Abstract
Cirrhosis is a chronic liver disease stage that encompasses a variety of etiologies resulting in liver damage. This damage may induce secondary complications such as portal hypertension, esophageal variceal bleeding, spontaneous bacterial peritonitis, and hepatic encephalopathy. Screening for and management of these complications incurs substantial health care costs; thus, determining the most economical and beneficial treatment strategies is essential. This article reviews the economic impact of a variety of prophylactic and treatment regimens employed for cirrhosis-related complications. Prophylactic use of β-adrenergic blockers for portal hypertension and variceal bleeding appears to be cost-effective, but the most economical regimen for treatment of initial bleeding is unclear given that cost comparisons of pharmacologic and surgical regimens are lacking. In contrast, prophylaxis for spontaneous bacterial peritonitis cannot be recommended. Standard therapy for spontaneous bacterial peritonitis includes antibiotics, and the overall economic impact of these medications depends largely on their direct cost. However, the potential development of bacterial antibiotic resistance and resulting clinical failure should also be considered. Nonabsorbable disaccharides are standard therapies for hepatic encephalopathy; however, given their questionable efficacy, the nonsystemic antibiotic rifaximin may be a more cost-effective, long-term treatment for hepatic encephalopathy, despite its increased direct cost, because of its demonstrated efficacy and prevention of hospitalization. Further studies evaluating the cost burden of cirrhosis and cirrhosis-related complications, including screening costs, the cost of treatment and maintenance therapy, conveyance to liver transplantation, liver transplantation success, and health-related quality of life after transplantation, are essential for evaluation of the economic burden of hepatic encephalopathy and all cirrhosis-related complications.
Introduction
Cirrhosis is a chronic liver disease with a multifactorial etiology (eg, chronic hepatitis viral infection, alcoholic liver disease, nonalcoholic fatty liver disease) that results in liver damage.Citation1 Resistance to liver blood flow because of fibrosis leads to portal hypertension (PH) and the formation of portosystemic collaterals,Citation2 which reduce blood flow through the liverCitation3 and lead to the accumulation of toxins in the systemic circulation. Symptoms of cirrhosis occur along a continuum, from no symptoms in the early stages of the disease to severe complications resulting from PH (eg, esophageal variceal bleeding [EVB]) and reduced toxin clearance (eg, hepatic encephalopathy [HE]).
The primary goals of therapy for cirrhosis are to manage patients’ symptoms and prevent the occurrence of cirrhosis-related complications until liver transplantation can be performed.Citation2,Citation4 The standard of care strategy includes appropriate screening for and treatment of complications secondary to cirrhosis (eg, PH, EVB, spontaneous bacterial peritonitis [SBP], and HE).Citation2 Although the incidence of these disorders has remained relatively stable in recent years, increases in the cost associated with hospitalization, screening, and treatment of these cirrhosis-related complications have raised concerns about the economic burden of these disorders. This review briefly discusses the economic impact of cirrhosis and highlights the health care cost burden of cirrhosis-related complications with a particular emphasis on HE.
Economic impact of cirrhosis
In the general population worldwide, up to 10% of people are estimated to have liver cirrhosis, although precise estimation is confounded because the disease may remain undiagnosed until related complications (eg, ascites, edema, bruising, encephalopathy) become apparent.Citation5 Cirrhosis is one of the leading causes of death worldwide, resulting in morbidity, a significant reduction in health-related quality of life (HRQOL), and a direct cost (excluding hepatitis C virus [HCV]) of approximately $1.4 billion in the US. The main causes of liver cirrhosis are HCV, hepatitis B virus, and alcohol consumption, suggesting that the disease may be preventable. Indeed, several practices have been implemented to reduce the risk of hepatitis infections, including screening of blood donors and pregnant women for infection, vaccination programs for infants and high-risk populations, and the establishment of infection control practices in hospitals.Citation6 Unfortunately, the cost of some of these programs may not justify their implementation despite the fact that the effects of chronic infection (eg, cirrhosis) could be avoided.Citation7 In addition, despite a reduction in new infections, the cost of managing aging patients with chronic liver disease may be substantial. For example, in 2009 the per-patient cost of managing chronic HCV infection was projected to increase 3.5 times over the next 20 years.Citation8
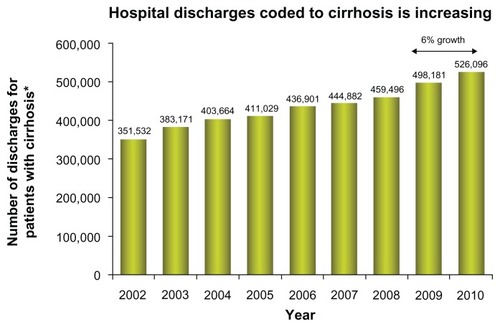
Currently, the management of patients with cirrhosis focuses on treating underlying diseases (eg, HCV, hepatitis B virus)Citation2, and associated symptoms, while maintaining patients’ health until liver transplantation is possible.Citation4 In general, according to the Healthcare Cost and Utilization Project (HCUP), the number of hospital discharges for chronic liver disease and cirrhosis from 2002 until 2010 has increased from roughly 400,000 to 525,000 ().Citation9 The cost of hospitalization decreased slightly from 2005 to 2006, but still far exceeded that reported in 1993. The substantial increase in hospitalization costs may be attributable to inflation of overall health care expenditures and alterations in therapeutic regimens for the treatment of cirrhosis-related diseases (eg, HCV) in addition to normal rates of inflation.
Figure 1 Trends in hospital discharges and in-hospital cost for cirrhosis from 2002 to 2010.
After a preliminary trial in 1986, standard therapy for HCV consisted of subcutaneous injections of 3 million units of interferon-α three times a week for 24 weeks,Citation10 but subsequent optimization of treatment recommended the extension of therapy duration to 48 weeks in some patients and the addition of ribavirin 800 mg/day.Citation10,Citation11 In an economic evaluation of randomized controlled trials of patients with HCV, the net cost of interferon-α therapy was $1827.48 and $3654.96 for 24 and 48 weeks of therapy, respectively.Citation12 When combined with ribavirin, the net cost of 48 weeks of therapy substantially increased to approximately $13,885.08. These more effective and more costly treatment regimens may allow more patients to survive to undergo liver transplantation. Economically, this translates into a greater number of patients who require medical care and incur the substantial cost of liver transplantation and follow-up management.
The present standard of care, direct antiviral agents telaprevir and boceprevir, has offered higher sustained virological response rates but with a substantial increase in cost.Citation13 For instance, telaprevir tends to cost $49,200 for the entire course of treatment, 12 weeks of therapy. Boceprevir costs are between $31,000 and $53,000 for 24–44 weeks of therapy.Citation14 Important to note is that these financial numbers do not include the cost of peginterferon-α or ribavirin.
In addition, patients awaiting transplantation must be evaluated and treated for cirrhosis-related complications to maintain overall health and be candidates for liver transplantation.Citation4 Maintenance of these patients represents a significant health care cost; the average annual cost for treatment of decompensated cirrhosis patients is substantially higher than that of patients with compensated cirrhosis.Citation15 Thus, cost-effective management of cirrhosis-related complications should be an economic priority.
Lastly, at a time of global economic crisis, the medical community must become more cognizant of the cost-effectiveness of any drug. Recently, two studies reported on telaprevir and boceprevir therapy in treatment-naïve patients and those whose condition had relapsed or not responded to previous treatment.Citation16,Citation17 The marked increase in sustained virological response has shown both drugs combined with pegylated interferon and ribavirin are cost-effective when considering the decrease in lifetime incidence of liver complications, quality-adjusted life years, and the incremental cost-effectiveness ratio.Citation18,Citation19
Economic burden of cirrhosis-related complications
Cirrhosis remains clinically “silent” in approximately 40% of patients and becomes apparent only through routine medical examination or complaints of diffuse nonspecific symptoms (eg, anorexia, weakness, fatigue).Citation1 These patients are said to have compensated cirrhosis and have a 5-year survival rate of approximately 90%.Citation20 However, compensated cirrhosis may deteriorate into a decompensated state, resulting in PH, EVB, SBP, or HE.Citation1 Without proper management of these complications and subsequent liver transplantation, <50% of patients with decompensated cirrhosis survive for 5 years.Citation20
PH, which is often the first cause for hospitalization in patients with cirrhosis, may contribute to the development of other complications, including ascites, hepatorenal syndrome, variceal bleeding, and HE because of sinusoidal hypertension, peripheral vasodilatation, and the development of portosystemic collaterals.Citation2 According to HCUP, the total number of hospital discharges receiving a principal diagnosis of PH or EVB has tended to decrease since 1993.Citation9 In contrast, the number of hospital discharges with cirrhosis-related HE and SBP has increased.
Interestingly, hospital charges associated with cirrhosis-related complications have increased from 1993 to 2009, despite either stability or reductions in the mean length of hospital stay.Citation9 For example, mean cost associated with PH in 1993 was approximately $19,872 versus $47,231 in 2009, although the average length of hospital stay was 7.3 and 5.3 days, respectively. Similarly, in-hospital charges associated with HE have increased from $11,808 in 1993 to $35,875 in 2009 despite a reduction in the mean length of hospital stay (8.1 days in 1993 versus 5.8 days in 2009). These estimates of hospital cost associated with cirrhosis-related complications may be skewed because patients with these disorders are likely to require more than one hospital stay per year; however, the actual cost burden is probably greater than the numbers reported by HCUP because of costs associated with nonhospital care (ie, physician office visits, medications, screening for additional complications or deterioration of complications, and use of nonhospital caregiving facilities). Indeed, the percentage of patients with HE who were discharged to nursing homes or rehabilitation centers increased from 2.6% to 23.3% between 1993 and 2009.
Indirect costs (eg, lost work productivity, car accidents resulting from patients with minimal HE) also have an impact on the economic burden of cirrhosis-related complications and are, to date, unexamined. Further, the cost of ineffective therapeutic and maintenance regimens, including expenditures associated with reductions in HRQOL, increase the economic burden of cirrhosis-related diseases. The evaluation and use of cost-effective therapeutic strategies to prevent the occurrence and halt the progression and relapse of various cirrhosis-related complications is essential in reducing the economic impact of these disorders.
Cost analysis of therapy for cirrhosis-related complications
EVB
EVB is a lethal consequence of PH.Citation21 Varices form at a rate of 8% per year in patients with cirrhosis and are associated with a hepatic venous pressure gradient > 10 mmHg.Citation22 According to guidelines provided by the American Association for the Study of Liver Diseases and the American College of Gastroenterology, esophagogastroduodenoscopy should be performed upon diagnosis of cirrhosis. If small varices are present but not bleeding, nonselective β-blockers may be used to prevent rupture. Patients with medium or large nonbleeding varices either may be administered β-blockers (eg, propranolol) or may have endoscopic variceal ligation (EVL) performed, depending on other diagnostic factors (eg, Child–Pugh class). Other options include surgical shunt (eg, transjugular intrahepatic portosystemic shunt [TIPS]), endoscopic sclerotherapy, although neither of these is recommended as prophylaxis for nonbleeding varices.
Primary prophylaxis with β-blocker therapy appears to be more cost-effective than EVL, sclerotherapy, shunt surgery, and “do nothing” strategies.Citation21,Citation23 There is some debate, however, as to whether prophylactic β-blocker therapy should be administered in patients who are at risk for variceal bleeding (eg, those with large varices, Child–Pugh class B or C cirrhosis, and presence of red wale marks upon endoscopy) or whether treatment should be given to all patients with cirrhosis, regardless of endoscopic results. In a decision model analysis, the total costs (in 2000 US dollars) associated with no prophylaxis, endoscopy screening before prophylaxis, and universal prophylaxis strategies were $36,700, $37,300, and $34,100 per patient, respectively.Citation24 Universal prophylaxis was associated with the highest number of quality-adjusted life years (QALYs; 6.65) compared with no prophylaxis (4.84 QALYs) and endoscopy screening (5.72 QALYs) strategies.Citation24 These data suggest that universal prophylaxis therapy in patients with cirrhosis offers a substantial economic benefit.Citation21,Citation24
Once variceal bleeding occurs, antibiotics (eg, ciprofloxacin) are administered to reduce the risk of SBP or other infections in addition to pharmacologic (eg, vasopressin with or without nitroglycerin, terlipressin, somatostatin, octreotide) or endoscopic (eg, EVL, sclerotherapy) therapies to control bleeding.Citation22 To date, no cost comparisons between these strategies (pharmacologic versus endoscopic therapy) are available, although a Cochrane meta-analysis concluded that endoscopic therapy (eg, sclerotherapy) was not superior to pharmacotherapy in patients with EVB and was associated with additional risks for adverse events versus pharmacologic intervention.Citation25 In cases where patients’ symptoms are nonresponsive to pharmacologic or endoscopic therapies, surgical techniques (eg, shunt surgery, TIPS) may be used to control variceal bleeding; however, cost comparisons of surgical techniques versus pharmacologic or endoscopic therapies have not been performed. Although surgical intervention may intuitively appear to be less cost-effective than pharmacologic or endoscopic therapies because of the occurrence of prolonged hospitalization, utilization of the intensive care unit, and perioperative blood requirements, the resulting reductions in rehospitalization and improvement in HRQOL in surgically managed patients suggest that such procedures should not be economically discounted.Citation26 In addition, patients who received surgical intervention would not be required to undertake preventive measures for rebleeding, further reducing the overall cost burden.Citation2
After initial variceal bleeding is controlled, patients who receive pharmacologic or endoscopic therapy must be actively managed to prevent recurrence.Citation22 Nonselective β-blockers in combination with EVL are recommended, but shunt surgery or TIPS may also be implemented in cases of recurrent bleeding that do not respond to pharmacologic or endoscopic therapy. A cost analysis of observation alone, pharmacologic therapy, EVL, EVL combined with pharmacologic therapy, or TIPS demonstrated that combined treatment with β-blockers and EVL was more effective and more economical than any of the other strategies.Citation27 In contrast, a study evaluating the cost of TIPS versus sclerotherapy or EVL demonstrated that TIPS was the most cost-effective strategy because some patients treated with sclerotherapy or EVL required subsequent TIPS due to nonresponse.Citation28 However, this study may not have taken into account that patients receiving TIPS have a higher incidence of HE compared with those not receiving TIPS.Citation2 Given this observation, an accurate representation of the total economic cost burden of TIPS would have to encompass the cost of treatment related to the management of HE in addition to variceal bleeding. Although no such cost analyses have been performed, it is likely that the additional cost of HE management prohibits TIPS as a first-line therapy for secondary prophylaxis.
SBP
SBP is a frequent, life-threatening complication of cirrhosis.Citation29 This infection of ascitic fluid occurs without an intraabdominal infection or inflammation. The main causative agents of SBP, determined by culturing the ascitic fluid, are bacteria (eg, Escherichia coli, streptococci, Klebsiella spp). Patients with cirrhosis and ascites who develop symptoms of SBP (ie, fever, chills, abdominal pain, tenderness) with or without worsening of kidney or liver function may be screened for SBP, which is diagnosed when the polymorphonuclear cell count of ascitic fluid is >250/mm3.Citation2
Because of the bacterial origin of SBP, antibiotics (eg, cefotaxime, third-generation cephalosporins) are administered as first-line therapies and have been used as prophylaxis for SBP, although the efficacy of prophylaxis is controversial.Citation30,Citation31 Quinolones (eg, ciprofloxacin, norfloxacin) may be used for SBP prophylaxis in patients and may be cost-effective, especially in high-risk patients (ascitic fluid total protein < 1.5 g/dL).Citation2,Citation24,Citation32–Citation34 Universal prophylaxis in all patients with cirrhosis also may be economically favorable if the cost of the antibiotic prophylaxis is minimal.Citation30 Unfortunately, widespread prophylaxis may elicit bacterial antibiotic resistance and reduce clinical efficacy.Citation35 Indeed, bacterial antibiotic resistance to cefotaxime is increasing in prevalence, thus warranting the use of other antibiotics as prophylaxis and potentially impacting cost.Citation36,Citation37
Overall, direct cost analyses of antibiotic treatment for SBP are lacking. However, a clinical trial evaluating oral ofloxacin versus intravenous cefotaxime demonstrated comparable efficacy, disease resolution, treatment duration, and incidence of mortality.Citation38 Given that oral ofloxacin is less expensive and does not require intravenous administration, it is more cost-effective than cefotaxime. Similar results have been reported for oral amoxicillin–clavulanic acid and ciprofloxacin versus cefotaxime.Citation39,Citation40
Intravenous albumin may be added as a supplement to antibiotic therapy to improve arterial blood volume and reduce the endotoxin and cytokine levels in patients. The addition of albumin to antibiotic therapy reduces the risk of renal impairment and death versus antibiotic therapy alone, but the necessity for in-hospital intravenous administration may cast doubt on the economic benefit of this strategy.Citation41
HE
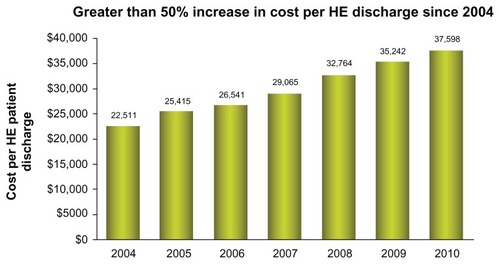
HE is a common condition in patients with cirrhosis, occurring in approximately 10%–60% of patients.Citation42 The frequency of HE continues to increase, with a 21% increase in 2010 (). In addition, the costs of HE continues to escalate to a level greater than $37,500 per single hospitalization ().
Figure 2 Increase trend in hepatic encephalopathy admissions since 2004, with 21% during the period of 2009 to 2010.
Abbreviations: HE, hepatic encephalopathy; ICD, International Classification of Diseases.
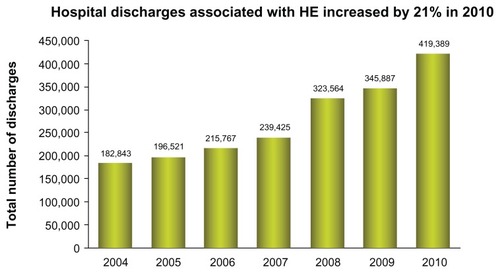
Figure 3 In-hospital cost (in US dollars) and duration of hospital stay for patients discharged with hepatic encephalopathy (International Classification of Diseases, Clinical Modification, Ninth Edition code, 572.2) or portal hypertension (International Classification of Diseases, Clinical Modification, Ninth Edition code, 572.3) from 2004 to 2010.
Abbreviations: HE, hepatic encephalopathy; ICD, International Classification of Diseases.
HE is caused by the accumulation of ammonia and other neuroactive substances within the systemic circulation due to reduced clearance by the liver as a result of portosystemic collateral formation or surgical alteration (eg, TIPS).Citation43 This accumulation of neurotoxic substances results in altered consciousness and motor abnormalities. The disease is progressive, and the symptoms range from minimal, with patients displaying only subtle disruptions in aspects of everyday life, to clinically severe. Minimal HE occurs in at least 20%–60% of patients with cirrhosis and is associated with the development of overt HE; therefore, effective management of minimal HE may prevent HE and its associated costs (eg, hospitalization).Citation42,Citation44 Current treatment of HE focuses on reducing the levels of ammonia and other neuroactive substances by altering or eliminating the source of these compounds (eg, intestinal bacteria).Citation43
The standard of care for HE is nonabsorbable disaccharides (eg, lactulose, lactitol), which are metabolized into propionic acid and lactic acid within the colon, resulting in colonic acidification and entrapment and excretion of ammonia.Citation2,Citation43 Clinical evaluations of nonabsorbable disaccharides for the treatment of HE have produced contradictory results, calling into question the overall efficacy and cost-effectiveness of these therapies despite their relatively low cost.Citation45–Citation48 Long-term maintenance therapy with lactulose to prevent recurrence of overt episodes appears to be effective, but patient compliance may be substantially hindered by adverse events (eg, diarrhea, flatulence).Citation49,Citation50 Noncompliance with these therapeutic regimens would increase the possibility of HE recurrence and thus increase health care costs. There is also a lack of evidence to support the claim that nonabsorbable disaccharides prevent the progression of minimal HE to overt HE, which suggests that costs associated with overt HE, and thus treatments that perturb this progression might be more cost-effective despite a more expensive direct cost. To date, no such therapies have been identified. However, nonsystemic antibiotics may offer a beneficial long-term economic alternative to nonabsorbable disaccharides.Citation50
Nonsystemic antibiotics (eg, neomycin, rifaximin) target the gastrointestinal flora and alter the bacterial count, thereby reducing production and accumulation of neuroactive substances.Citation51 The efficacy of neomycin in HE alone or in combination with lactulose is questionable,Citation52–Citation57 and tolerability concernsCitation53,Citation55 prohibit its long-term use. In contrast, rifaximin alone is more effective than placebo,Citation58 and rifaximin alone or in combination with nonabsorbable disaccharides is at least as effective as nonabsorbable disaccharides in reducing HE symptoms.Citation46,Citation54,Citation59–Citation64 In addition, rifaximin alone or in combination with nonabsorbable disaccharides alleviates HE symptoms more rapidly than lactulose, signifying the potential of rifaximin to reduce hospitalization costs.Citation59,Citation61,Citation62,Citation64
A 2007 decision model analysis examined several competing therapeutic strategies for HE, including a “do nothing” strategy, lactulose monotherapy, lactitol monotherapy, neomycin monotherapy, rifaximin monotherapy, and rifaximin as salvage therapy in patients intolerant of lactulose. It demonstrated that the “do nothing” strategy was the least effective and rifaximin salvage therapy was the most effective.Citation65 Lactulose monotherapy and rifaximin salvage therapy were the least expensive and most effective compared with the other strategies, but rifaximin salvage therapy cost an incremental $2315 per QALY gained versus lactulose. These findings suggest that, based on hypothetical assumptions, rifaximin therapy is more effective than no treatment but more expensive than other treatment strategies. However, overall cost-effectiveness must take into account not only the direct cost of therapy but also indirect costs, such as length of hospitalization, patient compliance or lack thereof, clinical status of patients at the end of therapy, and the potential for recurrence of an HE episode. These factors were not examined in the aforementioned study. For example, based on data from a Phase III clinical trial evaluating the efficacy of rifaximin in maintaining remission of HE episodes, for every four patients treated with rifaximin, approximately one case of breakthrough HE can be prevented.Citation66 In addition, one case of HE-related hospitalization can be prevented for every nine patients treated with rifaximin for up to 6 months. These data suggest that even though the direct cost of rifaximin is higher than placebo or “do nothing” strategies, benefits to the patient and a potential reduction in the need for long-term health care support the use of rifaximin as a cost-effective strategy.
In addition, several studies have evaluated the overall cost difference incurred by patients treated with rifaximin versus lactulose therapy.Citation67,Citation68 A retrospective chart review, published in 2006, of patients presenting with stage II HE who received either lactulose or rifaximin therapy demonstrated that the number and duration of hospitalizations was reduced in the rifaximin group compared with the lactulose group ().Citation68 Further, the total cost of therapy per patient per year was reduced by $5327 with the use of rifaximin. Similar results were obtained in a 2005 retrospective chart review of patients with HE who initially received lactulose but were switched to rifaximin after its introduction in the US in 2004.Citation50 Compliance rate, clinical status at the end of treatment, and adverse events associated with rifaximin were also significantly more favorable than with lactulose (P < 0.001 for all) in this study. As a whole, these results imply that despite the increased direct cost of rifaximin compared with nonabsorbable disaccharides, the overall benefits of rifaximin in terms of prevention of HE-related hospitalization, breakthrough HE episodes, reduction in length of hospital stay, and increased compliance and tolerability support the economic benefit of rifaximin over nonabsorbable disaccharides.
Table 1 Demographic and hospitalization information for both groups
When evaluating medical economics, all components must be considered. In the management of cirrhosis, drug costs, physician visits, procedures, and hospitalizations are the common economic components that determine the economic costs in treating the disease. HE management, in particular, rehospitalization, and length of hospital stays have been reported with economic costs recently presented.Citation66 Decreased length of stay, time to full diet, and frequency of readmission were found in patients treated with rifaximin prior to admission ().Citation69
Table 2 Overt hepatic encephalopathy hospitalizations identified by therapy upon admission and then in-house continuous or addition of medication
As the health care system transforms into a payer system guided towards bundle reimbursements, the health care provider will need to remain cognizant of lowering hospitalization frequency and shortening length of stay. In a retrospective review presented at the 2012 American College of Gastroenterology Annual Scientific Meeting, hospital readmissions were more frequent in patients on lactulose therapies. Although overall medication costs were much higher in the rifaximin groups, hospitalizations drove up cost to a significant amount culminating in rifaximin monotherapy group overall costs being 40% less than lactulose therapy (). The primary indicators for readmission were Model for End-Stage Liver Disease-driven international normalized ratio values, and an increase in Model for End-Stage Liver Disease score of 3.8 points.Citation70
Conclusion
Despite the implementation of preventive measures, the incidence of cirrhosis has remained stable, although the health care cost associated with cirrhosis has risen. The cause of this cost increase is unknown but may be related to the cost differential between previous therapeutic regimens and new, more beneficial therapies. These more beneficial therapies, in addition to being associated with a higher direct cost, may allow a greater percentage of patients to survive to receive liver transplantation, itself a costly medical procedure requiring hospitalization and posttransplantation outpatient care. These new therapies may also increase the number of patients who will develop and require management for cirrhosis-related complications while awaiting liver transplantation. Because of this, cost-effective management of cirrhosis-related complications is essential. Analyses of drug efficacy and patient compliance and tolerability, in addition to direct costs of treatment regimens, are essential for accurate determination of the cost benefit of individual therapies; however, to date, few adequately designed studies have undertaken such analyses. Further, studies evaluating the overall cost burden, including screening costs, cost of treatment and maintenance therapy, conveyance to liver transplantation, liver transplantation success, and the economic aspect of HRQOL after transplantation, are essential for evaluation of the economic burden of cirrhosis-related complications. Until these data are assessed, it is difficult to determine the overall economic burden of cirrhosis-related complications and, more importantly, provide the most economical and beneficial treatment strategies to patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- HeidelbaughJJBruderlyMCirrhosis and chronic liver failure: part I. Diagnosis and evaluationAm Fam Physician200674575676216970019
- Garcia-TsaoGLimJKManagement and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C ProgramAm J Gastroenterol200910471802182919455106
- SchuppanDAfdhalNHLiver cirrhosisLancet2008371961583885118328931
- CardenasAGinesPManagement of complications of cirrhosis in patients awaiting liver transplantationJ Hepatol200542Suppl 1S124S13315777567
- LimYSKimWRThe global impact of hepatic fibrosis and end-stage liver diseaseClin Liver Dis2008124733746vii18984463
- WasleyAGrytdalSGallagherKCenters for Disease Control and PreventionSurveillance for acute viral hepatitis – United States, 2006MMWR Surveill Summ200857212418354374
- LavanchyDThe global burden of hepatitis CLiver Int200929Suppl 1748119207969
- PyensonBFitchKIwasakiKConsequences of Hepatitis C Virus (HCV): Costs of a Baby Boomer Epidemic of Liver DiseaseNew York, NYMilliman, Inc2009 Available from: http://publications.milliman.com/research/health-rr/pdfs/consequences-hepatitis-c-virus-RR05-18-09.pdfAccessed January 18, 2011.
- LecuyerALevyCGaudelusJHospitalization of newborns and young infants for chickenpox in FranceEur J Pediatr2010169101293129720461528
- VrolijkJMde KnegtRJVeldtBJOrlentHSchalmSWThe treatment of hepatitis C: history, presence and futureNeth J Med2004623768215209471
- DienstagJLMcHutchisonJGAmerican Gastroenterological Association technical review on the management of hepatitis CGastroenterology2006130123126416401486
- ShepherdJWaughNHewitsonPCombination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic reviewHealth Technol Assess200043316711134916
- ShiffmanMLEstebanRTriple therapy for HCV genotype 1 infection: telaprevir or boceprevir?Liver Int201232Suppl 1546022212573
- McQueenCFDA approves Vertex’s new drug Incivek for treatment of hepatitis C [webpage on the Internet]Princeton, NJThe AIDS Beacon5232011 Available from: http://www.aidsbeacon.com/news/2011/05/23/fda-approves-vertexs-new-drug-incivek-telaprevir-for-treatment-of-hepatitis-c/Accessed January 21, 2013.
- BrownREDe CockEColinXAntonanzasFIloejeUHHepatitis B management costs in France, Italy, Spain, and the United KingdomJ Clin Gastroenterol20043810 Suppl 3S169S17415602166
- DenizBBroganAJMillerJDTalbirdSEThompsonJRProjections using decision-analytic modeling of long-term clinical value of telaprevir for the treatment of HCV patients who had failed prior peginterferon/ribavirin treatment [abstract]Hepatology201154S1802A803A
- CammaCPettaSEneaMCost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis CHepatology201256385086022454336
- ChhatwalJFerranteSADasbachEJCost-effectiveness of boceprevir use in patients with chronic hepatitis C genotype-1 who failed prior treatment with peginterferon/ribavirin [abstract]Hepatology201154Suppl 1801A802A21618565
- FerranteSAChhatwalJElbashaECost-effectiveness of boceprevir based regimens in previously untreated adult subjects with chronic hepatitis C genotype 1 [abstract]Hepatology201154Suppl 1795A796A
- GrewalPMartinPCare of the cirrhotic patientClin Liver Dis200913233134019442922
- ArguedasMRHeudebertGREloubeidiMAAbramsGAFallonMBCost-effectiveness of screening, surveillance, and primary prophylaxis strategies for esophageal varicesAm J Gastroenterol20029792441245212358270
- Garcia-TsaoGSanyalAJGraceNDCareyWPrevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosisHepatology200746392293817879356
- TeranJCImperialeTFMullenKDTavillASMcCulloughAJPrimary prophylaxis of variceal bleeding in cirrhosis: a cost-effectiveness analysisGastroenterology199711224734829024301
- SaabSDeRosaVNietoJDurazoFHanSRothBCosts and clinical outcomes of primary prophylaxis of variceal bleeding in patients with hepatic cirrhosis: a decision analytic modelAm J Gastroenterol200398476377012738453
- D’AmicoGPietrosiGTarantinoIPagliaroLEmergency sclerotherapy versus medical interventions for bleeding oesophageal varices in cirrhotic patientsCochrane Database Syst Rev20021CD00223311869632
- O’DonnellTFJrGembarowiczRMCallowADPaukerSGKellyJJDeterlingRAThe economic impact of acute variceal bleeding: cost-effectiveness implications for medical and surgical therapySurgery19808856937016776645
- RubensteinJHEisenGMInadomiJMA cost–utility analysis of secondary prophylaxis for variceal hemorrhageAm J Gastroenterol20049971274128815233665
- RussoMWZacksSLSandlerRSBrownRSCost-effectiveness analysis of transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic therapy for the prevention of recurrent esophageal variceal bleedingHepatology200031235836310655258
- RibeiroTCChebliJMKondoMGaburriPDChebliLAFeldnerACSpontaneous bacterial peritonitis: how to deal with this life-threatening cirrhosis complication?Ther Clin Risk Manag20084591992519209274
- DasAA cost analysis of long term antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhosisAm J Gastroenterol19989310189519009772051
- SabatMKolleLSorianoGParenteral antibiotic prophylaxis of bacterial infections does not improve cost-efficacy of oral norfloxacin in cirrhotic patients with gastrointestinal bleedingAm J Gastroenterol19989312245724629860409
- LoombaRWesleyRBainACsakoGPucinoFRole of fluoroquinolones in the primary prophylaxis of spontaneous bacterial peritonitis: meta-analysisClin Gastroenterol Hepatol20097448749319250986
- FernandezJRuiz del ArbolLGomezCNorfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhageGastroenterology200613141049105617030175
- SorianoGGuarnerCTomasANorfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhageGastroenterology19921034126712721397884
- DupeyronCMangeneyNSedratiLCampilloBFouetPLeluanGRapid emergence of quinolone resistance in cirrhotic patients treated with norfloxacin to prevent spontaneous bacterial peritonitisAntimicrob Agents Chemother19943823403448192461
- UmgelterAReindlWMiedanerMSchmidRMHuberWFailure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitisInfection20093712819169633
- YakarTGucluMSerinEAliskanHHusamettinEA recent evaluation of empirical cephalosporin treatment and antibiotic resistance of changing bacterial profiles in spontaneous bacterial peritonitisDig Dis Sci20105541149115419424797
- NavasaMFolloALlovetJMRandomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitisGastroenterology19961114101110178831596
- RicartESorianoGNovellaMTAmoxicillin-clavulanic acid versus cefotaxime in the therapy of bacterial infections in cirrhotic patientsJ Hepatol200032459660210782908
- TuncerITopcuNDurmusATurkdoganMKOral ciprofloxacin versus intravenous cefotaxime and ceftriaxone in the treatment of spontaneous bacterial peritonitisHepatogastroenterology200350531426143014571754
- SortPNavasaMArroyoVEffect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitisN Engl J Med1999341640340910432325
- PoordadFFThe burden of hepatic encephalopathyAliment Pharmacol Ther200725Suppl 13917295846
- GerberTSchomerusHHepatic encephalopathy in liver cirrhosis: pathogenesis, diagnosis and managementDrugs20006061353137011152016
- HartmannIJGroenewegMQueroJCThe prognostic significance of subclinical hepatic encephalopathyAm J Gastroenterol20009582029203410950053
- Als-NielsenBGluudLLGluudCNon-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trialsBMJ20043287447104615054035
- PaikYHLeeKSHanKHComparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized studyYonsei Med J200546339940715988813
- PooJLGongoraJSanchez-AvilaFEfficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled studyAnn Hepatol20065428128817151582
- SharmaPAgrawalASharmaBCSarinSKProphylaxis of hepatic encephalopathy in acute variceal bleed: a randomized controlled trial of lactulose versus no lactuloseJ Gastroenterol Hepatol2011266996100321129028
- SharmaBCSharmaPAgrawalASarinSKSecondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placeboGastroenterology2009137388589119501587
- LeevyCBPhillipsJAHospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathyDig Dis Sci200752373774117245628
- Abou-AssiSVlahcevicZRHepatic encephalopathy. Metabolic consequence of cirrhosis often is reversiblePostgrad Med20011092525411272694
- AtterburyCEMaddreyWCConnHONeomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double-blind clinical trialAm J Dig Dis1978235398406354373
- BlancPDauresJPLiautardJLactulose-neomycin combination versus placebo in the treatment of acute hepatic encephalopathy. Results of a randomized controlled trialGastroenterol Clin Biol1994181210631068 French7750678
- ConnHOLeevyCMVlahcevicZRComparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trialGastroenterology1977724 Pt 157358314049
- DawsonAMMcLarenJSherlockSNeomycin in the treatment of hepatic comaLancet195727370081262126813492624
- RothenbergMEKeeffeEBAntibiotics in the management of hepatic encephalopathy: an evidence-based reviewRev Gastroenterol Disord20055Suppl 3263517713457
- StraussETramoteRSilvaEPDouble-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathyHepatogastroenterology19923965425451483668
- SidhuSSGoyalOMishraBPSoodAChhinaRSSoniRKRifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial)Am J Gastroenterol2011106230731621157444
- BucciLPalmieriGCDouble-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathyCurr Med Res Opin19931321091188325041
- FestiDMazzellaGOrsiniMRifaximin in the treatment of chronic hepatic encephalopathy: results of a multicenter study of efficacy and safetyCurr Ther Res1993545598609
- GiacomoFFrancescoAMicheleNOronzoSAntonellaFRifaximin in the treatment of hepatic encephalopathyEur J Clin Res199345766
- LoguercioCFedericoADe GirolamoVFerrieriADel Vecchio BlancoCCyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical studyMinerva Gastroenterol Dietol2003491536216481971
- MasARodesJSunyerLComparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trialJ Hepatol2003381515812480560
- MassaPVallerinoEDoderoMTreatment of hepatic encephalopathy with rifaximin: double-blind, double dummy study versus lactuloseEur J Clin Res19934718
- HuangEEsrailianESpiegelBMThe cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy – a decision analysisAliment Pharmacol Ther20072681147116117894657
- BassNMMullenKDSanyalARifaximin treatment in hepatic encephalopathyN Engl J Med2010362121071108120335583
- LeevyCBEconomic impact of treatment options for hepatic encephalopathySemin Liver Dis200727Suppl 22631
- NeffGWKemmerNZachariasVCAnalysis of hospitalizations comparing rifaximin versus lactulose in the management of hepatic encephalopathyTransplant Proc200638103552355517175328
- NeffGWKemmerNParkinsonEOutcomes in length of hospital stay in cirrhotics admitted for overt hepatic encephalopathy [abstract]Am Coll Gastroeneterol2012107SupplS601
- NeffGWKemmerNParkinsonEReadmission rates and maintenance overt hepatic encephalopathy [abstract]Am Coll Gastroeneterol2012107Suppl 1S184S185
- Healthcare Cost and Utilization ProjectAgency for Healthcare Research and Quality [homepage on the Internet]. http://hcupnet.ahrq.govAccessed June 20, 2012.