent target of HbA1c ≤6.5% was used, and when patient-centered outcomes of hypoglycemia risk and impact on weight were included. IDegLira was shown to be a cost-effective treatment vs insulin glargine U100 plus insulin aspart for patients with type 2 diabetes not achieving glycemic targets on basal insulin in the USA.
Introduction
Estimates suggest that, in 2017, 30.2 million people were living with diabetes in the USA, and that this number will increase by almost 20% to 35.6 million by 2045.Citation1 Patients with diabetes are at increased risk of developing serious complications, including cardiovascular disease, eye disease, renal disease, neuropathy, and amputation.Citation2 Moreover, the disease causes 177,000 deaths per year in the USA, with 46.1% of these occurring in people under 60 years of age.Citation1
In addition to the clinical burden, diabetes is associated with significant costs, with diabetes-related health expenditure of $348 billion in 2017, which is expected to increase to $372 billion in 2045. Treating diabetes-related complications makes up the majority of the total cost over the lifetime of a patient, at 48–64% depending on the age at diagnosis.Citation3 Hypoglycemic events can also lead to significant costs, with average direct costs of a severe hypoglycemic event requiring medical assistance of $1,161, and an annual cost of hypoglycemic events of $250 in patients with type 2 diabetes for >5 years.Citation4
Maintaining glycemic control remains a focus for patients with type 2 diabetes, with a number of studies showing that improving glycemic control can lead to a reduced incidence of microvascular and macrovascular complications.Citation5–Citation11 In addition, there is growing evidence that patients with diabetes benefit from a more comprehensive, patient-centered approach, which considers hypoglycemia risk and impact on weight, rather than a purely glucocentric approach and this is captured in recommendations from the American Diabetes Association (ADA).Citation12–Citation17 Therefore, treatment decisions aim to minimize the risk of hypoglycemia and weight gain. Increases in body weight and high rates of hypoglycemia can reduce adherence to diabetes medications, which may impede glycemic control, and are associated with poorer cardiovascular outcomes.Citation18–Citation21
Owing to the progressive nature of type 2 diabetes, patients will commonly transition through the treatment algorithm from lifestyle intervention to oral anti-diabetes agents to basal insulin to multiple daily insulin injections.Citation17 While basal insulin doses can be titrated to maintain glycemic control, additional therapies may over time be required to maintain glycemic control. The ADA recommends addition of an additional injectable therapy if the basal insulin dose is >0.5 units/kg/day and HbA1c remains above target.Citation17 Commonly, this is through the addition of rapid-acting insulin at meal times to form a basal-bolus insulin regimen. However, this approach is associated with weight gain and increased hypoglycemia.Citation22 In addition, clinical inertia around the initiation of more complex treatment regimens requiring multiple daily injections may represent a barrier to intensification.Citation23
IDegLira represents an alternative therapy for patients not adequately controlled on basal insulin. IDegLira is a fixed-ratio combination of insulin degludec and the glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide. Through the complementary mechanisms of action of a basal insulin and a GLP-1 receptor agonist, a previous study has shown that IDegLira is associated with reductions in HbA1c, reductions in body weight, and low risk of hypoglycemia compared with basal-bolus therapy in patients with diabetes failing to achieve glycemic control on basal insulin.Citation24
The aim of the present analysis was to evaluate, in a simple and transparent manner, the short-term cost-effectiveness of IDegLira vs insulin glargine U100 plus insulin aspart in patients with type 2 diabetes failing to achieve glycemic control on basal insulin in the US setting. The analysis assessed the cost per patient achieving HbA1c-focused and composite (capturing weight gain and hypoglycemia) treatment targets. This approach puts the cost of two treatments into perspective relative to the achievement of relevant outcomes for health-care payers and prescribers, and has been used in previous peer-reviewed publications.Citation25–Citation29
Material and methods
Clinical data
All clinical data to inform the cost-effectiveness analysis were taken from the DUAL VII trial.Citation24 DUAL VII was an open-label, two-arm parallel, randomized, treat-to-target phase IIIb, 26-week trial comparing the efficacy and safety of IDegLira with insulin glargine U100 plus insulin aspart in patients with type 2 diabetes not achieving glycemic control targets (HbA1c 7.0–10.0%) on insulin glargine U100 20–50 U and a stable dose of metformin. In total, 505 adults were enrolled, with mean age of 58.3 years, mean duration of diabetes of 13.2 years, mean HbA1c of 8.2%, and a mean pre-trial insulin glargine U100 dose of 33.4 U. Participants were randomly allocated in a 1:1 ratio to receive IDegLira or insulin glargine U100 plus insulin aspart (with patients in both arms continuing concomitant metformin), with outcomes assessed over 26 weeks of treatment.
The primary endpoint was change in HbA1c from baseline at 26 weeks. Prespecified secondary endpoints included the percentage of patients achieving targets of HbA1c <7.0%, HbA1c <7.0% without weight gain, HbA1c <7.0% without hypoglycemia during the maintenance period of the trial (the last 12 weeks), HbA1c <7.0% without weight gain and hypoglycemia in the last 12 weeks of the trial, and the equivalent targets with a more stringent glycemic control target of HbA1c ≤6.5%. In addition, post-hoc analyses assessed the percentage of patients achieving targets of HbA1c <7.0% without hypoglycemia over the 26 weeks of the trial, HbA1c <7.0% without weight gain and hypoglycemia over the 26 weeks of the trial, and two equivalent targets with a glycemic control target of HbA1c ≤6.5%. These glycemic control targets reflect recommendations by the ADA (HbA1c <7.0% for most patients; HbA1c <6.5% if this can be achieved without significant hypoglycemia or other adverse effects of treatment) and the American Association of Clinical Endocrinologists (AACE) (HbA1c ≤6.5 for most patients, with an individualized higher target if the lower target cannot be achieved without adverse outcomes).Citation15,Citation30 Hypoglycemia was defined as treatment-emergent severe or blood-glucose confirmed symptomatic hypoglycemic events. The percentage of patients achieving each endpoint is shown in . There was no difference in the proportion of patients achieving glycemic control targets of HbA1c <7.0% and HbA1c ≤6.5%, but more subjects receiving IDegLira reached the composite endpoints including weight gain and/or hypoglycemia (both in the last 12 weeks of the trial and over the full 26 weeks) compared with insulin glargine U100 plus insulin aspart.Citation24
Table 1 Percentage of patients achieving treatment targets
Cost data
Costs were accounted from a US healthcare payer perspective in 2017 US dollars ($), capturing the study drugs (IDegLira, insulin glargine U100, and insulin aspart), needles for subcutaneous injection, and self-monitoring of blood glucose (SMBG) testing. No other costs (such as costs of diabetes-related complications or costs related to severe hypoglycemia) were included in the analysis, as it assessed the treatment cost per patient achieving a responder endpoint. Doses of IDegLira (40.06 units [1 unit contains 1 U insulin degludec and 0.036 mg liraglutide]), insulin glargine U100 (52.65 U), and insulin aspart (32.30 U) were taken from the DUAL VII study at the end of the trial.Citation24 Patients receiving IDegLira required one needle per day for subcutaneous injection, and patients receiving insulin glargine U100 plus insulin aspart were assumed to use four needles per day, as once-daily injection of insulin glargine U100 and three-times daily injection of insulin aspart was the most common dosing schedule in the DUAL VII trial, with 66.5% of participants using this approach. Wholesale acquisition costs were used to calculate the daily cost of treatment.Citation31 Costs of SMBG testing were based on an analysis of insurance claims in the USA, and were inflated using the consumer price index for medical care.Citation32 This reflects the cost to third party payers, and the true cost of needles and SMBG testing may be higher if patient out-of-pocket expenditure is considered. Annual costs were calculated by multiplying the daily cost by 365.25.
Evaluation of cost-effectiveness
The annual cost per patient achieving target (cost of control) was assessed for the eight prespecified endpoints and the four post-hoc endpoints in an economic model developed in Microsoft Excel. The annual cost of control was calculated by dividing the annual cost of treatment by the proportion of patients achieving the target. The spending required with insulin glargine U100 plus insulin aspart to achieve an equivalent outcome of one patient achieving target relative to $1 spent on IDegLira was calculated by dividing the cost of control with insulin glargine U100 plus insulin aspart by the cost of control with IDegLira. An example calculation is shown in , and the methodology has been used in previously published analyses.Citation25–Citation29 No discounting was applied as costs were not projected beyond a 1-year time horizon.
Table 2 Example cost of control calculation
Sensitivity analyses
Sensitivity analyses were conducted to assess the impact of variation in the model inputs on the calculated cost-effectiveness outcomes. Eight analyses were performed with the proportion of patients achieving target, intervention costs, needle costs, and SMBG testing costs increased and decreased by 10% in turn. The cost of needles and SMBG test strips may vary between patients and between healthcare payers (depending on the coverage of the healthcare plan), and therefore a conservative sensitivity analysis was conducted with these costs excluded in both treatment arms. This allows the importance of resource use relating to needles and SMBG testing in driving cost-effectiveness outcomes to be tested, by comparing the results of this analysis with those in the base case. The impact of applying alternative basal insulin costs in the insulin glargine U100 plus insulin aspart arm was assessed by applying the costs of biosimilar insulin glargine U100 and neutral protamine Hagedorn (NPH) insulin in place of insulin glargine U100, with equivalent clinical effectiveness in terms of bringing patients to all treatment targets to insulin glargine U100 plus insulin aspart assumed. As the DUAL VII trial allowed patients to dose insulin aspart up to four times daily, sensitivity analyses were conducted with variation in the insulin aspart dosing schedule in the insulin glargine U100 plus insulin aspart arm. A sensitivity analysis was performed with the costs of non-severe and severe hypoglycemic events included in each treatment arm. The rates of non-severe and severe hypoglycemic events were taken from the DUAL VII trial, and were 2.28 and 0.0003 events per patient per year, respectively, with IDegLira, and 10.91 and 0.0011 events per patient per year, respectively, with insulin glargine U100 plus insulin aspart. Costs of events ($7.10 and $4,779.82, respectively) were taken from a previously published economic evaluation in the US setting.Citation33 These event rates and costs have also been used in published short- and long-term cost-effectiveness analyses based on the DUAL VII trial.Citation34,Citation35 These costs were included in a sensitivity analysis, rather than the base case, as IDegLira was associated with lower rates of non-severe and severe hypoglycemic events, and therefore the base case remains a conservative estimate of cost-effectiveness. DUAL VII was a 26-week trial and therefore a sensitivity analysis was conducted with cost of control assessed over 6 months, rather than over a 1-year time horizon as used in the base-case analysis.
Probabilistic sensitivity analysis was performed with sampling around both costs and clinical inputs. The proportion of patients achieving each target and treatment costs were sampled for each intervention from normal distributions. Following sampling of values for both treatments, the therapy with the lowest cost of control for each target was recorded. The process was then repeated 1,000 times, as outcomes were stable at these settings, with all inputs resampled for each iteration.
Results
Cost outcomes
Total annual medication and consumable costs were $678 lower with IDegLira than with insulin glargine U100 plus insulin aspart (). Annual drug costs were $333 higher for patients receiving IDegLira owing to the higher acquisition cost of IDegLira compared with insulin glargine U100 and insulin aspart. However, this was entirely offset by cost savings resulting from reduced needle use (annual cost saving of $498 per patient) and SMBG use (annual cost saving of $513 per patient). Reduced needle costs were driven by the once-daily administration of IDegLira, compared with the once-daily administration of insulin glargine U100 and three-times-daily administration of insulin aspart. The lower costs of SMBG testing identified in the analysis of insurance claims in the USA reflects the higher risk of hypoglycemia with basal-bolus insulin therapy, which therefore necessitates a higher frequency of SMBG testing.Citation15
Table 3 Annual treatment costs
Number needed to treat
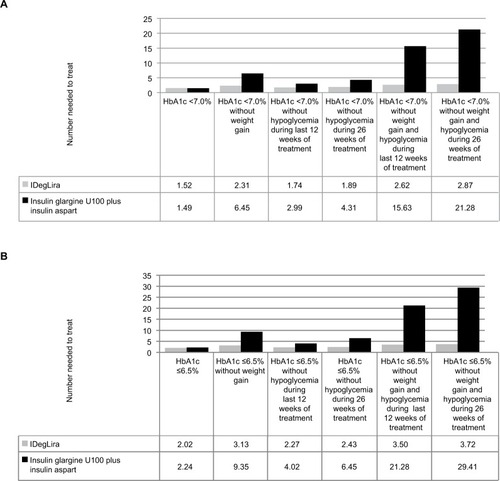
When glycemic control targets of HbA1c <7.0% and HbA1c ≤6.5% were considered, IDegLira and insulin glargine U100 plus insulin aspart were associated with similar numbers needed to treat to bring one patient to target, at 1.52 vs 1.49 patients requiring treatment to bring one patient to target and 2.02 vs 2.24 patients requiring treatment to bring one patient to target, respectively (). However, when weight gain and/or hypoglycemia were included in patient-centered treatment targets, IDegLira was consistently associated with a lower number needed to treat to bring one patient to target. The difference was greatest for the treatment targets of HbA1c ≤6.5% without weight gain and hypoglycemia during 26 weeks of treatment (3.72 vs 29.41 patients requiring treatment to bring one patient to target) and HbA1c <7.0% without weight gain and hypoglycemia during 26 weeks of treatment (2.87 vs 21.28 patients requiring treatment to bring one patient to target).
Figure 1 Number needed to treat to bring one patient to target.
Cost of control
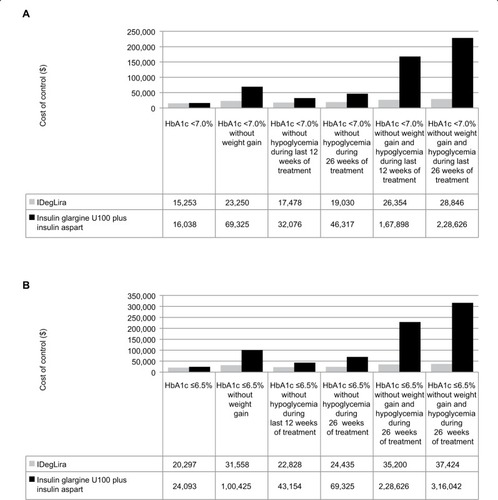
IDegLira was associated with a lower cost of control than insulin glargine U100 plus insulin aspart for all endpoints included in the analysis (). The difference was smallest for the glycemic control target of HbA1c <7.0%, with the annual cost of control only $785 lower with IDegLira than with insulin glargine U100 plus insulin aspart. IDegLira was associated with substantially lower cost of control values when the more stringent target of HbA1c ≤6.5% was used, and when patient-centered treatment targets including weight gain and/or hypoglycemia were assessed. The difference in cost of control was greatest for the post-hoc targets of HbA1c ≤6.5% without weight gain and hypoglycemia over the 26 weeks of the trial ($37,424 vs $316,042, showing that for every $1 spent on IDegLira spending of $8.44 was required with insulin glargine U100 plus insulin aspart to achieve an equivalent outcome) and HbA1c 7.0% without weight gain and hypoglycemia over the 26 weeks of the trial ($28,846 vs $228,626, showing that for every $1 spent on IDegLira spending of $7.93 was required with insulin glargine U100 plus insulin aspart to achieve an equivalent outcome). IDegLira was associated with substantially lower cost of control values when either time period was used to capture hypoglycemic events (prespecified target of hypoglycemia in the last 12 weeks of the trial or the post-hoc target of hypoglycemia over the 26 weeks of the trial).
Figure 2 Cost of control.
Sensitivity analyses
Cost of control values remained lower with IDegLira than insulin glargine U100 plus insulin aspart in the majority of sensitivity analyses conducted (). Increasing the proportion of patients achieving the targets (applied in both arms simultaneously) resulted in greater differences in the cost of control, while reducing the proportion of patients achieving the targets had the converse effect. Cost of control values remained lower with IDegLira for all endpoints across these two analyses. Variation in the pack prices of the interventions did not change the conclusion that the cost of control was lower for all endpoints with IDegLira. Similarly, varying the cost of needles and SMBG testing did not change the conclusions of the analysis, although differences were smaller when the costs of needles and SMBG testing were reduced. Removing the costs of needles and SMBG testing altogether resulted in a lower cost of control with insulin glargine plus insulin aspart for HbA1c <7.0%, but IDegLira remained associated with a lower cost of control for all other endpoints.
Table 4 Sensitivity analysis results: difference in cost of control for IDegLira vs insulin glargine U100 plus insulin aspart
Applying the cost of biosimilar insulin glargine U100 in place of insulin glargine U100 reduced the annual treatment cost and the cost of control in the comparator arm, owing to the lower acquisition cost. In this analysis, biosimilar insulin glargine U100 plus insulin aspart and insulin glargine U100 plus insulin aspart were assumed to have equivalent clinical efficacy. However, IDegLira remained associated with a lower cost of control for all endpoints except HbA1c <7.0%, where the cost of control was $285 lower with biosimilar insulin glargine U100 plus insulin aspart. Replacing the cost of insulin glargine U100 with the cost of NPH insulin, with no changes in clinical efficacy, had a similar effect. The cost of control was lower with insulin NPH plus insulin aspart for HbA1c <7.0% and HbA1c ≤6.5%, but the cost of control was lower with IDegLira for all other endpoints.
Reducing the frequency of insulin aspart injection resulted in lower cost of control values in the insulin glargine U100 plus insulin aspart arm, owing to the reduced needle resource use. Conversely, increasing the frequency of insulin aspart injection increased the cost of control in the insulin glargine U100 plus insulin aspart arm. The cost of control remained lower with IDegLira compared with insulin glargine U100 plus insulin aspart for all endpoints in both sensitivity analyses. Inclusion of the cost of non-severe and severe hypoglycemic events resulted in an increased cost of control for IDegLira and insulin glargine U100 plus insulin aspart for all treatment targets, due to the additional cost included. Differences were greater than in the base-case analysis, with IDegLira associated with a lower cost of control for all targets. This resulted from the lower rates of non-severe and severe hypoglycemic events with IDegLira compared with insulin glargine U100 plus insulin aspart in the DUAL VII study.
Calculating cost of control values over 6 months (rather than 1 year in the base case) did not change the conclusion that IDegLira was associated with a lower cost of control compared with insulin glargine U100 plus insulin aspart for all endpoints.
Probabilistic sensitivity analysis, with sampling around clinical and cost inputs, found that the probability that IDegLira was associated with a lower cost of control than insulin glargine U100 plus insulin aspart was 63% for the target of HbA1c <7.0%, 84% for the target HbA1c ≤6.5%, and 100% for all other targets, confirming that the greater clinical efficacy and lower total annual cost resulted in a lower cost of control with IDegLira compared with insulin glargine U100 plus insulin aspart.
Discussion
The DUAL VII trial demonstrated that IDegLira and insulin glargine U100 plus insulin aspart were associated with equivalent percentages of patients achieving glycemic control targets of HbA1c <7.0% and HbA1c ≤6.5% (due to the treat-to-target, non-inferiority design of the trial), but when weight gain and/or hypoglycemia were included, IDegLira was more effective, bringing a greater proportion of patients to treatment targets. This reflects the benefits of patient-centered treatment with IDegLira, not only targeting glycemic control but also reducing the risk of hypoglycemia and reducing body weight. The use of IDegLira led to increased drug costs compared with insulin glargine U100 plus insulin aspart, but cost savings resulting from reduced needle use and SMBG testing entirely offset this, leading to lower annual treatment costs with IDegLira. Annual costs of control per patient were similar with IDegLira and insulin glargine U100 plus insulin aspart, achieving a target of HbA1c <7.0%, but the annual cost of control was lower with IDegLira for a glycemic control target of HbA1c ≤6.5% and when patient-centered treatment targets were assessed. Across extensive sensitivity analysis with variation in the model inputs, the cost of control with IDegLira remained lower for composite treatment targets in all scenarios, and the cost of control was only higher for glycemic control targets when needle and SMBG testing costs were not included and when alternative basal insulin costs were applied (a highly conservative scenario with NPH insulin assumed to have equivalent efficacy to insulin glargine U100). The findings of the analysis were shown to be robust, and IDegLira is likely to represent a cost-effective treatment for patients with type 2 diabetes not achieving glycemic control on basal insulin in the USA. This treatment option addresses not only glycemic control but also avoidance of weight gain and reduced frequency of hypoglycemic events.
In the USA, 55.2% of patients with type 2 diabetes receiving basal insulin have an HbA1c ≥8.0%, and 33.1% have an HbA1c ≥9.0%.Citation36 Expanding treatment options that aid these patients in achieving glycemic control may reduce the frequency of diabetes-related complications and associated costs. Achieving these improvements in a cost-effective manner represents a key goal for healthcare payers.
An alternative treatment option for patients not achieving glycemic control on basal insulin is up-titration of the basal insulin dose. A previous analysis based on the head-to-head DUAL V trial compared the cost of control with IDegLira with up-titration of insulin glargine U100.Citation27,Citation37 This study found that the cost per patient achieving glycemic control and patient-centered treatment targets was lower with IDegLira than with insulin glargine U100, with similar patterns observed as in the present analysis, with greatest differences when weight and/or hypoglycemia were included. As well as assessing outcomes in all patients with type 2 diabetes, the previous analysis assessed outcomes in subgroups of patients with HbA1c >8.0% and HbA1c >9.0% at baseline, with IDegLira shown to be more cost-effective in patients with poorer glycemic control at baseline. The previous analysis based on the DUAL V trial and the present analysis based on the DUAL VII study have shown that the cost of control is lower with IDegLira than both up-titration of the basal insulin dose and the addition of bolus insulin.
The present analysis focused on glycemic control targets of HbA1c ≤6.5% and HbA1c <7.0%, as recommended by the AACE and the ADA.Citation15,Citation30 However, these stringent goals may not be applicable for all patients with type 2 diabetes. There is increasing focus on individualizing glycemic control targets, depending on age, frailty, and existing complications, and the American College of Physicians recommends an HbA1c target of 7.0–8.0% for most patients with type 2 diabetes.Citation38 Additional cost-effectiveness analyses based on less aggressive HbA1c goals are planned by the author group, aiming to assess cost-effectiveness across a wider range of glycemic control targets. The intention is to publish these additional analyses in a separate manuscript.
A key advantage of the present analysis is its simplicity and transparency. The analysis can be easily replicated and quickly updated when acquisition costs of interventions change or if additional data become available. Furthermore, no projections of cost and clinical outcomes are made over patient lifetimes, as is often the case when the cost-effectiveness of interventions for diabetes is assessed.Citation39 It should be noted that the present analysis is intended to complement conventional modeling approaches. The same author group has published a long-term cost-effectiveness analysis and a 1-year cost-effectiveness analysis based on the DUAL VII trial.Citation34,Citation35 In the long-term cost-effectiveness analysis, the frequency and time to onset of diabetes-related complications were projected over patient lifetimes, with costs and health state utilities assigned to these events.Citation34 The 1-year analysis captured the impact of hypoglycemia rates and changes in body mass index on quality of life over the short term.Citation35 In both of these analyses, IDegLira was associated with improved quality of life and reduced costs, compared with treatment with insulin glargine U100 plus insulin aspart. The present analysis, when considered in conjunction with other published analyses based on the DUAL VII trial, provides useful information to healthcare payers and patients to allow selection of cost-effective treatments for patients with type 2 diabetes.
The randomized clinical trial data represent a robust data source to inform the study, increasing the confidence in the findings of the short-term cost-effectiveness analysis. An additional advantage is that the target endpoints are based on guidance from the ADA, and therefore the analysis is highly relevant to patients with type 2 diabetes and healthcare payers in the USA.Citation14,Citation15,Citation17
The primary limitation of the cost of control approach is that it does not offer a willingness-to-pay context, as there are no accepted thresholds around how much a healthcare payer is prepared to pay per patient achieving the glycemic control and composite endpoints included in the analysis. Furthermore, it is not possible to compare the results with other analyses or to compare the relative cost-effectiveness of interventions included in the present analysis with interventions in different therapeutic areas. Conventional cost–utility analysis calculates the cost per quality-adjusted life-year gained, a key strength of which is allowing comparisons to be made across studies and diseases. Therefore, the cost of control data in the present analysis are intended to complement, not replace, conventional cost–utility analyses, providing short-term data which may be useful to healthcare decision-makers.
A further limitation of the present analysis was that the adverse impacts of treatment, other than weight gain and hypoglycemia, were not included in the composite endpoints. Data on the proportion of patients achieving glycemic control targets without experiencing additional adverse events were not collected in the DUAL VII trial, and therefore these events were not captured in the present cost-effectiveness analysis. The most common adverse event in the IDegLira arm of the DUAL VII study was nausea, affecting 11.1% of patients receiving IDegLira compared with 1.6% of patients receiving insulin glargine U100 plus insulin aspart.Citation24 In the insulin glargine U100 plus insulin aspart arm, the most common adverse event was nasopharyngitis, with 11.9% of patients affected compared with 4.8% of patients receiving IDegLira.Citation24 Overall, adverse event rates were similar in both arms of the DUAL VII study, and therefore the conclusions of the present analysis would be unlikely to change if additional adverse events were included. Future cost of control analyses could look to capture a wider range of adverse events, thereby providing a broader assessment of cost-effectiveness.
Conclusion
The present analysis assessed the cost per patient of achieving single and composite treatment targets with IDegLira and insulin glargine U100 plus insulin aspart in patients with type 2 diabetes failing to achieve glycemic targets on basal insulin alone. Clinical trial data have shown that an equivalent or increased proportion of patients achieved recommended treatment targets with IDegLira compared with insulin glargine U100 plus insulin aspart. The use of IDegLira led to reduced medication and supply costs. Therefore, cost of control values were lower with IDegLira than with insulin glargine U100 plus insulin aspart, with the largest differences when patient-centered targets capturing weight gain and/or hypoglycemia were considered. This reflects the wide-ranging benefits of treatment with IDegLira compared with basal-bolus insulin. The present analysis suggests that IDegLira is likely to be a cost-effective treatment option vs insulin glargine U100 plus insulin aspart for patients with type 2 diabetes not achieving glycemic targets on basal insulin in the USA.
Ethics approval
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data sharing statement
Supporting data can be obtained from the corresponding author.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, and take responsibility for the integrity of the work as a whole. The study was designed by all authors, and conducted by BH. BH drafted the manuscript, and all other authors critically revised it for intellectual content. All authors approve of the final version and agree to be accountable for all aspects of the work.
Acknowledgments
The present cost-effectiveness analysis was supported by funding from Novo Nordisk A/S.
Disclosure
LKB has participated in advisory boards and received speaker’s honoraria from Novo Nordisk Inc. MM is an employee of Novo Nordisk Inc. AB is an employee of Novo Nordisk Pharma Gulf FZ-LLC. BH and WJV are employees of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S and Novo Nordisk Inc. to support preparation of the analysis. EJ has participated in advisory boards and received speaker’s honoraria from Novo Nordisk A/S. The authors report no other conflicts of interest in this work.
References
- International Diabetes FederationIDF diabetes atlas8th ed http://www.diabetesatlas.org/across-the-globe.htmlAccessed November 22, 2017
- GreggEWLiYWangJChanges in diabetes-related complications in the United States, 1990–2010N Engl J Med2014370161514152324738668
- ZhuoXZhangPHoergerTJLifetime direct medical costs of treating type 2 diabetes and diabetic complicationsAm J Prev Med201345325326123953350
- FoosVVarolNCurtisBHEconomic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United StatesJ Med Econ201518642043225629654
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) GroupLancet199835291318378539742976
- HolmanRRPaulSKBethelMAMatthewsDRNeilHAW10-year follow-up of intensive glucose control in type 2 diabetesN Engl J Med2008359151577158918784090
- Ismail-BeigiFCravenTBanerjiMAEffect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trialLancet2010376973941943020594588
- PatelAMacmahonSChalmersJADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetesN Engl J Med2008358242560257218539916
- DuckworthWAbrairaCMoritzTVADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetesN Engl J Med2009360212913919092145
- StettlerCAllemannSJüniPGlycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trialsAm Heart J20061521273816824829
- TurnbullFMAbrairaCAndersonRJIntensive glucose control and macrovascular outcomes in type 2 diabetesDiabetologia200952112288229819655124
- MarsoSPDanielsGHBrown-FrandsenKLEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetesN Engl J Med2016375431132227295427
- ZinmanBWannerCLachinJMEMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetesN Engl J Med2015373222117212826378978
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care201538114014925538310
- American Diabetes Association6. Glycemic targetsDiabetes Care201740Suppl 1S48S5627979893
- American Diabetes Association7. Obesity management for the treatment of type 2 diabetesDiabetes Care201740Suppl 1S57S6327979894
- American Diabetes Association8. Pharmacologic approaches to glycemic treatmentDiabetes Care201740Suppl 1S64S7427979895
- WalzLPetterssonBRosenqvistUDeleskogAJournathGWändellPImpact of symptomatic hypoglycemia on medication adherence, patient satisfaction with treatment, and glycemic control in patients with type 2 diabetesPatient Prefer Adherence20148859360124812495
- GrandySFoxKMHardyESHIELD Study GroupAssociation of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes)Curr Ther Res Clin Exp201375778224465048
- LimSChoiSHKimKMThe association of rate of weight gain during early adulthood with the prevalence of subclinical coronary artery disease in recently diagnosed type 2 diabetes: the MAXWEL-CAD studyDiabetes Care20143792491249924914242
- PieberTRMarsoSPMcguireDKDEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortalityDiabetologia2018611586528913543
- AbrahamsonMJPetersAIntensification of insulin therapy in patients with type 2 diabetes mellitus: an algorithm for basal-bolus therapyAnn Med201244883684622822902
- KhuntiKNikolajsenAThorstedBLAndersenMDaviesMJPaulSKClinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulinDiabetes Obes Metab201618440140926743666
- BillingsLKDoshiAGouetDEfficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trialDiabetes Care20184151009101629483185
- LangerJHuntBValentineWJEvaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United StatesJ Manag Care Pharm201319323724623537458
- SkovgaardRJon PlougUHuntBValentineWJEvaluating the cost of bringing people with type 2 diabetes mellitus to multiple targets of treatment in CanadaClin Ther20153781677168826186809
- HuntBMocarskiMValentineWJLangerJEvaluation of the short-term cost-effectiveness of IDegLira versus continued up-titration of insulin glargine U100 in patients with type 2 diabetes in the USAAdv Ther201734495496528281218
- HuntBMcconnachieCCGambleCDang-TanTEvaluating the short-term cost-effectiveness of liraglutide versus lixisenatide in patients with type 2 diabetes in the United StatesJ Med Econ201720111117112028651479
- LopezJMSMacomsonBEktareVPatelDBottemanMEvaluating drug cost per response with SGLT2 inhibitors in patients with type 2 diabetes mellitusAm Health Drug Benefits20158630931826557225
- GarberAJAbrahamsonMJBarzilayJIConsensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summaryEndocr Pract201723220723828095040
- Medi-Span Price Rx Available from: http://www.wolterskluwercdi.com/price-rx/Accessed August 16, 2017
- YeawJLeeWCAagrenMChristensenTCost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetesJ Manag Care Pharm2012181213222235952
- WeatherallJBloudekLBuchsSBudget impact of treating commercially insured type 1 and type 2 diabetes patients in the United States with insulin degludec compared to insulin glargineCurr Med Res Opin201733223123827764979
- DempseyMMocarskiMLangerJHuntBLong-term cost-effectiveness analysis shows that IDegLira is associated with improved outcomes and lower costs compared with insulin glargine U100 plus insulin aspart in the USJ Med Econ201821111110111830114954
- DempseyMMocarskiMLangerJHuntBIDegLira is associated with improved short-term clinical outcomes and cost savings compared with insulin glargine U100 plus insulin aspart in the U.SEndocr Pract201824979680430308134
- LangerJTianYWengWGambleCMocarskiMAssessing unmet needs for type 2 diabetes patients treated with basal insulin in the United StatesEndocrine Practice201622S27879
- LingvayIPérez ManghiFGarcía-HernándezPEffect of insulin glargine up-titration vs insulin Degludec/Liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trialJAMA2016315989890726934259
- QaseemAWiltTJKansagaraDHorwitchCBarryMJForcieaMAClinical Guidelines Committee of the American College of PhysiciansHemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of PhysiciansAnn Intern Med2018168856957629507945
- TarrideJ-EHopkinsRBlackhouseGA review of methods used in long-term cost-effectiveness models of diabetes mellitus treatmentPharmacoeconomics201028425527720222752
