Abstract
Background
First-line maintenance erlotinib in patients with locally advanced or metastatic nonsmall cell lung cancer (NSCLC) has demonstrated significant overall survival and progression-free survival benefits compared with best supportive care plus placebo, irrespective of epidermal growth factor receptor (EGFR) status (SATURN trial). The cost-effectiveness of first-line maintenance erlotinib in the overall SATURN population has been assessed and published recently, but analyses according to EGFR mutation status have not been performed yet, which was the rationale for assessing the cost-effectiveness of first-line maintenance erlotinib specifically in EGFR wild-type metastatic NSCLC.
Methods
The incremental cost per life-year gained of first-line maintenance erlotinib compared with best supportive care in patients with EGFR wild-type stable metastatic NSCLC was assessed for five European countries (the United Kingdom, Germany, France, Spain, and Italy) with an area-under-the-curve model consisting of three health states (progression-free survival, progressive disease, death). Log-logistic survival functions were fitted to Phase III patient-level data (SATURN) to model progression-free survival and overall survival. The first-line maintenance erlotinib therapy cost (modeled for time to treatment cessation), medication cost in later lines, and cost for the treatment of adverse events were included. Deterministic and probabilistic sensitivity analyses using Monte Carlo simulation (1000 iterations) were performed.
Results
According to the model simulations, first-line maintenance erlotinib compared with best supportive care in EGFR wild-type stable metastatic NSCLC resulted in 4.57 months of life gained (17.82 months for erlotinib versus 13.24 months for best supportive care) and 1.14 months of life without progression gained (erlotinib 4.29 versus best supportive care 3.15), and incremental total costs of erlotinib from €7897 (UK) to €9580 (Germany). The corresponding mean incremental cost per life-year gained of erlotinib ranged between €20,711 (UK) and €25,124 (Germany). Sensitivity analyses confirmed these results.
Conclusion
First-line erlotinib maintenance treatment is cost-effective compared with best supportive care in EGFR wild-type stable metastatic NSCLC, irrespective of the country setting.
Introduction
Lung cancer is the most frequently diagnosed malignancy in the world, and the leading cause of cancer-related death.Citation1 Based on histology, lung cancer can be broadly divided into small cell lung cancer and nonsmall cell lung cancer (NSCLC).Citation2,Citation3 The latter accounts for about 80% of all lung cancer cases,Citation1 and is divided into three major histological subtypes, ie, squamous cell carcinoma, adenocarcinoma, and large cell (undifferentiated) carcinoma.Citation2,Citation3 About 40% of NSCLC patients present with locally advanced noncurable (stage IIIb) or metastatic (stage IV) disease,Citation4,Citation5 for which the prognosis is poor. Less than 2% of patients with metastatic NSCLC are alive after 5 years.Citation4,Citation5
A biological and genetic variation of lung cancer is activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR).Citation6 The prevalence of EGFR activating mutations in NSCLC varies by ethnicity, with rates of 10%–20% estimated in Caucasians and 30%–40% reported in Asian populations.Citation2 NSCLC with no EGFR mutation is generally referred to as EGFR wild-type.
Standard first-line platinum doublet chemotherapy has shown a median overall survival of around 8–10 months.Citation7,Citation8 First-line platinum doublet chemotherapy is the current standard of care for first-line treatment in patients with EGFR wild-type, but is recommended to be stopped at disease progression, or after 4–6 treatment cycles at the latest, due to cumulative toxicity and a plateau in effectiveness.Citation2,Citation3 Following discontinuation of chemotherapy, most patients experience disease progression within 2–3 months,Citation9,Citation10 after which second-line treatment is recommended.Citation2,Citation3 Studies have suggested that 30%–50% of patients do not receive second-line treatment due to rapid disease progression, increased symptom burden, and decreasing performance status.Citation10 Hence the use of active maintenance therapy introduced immediately after first-line platinum doublet chemotherapy in patients with complete, partial, or stable disease response to treatment has been proposed.Citation2,Citation3 One maintenance treatment option is erlotinib, indicated in squamous and nonsquamous cell metastatic NSCLC after 4–6 cycles of platinum-based chemotherapy.Citation11,Citation12 Besides erlotinib, pemetrexed is the only other switch first-line maintenance option, but is only indicated for patients with nonsquamous cell disease.
Erlotinib is an EGFR tyrosine kinase inhibitor. Its efficacy as first-line maintenance therapy has been established in the randomized, multicenter, placebo-controlled Phase III SATURN trial.Citation13 The SATURN study compared first-line maintenance therapy with either erlotinib or best supportive care plus placebo (n = 889) following four cycles of platinum-based chemotherapy in patients with locally advanced or metastatic NSCLC. Patients who had not experienced disease progression after initial chemotherapy were randomized 1:1 to receive erlotinib 150 mg/day orally (plus best supportive care) or placebo (plus best supportive care) until disease progression, unacceptable toxicity, or death. The results demonstrated significant progression-free survival and overall survival benefits of erlotinib compared with placebo in the intention-to-treat population (progression-free survival hazard ratio [HR] 0.69, 95% confidence interval [CI] 0.58–0.82, P < 0.0001; overall survival HR 0.81, 95% CI 0.70–0.95, P = 0.0088).Citation13 The subpopulation of patients with stable disease following initial first-line chemotherapy appeared to benefit more from erlotinib than those with a previous complete or partial response,Citation13 and it is for this stable disease group that erlotinib is indicated as a maintenance treatment in the European Union.Citation11
Subgroup analyses of the EGFR wild-type population showed a significant progression-free and overall survival benefit (progression-free survival HR 0.78, 95% CI 0.63–0.96, P = 0.0185; overall survival HR 0.77, 95% CI 0.61–0.97, P = 0.0243).Citation13
The cost-effectiveness of first-line maintenance erlotinib in patients with metastatic NSCLC and stable disease, including all patients irrespective of EGFR mutation status, has been demonstrated across three countries in the European Union in recent analyses.Citation14 There has not yet been any assessment of whether the significant progression-free and overall survival benefit in EGFR wild-type patients observed in SATURN for first-line maintenance erlotinib corresponds to a cost-effective treatment regimen specifically in this patient group.
Thus, cost-effectiveness analyses were undertaken with the objective of determining the incremental cost-effectiveness of first-line maintenance erlotinib compared with best supportive care in patients with EGFR wild-type metastatic NSCLC and stable disease following first-line therapy, in five European countries.
Materials and methods
Cost-effectiveness analysis
A cost-effectiveness analysis using standard analytic decision methods was undertaken to assess the incremental cost per life-year gained from first-line maintenance erlotinib compared with best supportive care in patients with EGFR wild-type stable metastatic NSCLC. The model was programmed in Microsoft Excel 2003. The perspective of the analysis was that of national health care payers in five European countries, namely the UK, Germany, France, Spain, and Italy. For the base case analyses, costs, and health benefits were discounted at a 3.5% rate per annum.
Model structure
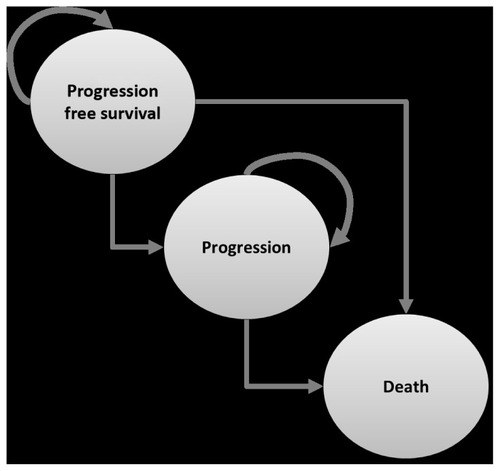
An area-under-the-curve (AUC) model (or partitioned survival model) was used, consisting of three health states, ie, progression-free survival, progression, and death (see ). Patients on first-line maintenance for EGFR wild-type stable metastatic NSCLC entering the model receive either erlotinib or best supportive care and were simulated over a lifetime horizon. All patients enter the model in the “progression-free survival” health state and in each month can either progress to a “worse” health state (ie, from “progression-free survival” to “progression” or “death”; or from “progression” to “death”) or remain in the same health state.
The AUC model calculates the likelihood of patients remaining in either progression-free survival or overall survival without discrete transitions using individual parametric survival curves fitted to the Kaplan–Meier curves from the SATURN study. The difference in the proportion of patients with overall and progression-free survival at any time is assumed to be the proportion of patients in the “progression” health state; death is calculated as the residual of the overall survival curve. Because an event (“progression” and “death”) can occur at any time and not just at the end of each month, a half-cycle correction was applied which assumed that both costs and effects occur half way through a model cycle.
Survival data
Progression-free and overall survival data from the EGFR wild-type stable metastatic NSCLC patients in SATURN were fitted with parametric functions to extrapolate the data beyond the trial period over patients’ lifetimes. Log-logistic functions [1/(1 + t × α)β] were used to simulate the time-dependent (t = time in months) probabilities of staying in the progression-free survival health state (erlotinib, α = 0.097, β = 2.151; best supportive care, α = 0.019, β = 2.151) and in the overall survival health state (erlotinib, α = 0.009, β = 1.901; best supportive care, α = 0.017, β = 1.901), respectively. These log-logistic functions provided the best fit to the data (assessed by the AUC statistic and log-likelihood ratio test) when testing various parametric functions (eg, log normal, exponential, gamma).
Cost input
For estimation of the erlotinib medication costs it was assumed that, as per the SATURN protocol, patients received a single daily dose of 150 mg of erlotinib orally (dispensed in packs of 30 tablets). Drug costs were modeled on the basis of current ex-factory prices. As in any trial, some patients in SATURN cease treatment prior to disease progression because of treatment-related toxicities; withdrawal due to adverse events occurred in 20 patients (5%) in the erlotinib group versus seven patients (2%) in the best supportive care group.Citation13 In the base case, erlotinib treatment was assumed for the time from when patients enter the model until treatment cessation, based on information from the SATURN trial. The potential costs of administering the oral drug erlotinib was considered to be small, with negligible total cost impact, and were thus not taken into account.
The costs of treating adverse events associated with erlotinib were modeled on the basis of incidence rates of adverse events ≥ grade 3 with a frequency ≥ 1% observed in the SATURN stable disease population prior to disease progression, ie, diarrhea (1.6%) and rash (5.2%). All adverse events ≥ grade 3 occurred in the erlotinib arm; in the placebo arm, no grade 3 or higher events were observed and thus adverse event costs were not considered for best supportive care. Incidence rates were multiplied with unit cost per adverse event episode to derive an average adverse event treatment cost, which was applied in the first model cycle (see ).
Table 1 Input values used in the cost-effectiveness model analyses for the five European countriesTable Footnotea
For patients experiencing disease progression during the simulation, it was assumed that 73% received active second-line treatment which was the proportion seen in the SATURN trial. Active treatment with docetaxel for 90 days was assumed (reflecting the median therapy duration in major randomized controlled Phase III trialsCitation15–Citation17), the costs of which were assessed on the basis of current ex-factory prices.
Sensitivity analyses
Model inputs and assumptions for the base case analysis were tested in both deterministic and probabilistic sensitivity analyses that investigated the impact of changes in key input parameters. Univariate deterministic sensitivity analyses were undertaken varying the following cost variables one by one, ie, administration costs, second-line treatment costs, discount rates for effects, and costs (see for parameter values tested). The base case setting on treatment duration of erlotinib (until treatment cessation) was tested by assuming treatment until disease progression (during the whole period of progression-free survival).
A probabilistic sensitivity analysis was also conducted to determine the overall influence of uncertainty within the model. Distributions around point estimates of key variables were defined that reflect parameter uncertainty. A second-order Monte Carlo simulation with 1000 iterations was run, drawing random values simultaneously from the predefined distributions. One thousand iterations were chosen because these provide a sufficiently high number to produce stable probabilistic health and economic outcomes, comparable with results of deterministic analyses. The following types of distributions were defined: gamma distributions that account for the impossibility of negative costs and which simulate potential high cost outliers (for cost of administering erlotinib, adverse event costs, and costs of second-line treatment), beta distribution generally used to describe proportions (for the proportion of patients receiving second-line treatment), and using Cholesky decomposition of the variance-covariance matrix within log-logistic survival functions (for progression-free survival and overall survival models).
Results
In patients with stable EGFR wild-type metastatic NSCLC, the model simulations showed that first-line maintenance erlotinib compared with best supportive care, resulted in 4.6 months of life gained (17.8 months for erlotinib versus 13.2 months for best supportive care); and 1.1 months of life without progression gained (4.3 months for erlotinib versus 3.2 months for best supportive care). The analyses showed total costs of erlotinib ranging from €10,542 (Italy) to €13,203 (Germany) and total costs of best supportive care from €2393 (Italy) to €3623 (Germany), resulting in total incremental costs ranging from €7897 (the UK) to €9580 (Germany). Mean incremental costs per life-year gained with first-line maintenance erlotinib compared with best supportive care in stable EGFR wild-type metastatic NSCLC ranged between €20,711 (the UK) and €25,124 (Germany); all well within ranges typically considered as cost-effective ().
Table 2 Results of cost-effectiveness analyses: base case and sensitivity analyses for five European countries
Sensitivity analyses results did not vary much because of changes in input values. The most sensitive variables were the discount rate for effects and duration of treatment. Lowest incremental cost-effectiveness ratios were found when lowering the discount rate for the treatment effect to 0%, highest values when assuming erlotinib treatment until progression and not until treatment cessation ().
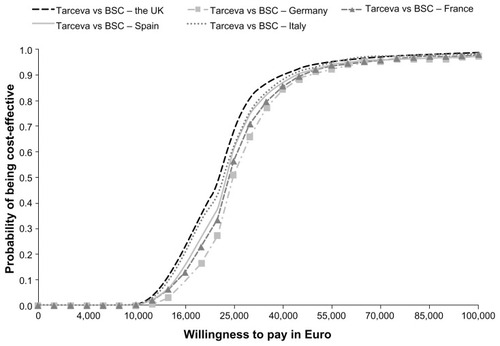
The cost-effectiveness acceptability curves resulting from probabilistic sensitivity analyses show that erlotinib would be considered cost-effective across the range of possible willingness-to-pay thresholds (see ). Looking exemplarily at a cost per life-year gained threshold of €50,000, the probability that erlotinib is cost-effective is 93.6% in the UK, 91.0% in Germany, 91.7% in France, 92.1% in Spain, and 92.9% in Italy.
Discussion
This analysis is the first to assess the cost-effectiveness of erlotinib as first-line maintenance treatment in patients with stable EGFR wild-type metastatic NSCLC. The results of the modeled analyses demonstrate an incremental costs per life year gained ranging between €20,711 and €25,124 comparing first-line maintenance erlotinib with best supportive care in five European countries (UK, Germany, France, Spain and Italy), and thus well within a range generally considered as cost-effective. Deterministic and probabilistic sensitivity analyses confirmed these findings, indicating that erlotinib first-line maintenance in stable EGFR wild-type metastatic NSCLC translates into improved overall survival and cost-effectiveness across jurisdictions in Europe. These analyses are based on empiric progression-free and overall survival data from the SATURN trial, and several parametric survival models were tested to identify the model with the best fit to the data.
In the SATURN trial, biomarker analyses for EGFR status showed that first-line maintenance erlotinib was efficacious irrespective of the EGFR status.Citation13 However, for the EGFR activating mutation-positive stable metastatic NSCLC population, patient numbers in SATURN were too small (n = 20) to fit survival models around the data reliably, and hence model the cost-effectiveness.
The cost-effectiveness of first-line maintenance erlotinib versus best supportive care stable metastatic NSCLC population, including all patients irrespective of EGFR mutation status, throughout European countries has recently been demonstrated in modeled analyses by Vergnenegre et al.Citation14 This analysis found that first-line maintenance erlotinib resulted in incremental costs per life year gained of between €27,885 and €39,783. Survival in this analysis was also modeled on the basis of the SATURN data. However, as the overall SATURN stable disease population showed a lower overall survival treatment effect than the stable EGFR wild-type sub-population, the incremental cost-effectiveness ratios reported by Vergnenegre et al were higher than in our analyses. The lower survival in the overall stable disease population could potentially be explained by the fact that 50% of patients in SATURN were of either “indeterminate” or “missing” EGFR mutation status, with 44% being wild-type and only 6% having EGFR activating mutations. This low proportion of patients tested for EGFR status may explain the observed variations in results.
The analyses presented are based on robust and transparent modeling and costing methods. Ex-factory drug prices were assumed for both erlotinib and second-line treatment (docetaxel), without considering potential pricing schemes available in the countries included. Administration costs were not considered for the base case analysis because they were regarded as negligible, given that erlotinib is administered orally. Sensitivity analyses varying administration costs have confirmed this assumption, because this parameter was not found to influence the results significantly. Costs of best supportive care were not considered in either the erlotinib or the best supportive care comparison group, because these costs are likely to be similar in both and thus their exclusion should not greatly affect the differences in costs and in the incremental cost-effectiveness ratios observed.
Assumptions on second-line treatment had to be made (for patients experiencing disease progression in the course of the simulation), whereby 90 days of docetaxel treatment was assumed for a proportion of patients. Other more expensive second-line agents such as pemetrexed are increasingly being utilized in first-line treatment, which was the reason for using docetaxel to estimate second-line treatment costs. Even higher second-line treatment costs would probably not affect incremental cost-effectiveness ratios, because these costs are likely to be comparable between the erlotinib and the best supportive care groups.
In addition, given that patients receiving erlotinib might progress later than patients in best supportive care, discounting costs might actually lead to higher overall second-line costs accrued in the best supportive care arm than in the erlotinib arm. This effect can be observed in the model, where second-line costs are spread pro rata across the progressive disease period. In order to test the impact on results of uncertain variables, all uncertain input parameters were tested in deterministic and probabilistic sensitivity analyses that demonstrated the robustness of base case results.
Besides erlotinib, other first-line maintenance options include targeted agents, such as bevacizumab, added to first-line platinum doublet chemotherapy and then continued until disease progression (continuous maintenance therapy).Citation2,Citation3 Besides erlotinib, pemetrexed is the only other switch first-line maintenance option, but it is only indicated for nonsquamous cell patients,Citation18 whereas erlotinib is indicated in both nonsquamous and squamous cell patients.Citation11
A recent population-matched indirect comparison of erlotinib and pemetrexed considering the intention-to-treat population of the SATURN and JMEN trials has found both treatments to be similarly efficacious.Citation19 In addition, Nuijten et al have demonstrated that erlotinib is less costly than pemetrexed.Citation20 Further considering other demonstrated advantages of erlotinib in terms of tolerability, administration, and patient convenience,Citation19 erlotinib appears to be the preferred treatment option over pemetrexed. Considering this, and taking into account that pemetrexed is only indicated in nonsquamous patients, whereas most EGFR wild-type patients are squamous, we did not regard pemetrexed as an appropriate comparator and therefore focused our analysis on comparing erlotinib with best supportive care.
In first-line NSCLC therapy, the choice of therapy according to EGFR mutation status has been proposed as a way to target treatment to the patient group that is most likely to benefit from erlotinib therapy. For example, somatic mutations in the EGFR gene have been proposed as the most robust biomarkers for EGFR-targeted choice of therapy in first-line NSCLC therapy.Citation21 However, for first-line maintenance, second-line and third-line therapy, there is no evidence supporting such an EGFR biomarker-based patient selection scheme for erlotinib.Citation21,Citation22 According to the clinical perspective shown above, our health economic analyses do not provide any indication for using EGFR activating mutation status as a predictor of more or less cost-effective subgroups for treatment with erlotinib, because patients with wild-type EGFR appear to benefit from first-line maintenance erlotinib at reasonable cost.
Conclusion
First-line erlotinib maintenance treatment is efficacious and represents good value for money compared with best supportive care, regardless of country setting, in patients with metastatic NSCLC and stable wild-type EGFR according to a cost-effectiveness model based on the Phase III SATURN trial data.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- NCCN Guidelines – Clinical Practice Guidelines in OncologyNon-small cell lung cancer Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfAccessed May 16, 2012
- D’AddarioGFruhMReckMBaumannPKlepetkoWFelipEMetastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol2010215116119
- MountainCFThe international system for staging lung cancerSemin Surg Oncol200018210611510657912
- GoldstrawPCrowleyJChanskyKThe IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumoursJ Thorac Oncol20072870671417762336
- GazdarAFActivating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitorsOncogene20092812431
- RamalingamSBelaniCSystemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directionsOncologist200813151318263769
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- BrodowiczTKrzakowskiMZwitterMCisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a Phase III trialLung Cancer200652215516316569462
- FidiasPMDakhilSRLyssAPPhase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancerJ Clin Oncol200927459159819075278
- European Medicines AgencyTarceva (erlotinib) – EU label and approval Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000618/WC500033994.pdfAccessed May 16, 2012
- US Food and Drug AdministrationTarceva (erlotinib) – FDA label and approval Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdfAccessed May 16, 2012
- CappuzzoFCiuleanuTStelmakhLErlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled Phase 3 studyLancet Oncol201011652152920493771
- VergnenègreARayJChouaidCCross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancerClinicoecon Outcomes Res201241313722347803
- ShepherdFADanceyJRamlauRProspective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapyJ Clin Oncol200018102095210310811675
- ShepherdFARodriguesPJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- HannaNShepherdFAFossellaFVRandomized Phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapyJ Clin Oncol20042291589159715117980
- European Medicines AgencyAlimta (pemetrexed) – EU label and approval Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000564/WC500025611.pdfAccessed May 16, 2012
- CascianoRBischoffHNuijtenMMalangoneERayJMaintenance erlotinib versus pemetrexed for the treatment of non-small cell lung cancer: indirect comparison applying real-life outcomesProceedings of the International Society for Pharmacoeconomics and Outcomes Research 13th Annual European CongressNovember 6–9, 2010Prague, Czech Republic
- NuijtenMJde CastroCJChouaidCA cross-market cost comparison of erlotinib versus pemetrexed for first-line maintenance treatment of patients with locally advanced or metastatic non-small-cell lung cancerLung Cancer201276346547122153602
- Ontario Health Technology Advisory CommitteeEpidermal growth factor receptor (EGFR) mutation testing for prediction of response to EGFR-targeting tyrosine kinase inhibitor (TKI) drugs in patients with advanced non-small-cell lung cancerPresentedat the Ontario Health Technology Advisory committeeAugust, 2010 Available at: http://www.health.gov.on.ca/english/providers/program/ohtac/tech/recommend/rec_EGFR_20101209.pdfAccessed May 16, 2012
- BradburyPATuDSeymourLEconomic analysis: randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancerJ Natl Cancer Inst2010102529830620160168