Abstract
Background
The purpose of this study was to identify a real-world US population having undergone surgery for malignant melanoma and describe treatment patterns, health care resource utilization, and costs for patients who subsequently received interferon alfa-2b (IFN) therapy or other standard of care chemotherapies.
Methods
A retrospective cohort study was conducted using administrative claims from MarketScan® databases among melanoma patients diagnosed between 2004 and 2008 who had surgery and were subsequently treated with IFN or other chemotherapies. Health care resource utilization and costs of services (converted to 2009 dollars) were evaluated. Cost refers to the amount paid to providers associated with the health service.
Results
Of 18,075 subjects with melanoma surgery claims, 1525 (8.4%) were treated with IFN and 1194 (6.6%) with other chemotherapies. Median duration (days) and number of doses of IFN therapy were 29 and 20, respectively. Approximately half of patients who received IFN discontinued therapy within or after the one-month induction phase. For IFN therapy patients, average total cost per patient for the last melanoma-related surgery prior to start of therapy, including costs of the surgery itself, pathology, anesthesia, and hospital care, was $2219. The average total cost per patient related to IFN therapy was $1188; this included costs for drug, office visits, blood work, and infusions. Other chemotherapy costs ranged from $146 to $2678.
Conclusion
There is an unmet treatment need, considering that this study observed that melanoma patients on IFN therapy post-surgery do not complete the recommended one-year course of treatment which may compromise its full therapeutic benefits. Further study to investigate reasons for discontinuation may be warranted. In addition, costs associated with adjuvant IFN therapy in post-surgical treatment of disease are likely acceptable.
Introduction
In the US in 2010, malignant melanoma was the fifth and seventh most common cancer diagnosed in men and women, respectively,Citation1 and the number of new cases (excluding basal cell and squamous cell carcinoma) was estimated at 68,130.Citation2 Among the available treatment options for malignant melanoma, surgical resection with or without lymph node sampling represents the standard of care and allows for staging, regional control of the disease, and, possibly, improved survival. In the US, patients with stage III melanoma undergo resection of primary tumors and all involved nodal basins with the aim of reducing the likelihood of tumor recurrence.Citation3–Citation5 Approximately 85%–90% of Stage III patients undergo surgery (lymphadenectomy or excision surgery). According to national guidelines, adjuvant therapy is offered to patients with stage III disease who have undergone surgery as the primary treatment and consists primarily of treatment with interferon alfa, but may also include observation, other treatments, or enrollment into clinical trials.Citation6
Interferon alfa-2b (Intron® A or IFN) is an immunotherapy indicated as adjuvant to surgical treatment in patients 18 years of age or older with malignant melanoma who are free of disease but at high risk of systemic recurrence within 56 days of surgery.Citation7 High-dose IFN over the long term has been shown to increase relapse-free survival.Citation8 It is approved as adjuvant therapy for high-risk (stages IIB, IIC, and III) melanoma.Citation7 On the other hand, chemotherapy is usually reserved for late-stage patients with metastatic disease (stage IV).
There are currently no published studies in the US on the real-world proportion of melanoma patients who receive IFN therapy following surgery and there are limited data on treatment patterns and costs specifically associated with adjuvant IFN treatment in melanoma patients following surgery.Citation9–Citation11 Using administrative claims data, the aims of this study were to: identify a real-world population which had undergone surgery for malignant melanoma and the proportion of those patients who had received IFN therapy post-surgery; describe the duration and frequency of IFN therapy; and describe the health care resource utilization (HCRU) and costs associated with the last surgery prior to start of IFN therapy, IFN therapy, and other selected chemotherapy agents used to treat melanoma post-surgery.
Materials and methods
Design and setting
The study was a retrospective cohort analysis of two Thomson Reuters MarketScan® Research databases.Citation12 The Thomson Reuters MarketScan Commercial Claims and Encounters database contains details of annual inpatient, outpatient, emergency room, and outpatient prescription drug claims of several million individuals covered under a variety of fee-for-service and capitated health plans. The Thomson Reuters MarketScan Medicare Supplemental and Coordination of Benefits database includes both the Medicare-paid portion and the employer-paid supplemental portion of payment. Neither database includes death data. Data were extracted in April 2011.
Subjects and treatments
Five cohorts were formed and are described with their inclusion criteria as follows:
Cohort A included patients over the age of 18 years with a confirmed melanoma diagnosis claim based on ICD9 diagnosis codes (172.0–172.9 and V10.82) between January 1, 2004 and December 31, 2008 in either the primary or secondary position of an insurance claim in any health care setting. The index melanoma diagnosis event was defined as the first medical event for melanoma based on the diagnosis code for a given patient occurring during the time window.
Cohort B consisted of patients from cohort A with confirmed claim(s) for a surgical intervention procedure related to melanoma. The index surgery event was defined as the first medical claim for a surgical intervention related to melanoma based on CPT4 reimbursement codes and ICD9 code 403. Cohort B was further examined to determine those patients whose surgery was performed following melanoma diagnosis (based on their index surgery date being after their index melanoma diagnosis date), and those who were diagnosed after surgery (based on their index surgery date being prior to a melanoma diagnosis).
Cohort C1 included patients from cohort B with confirmed claim(s) for IFN therapy within 60 days of a post-surgery claim. Patients needed to have started therapy prior to January 1, 2009.
Cohort C2 included the remaining patients from cohort B who did not have a claim for IFN therapy, and therefore consists of patients who received any other non-IFN therapy within 60 days post-surgery claim. Non-IFN therapy includes any medications taken by the patient, including those used to treat melanoma, such as chemotherapies but not IFN, or those to treat any other concomitant disease (not classified).
Cohort C3 consists of patients from cohort C2 who received chemotherapeutic drugs considered standard of care for regional and distant metastatic melanoma based on National Comprehensive Cancer Network guidelines,Citation6 specifically carboplatin, cisplatin, dacarbazine, paclitaxel, or temozolomide.
No continuous enrollment criteria were applied for the study. For cost data, only those related to a fee-for-service plan type were collected (average enrollee distribution from 2004 to 2008 was 81% for fee-for-service and 19% for the capitated health plan type).
Data analyses
The duration of IFN therapy 60 days post-surgery was analyzed separately by induction phase (defined as 0–29 days from start of IFN therapy) and maintenance phase (>29 days from start of IFN therapy). A window of 29 days was used from the start date of IFN therapy because induction therapy for Intron A consists of 4 weeks (28 days).Citation7 Thereafter, any claim was considered maintenance. Patients were censored at 12 months of IFN therapy.
The frequency of doses of IFN therapy among melanoma patients who received IFN therapy within 60 days post-surgery was estimated based on the number of claims recorded in the database. During the induction phase, it was assumed that patients also had claims related to office visits (for infusion administration).
For melanoma-related surgery, costs were collected for the last surgery claim prior to the start of drug therapy and using a ± one-day window. Services considered under melanoma-related surgery included the surgery itself, plus associated anesthesia, pathology, and hospital care captured from cohort C1. Surgery claims included those related to lymph node biopsy, removal or excision, and lymphadenectomy. Pathology claims included those related to any consultations, pathologist examinations, or procedures that occurred. Claims for observation, same day admissions, inpatient care, and associated consultations were considered hospital care.
Drug acquisition costs were based on costs related to the drug reimbursement (J) code for each product, including oral formulations where available. Claims associated with use of IFN according to the label were captured, reflecting tests normally required for monitoring with IFN use; any other routine laboratory tests that patients had done during their treatment period (that were not related to their melanoma treatment) were not considered.Citation7 Services related to the use of IFN therapy included complete blood count panels, thyroid-releasing hormone stimulation tests, infusion administrations, and associated office visits.
Cost refers to the amount paid to providers associated with the claim or health service including copayment(s) and/or coinsurance. All service costs are represented as 2009 costs. Costs were adjusted for yearly inflation based on the consumer price index medical care expenditure category, using 2009 as the base case.Citation13
The average unit cost per service per patient, across all claim codes (ICD9, CPT4, J) for that service, was calculated by averaging across all patients the cumulative sum of the costs incurred for a specific service by the total number of claims for the given service within each patient. A listing of all codes included in the analysis is available in Supplementary Table S1.
Results
Characteristics of patient population
Of the 178,817 melanoma patients identified in the Market Scan databases (), 18,075 had a confirmed claim for surgical intervention related to melanoma. Of these, 83.3% (n = 15,055) received surgery after a melanoma diagnosis. The mean and median duration from the time of melanoma diagnosis to the last melanoma-related surgery prior to the start of IFN therapy was 126 ± 288 days and 28 (range 0–2154) days, respectively. In total, 16.7% (n = 3020) of patients were diagnosed following surgery.
Figure 1 Identification of melanoma patients.
Abbreviation: IFN, interferon alfa-2b.
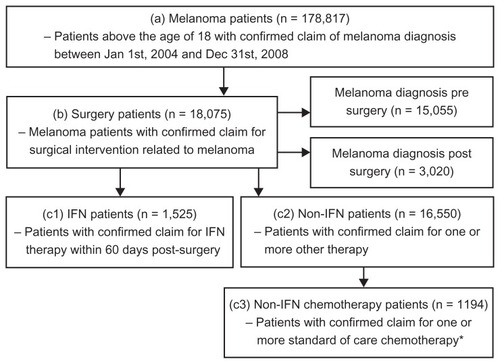
Among the 18,075 melanoma patients identified who had a confirmed claim of surgery related to melanoma, 8.4% (n = 1525) were treated with IFN therapy and 91.6% (n = 16,550) were treated with non-IFN therapy within 60 days post-surgery. Of the patients with a surgery claim, 6.6% (n = 1194) had a confirmed claim for at least one of the standard of care chemotherapeutic agents for metastatic melanoma. It should be noted that a subset of the CPT4 codes used to identify patients with a surgical intervention related to melanoma was labeled as “biopsy/lymph node removal”. Although it could not be distinguished whether the code pertained to a surgical procedure conducted for diagnostic or intervention purposes, exploratory analyses were conducted excluding this subset of patients and the distribution with regard to IFN therapy was similar. Of 17,607 patients, 8.6% (n = 1506) were treated with IFN and 91.4% (n = 16,101) patients were treated with non-IFN therapy following surgery.
The characteristics and demographics for the melanoma patient population with a surgery claim and treated with adjuvant IFN or non-IFN therapy, along with results for statistical tests conducted between the two groups, are summarized in .
Table 1 Patient demographics and other baseline characteristics, by type of treatment
Duration and frequency of IFN therapy
IFN therapy comprises two phases, ie, induction and maintenance, for a total duration of one year. The induction phase consists of intravenous injections administered five times a week for 4 weeks, for a maximum of 20 doses. This is followed by a maintenance phase of subcutaneous injections that are self-administered three times a week for 48 weeks, for a maximum of 144 doses.Citation7
Among the 1525 melanoma patients who received IFN therapy within 60 days post-surgery (cohort C1), about half (n = 779, 51.1%) discontinued therapy within or after induction and half (n = 746, 48.9%) continued on to the maintenance phase ().
Table 2 IFN therapy duration and frequency from claims data of melanoma patients post-surgery
For the induction phase, patients received IFN therapy for approximately one month, with a mean and median duration of IFN therapy of 22 days and 25 days, respectively, with broad ranges. Patients continued on IFN therapy into the maintenance phase, including induction, for approximately 3–5 months, with mean and median durations of 147 and 74 (range 30–707) days, respectively ().
Assessing the proportion of patients on IFN therapy for various durations, 51.1% were on therapy for one month, another 26.4% of patients continued to 3 months, and 17.8% from 6 months up to one year. The remaining 4.7% had at least one claim beyond one year, for reasons that cannot be obtained from an administrative claims database (). Ninety one percent of IFN patients were on therapy for some period of time during a one-year period.
Among melanoma patients who received IFN therapy within 60 days post-surgery, both the mean and median frequency of doses during the induction phase for IFN therapy was 14. During the maintenance phase, the mean and median dose frequency was 40 and 23, respectively, with broad ranges ().
HCRU and costs of melanoma-related services
Health care resource utilization and average unit costs per service per melanoma patient are reported for cohort C1 (n = 1525). Costs include those associated with the last surgery prior to IFN therapy and those related to the subsequent treatment after surgery ().
Table 3 Health care resource utilization and 2009 costs for treating adjuvant melanoma
The average total cost per patient for melanoma-related surgery was $2219 (n = 1525), with surgery cost being the most expensive component, averaging $1046 (n = 1525). The average cost per patient related to anesthesia, pathology, and hospital care was $858 (n = 639), $168 (n = 644), and $147 (n = 746), respectively.
The average total cost per patient specifically related to adjuvant IFN therapy amounted to $1188, including drug cost (but not including any concomitant drug treatment costs). Any IFN regimen represents treatment of malignant melanoma patients at Stage IIB to III. On average, for a melanoma patient being treated with IFN, it cost a total of $374 (n = 1525) for services related to drug therapy. The cost per patient for complete blood count and thyroid-releasing hormone tests were $18 (n = 1231) and $34 (n = 134), respectively, while the cost associated with drug infusion was $255 (n = 1525). An office visit cost $67 (n = 1464). The average cost for drug only for each patient was $814 (n = 1525).
In addition, the number of services and costs related to the drug-only portion of non-IFN standard of care chemotherapy for metastatic melanoma (cohort C3, n = 1194) was also captured. These regimens represent treatment for Stage IV metastatic disease. Dacarbazine, temozolomide, cisplatin, carboplatin, and paclitaxel all have differing dosage schedules, as reflected in the varying number of claims among the drugs. Temozolomide, with the fewest claims, was the most expensive standard of care agent ($2678; n = 39). Corresponding costs ranged from $146 (n = 298) for dacarbazine and $202 (n = 344) for cisplatin, to $715 (n = 530) and $886 (n = 578) for carboplatin and paclitaxel, respectively.
Discussion
Using real-world claims data, this study identified populations that had undergone surgery for malignant melanoma and subsequently received IFN therapy or non-IFN standard of care chemotherapy. According to the indications for IFN and non-IFN chemotherapies, these treatments describe adjuvant therapy for patients with Stage IIB to III malignant melanoma and with Stage IV metastatic melanoma, respectively.
For the population who received IFN therapy, the duration of total therapy (induction and maintenance combined) was on average 3–5 months. However, approximately 50% of patients discontinued therapy within or after induction, for reasons that cannot be obtained from an administrative claims database study like this. The median number of total doses for IFN therapy was 20, corresponding to the total number of doses for the induction phase according to the Intron A product label.Citation7 This indicates that patients seem to complete the induction phase of therapy which requires infusion, and then discontinue, not moving onto the self-administered maintenance phase of therapy. Further study to investigate reasons for discontinuation may be warranted. In addition, 91% of patients were on IFN therapy for some period of time during a one-year period. There is no known published source regarding the duration of IFN therapy as observed in the pivotal clinical trials, but the median relapse-free survival for IFN observed was 1.72 years compared with 0.98 years for observation.Citation14
To optimize treatment outcomes, it is important to adhere to recommended therapy regimens. A study by Agarwala et al indicated that 4 weeks of therapy with IFN, equivalent to the induction phase alone, did not show improvement in relapse-free survival (6.8 years compared with 7.3 years for observation) in patients with intermediate (81% of IFN-treated population) or high-risk (19% of IFN-treated population) melanoma.Citation15 This is in contrast with the increase observed in the pivotal trial, noted earlier.Citation14 However, there is no publicly available scientific source documenting the duration of IFN therapy from either of these trials. The study by Agarwala et al suggests that longer duration of IFN treatment may be important.Citation15 Adherence to the recommended dosage and administration of IFN in the high-risk population should be an important part of patient education to maximize the benefits of therapy.
Possible factors contributing toward discontinuation of IFN therapy may be inconvenience of infusion administration and frequency of therapy during the induction phase, as well as toxicity related to therapy.Citation16 Newer therapies are available for Stage III disease, such as a PEGylated version of IFN (Sylatron™) which, in contrast with non-PEGylated interferons, allows for less frequent dosing because of delayed renal clearance. In addition, dosing modification guidelines exist for PEGylated IFN which are intended to manage any associated toxicity and allow patients to stay on therapy for as long possible. The proposed course of treatment with PEGylated IFN for melanoma involves an induction phase of one dose per week subcutaneously for 8 weeks, followed by a maintenance phase of half that dose once per week subcutaneously for up to 5 years.Citation17 This dosing form allows for self-administration, thereby eliminating costs associated with infusion, such as for IFN.
Two of the largest cost components of the annual direct cost of diagnosing and treating melanoma in the US are reported from a modeling study to be adjuvant IFN therapies (27%, $151 million) and terminal care (35%, $197 million).Citation18 This was confirmed by a US economic model that found the most expensive elements of medical care among patients with melanoma to be adjuvant IFN therapy at about $76,000 per patient, and palliative care at $14,500 per patient.Citation19 This is supported by another US study which showed that hospital services were the main contributor to the high cost of malignant melanoma on a per patient basis among subjects aged 65 years and older who had a malignant melanoma diagnosis of at least stage IIB or higher.Citation9 Differences in methodology among studies preclude direct comparison of cost results.
For the current study, cost refers to the amount paid to providers associated with the health service; because these are reimbursement rates, actual costs could be lower than what is reported. Looking at the study results, average unit costs for surgery itself accounted for most of a patient’s total melanoma-related surgery costs at $1046 out of $2219. The average unit cost for IFN drug therapy only was $814, while average unit costs for other chemotherapy drugs ranged from $146 to $2678. However, these values should be taken in context with the patient populations they treat, IFN is used for patients with Stage IIB to III disease, which are a larger segment of the melanoma patient population compared with patients at Stage IV. In addition, patients at the earlier stages have a better chance for survival and continuing on treatment. Further, the number of single-agent or combination regimen chemotherapy cycles completed also contributes toward the cost. Therefore, comparisons cannot be made in this regard.
There were limitations in conducting this retrospective database study. The stage of disease could not be confirmed due to the absence of staging information in the database; it was assumed that receipt of IFN therapy following surgery defined patients with at least regional disease. Secondly, the proportion of patients that were part of a clinical trial was unknown and may influence claims generated if services were reimbursed by other sources, thus causing potential for underestimation of some costs. Also, inclusion of only fee-for-service patients may have introduced selection bias, though the majority of patients were enrolled in this plan type. Lastly, patient adherence could not be assessed due to lack of information on aspects such as disease management and how IFN was administered during the treatment phases.
Conclusion
This study observes that there is an unmet treatment need because melanoma patients on IFN therapy post-surgery enrolled in this claims database did not complete the recommended one-year course of treatment, and half of patients discontinued therapy within or after the induction phase; this may compromise the full therapeutic benefits of IFN therapy. Further, costs associated with adjuvant IFN therapy in post-surgical treatment of disease are likely acceptable.
Supplementary table
Table S1 CPT4 and J codes used in claims data analysis of melanoma patients
Disclosure
The authors are employees of Merck, Sharpe and Dohme Corporation, a subsidiary of Merck and Co, Inc, Whitehouse Station, NJ. Medical writing funded by Merck, Sharpe and Dohme Corporation was provided by BioMedCom Consultants Inc, Montreal, Canada.
References
- RigelDSEpidemiology of melanomaSemin Cutan Med Surg201029420420921277533
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin201060527730020610543
- AlgaziAPSoonCWDaudAITreatment of cutaneous melanoma: current approaches and future prospectsCancer Manag Res2010219721121188111
- CalifanoJNanceMMalignant melanomaFacial Plast Surg Clin North Am200917333734819698915
- PetrescuICondreaCAlexandruADiagnosis and treatment protocols of cutaneous melanoma: latest approach 2010Chirurgia (Bucur)2010105563764321141087
- National Comprehensive Cancer NetworkNCCN clinical practice guidelines in oncologyMelanoma Version 42011
- Intron® (Product information)Kenilworth, NJSchering-Plough Corporation2009
- KirkwoodJMManolaJIbrahimJSondakVErnstoffMSRaoUA pooled analysis of Eastern Cooperative Oncology Group and Intergroup trials of adjuvant high-dose interferon for melanomaClin Cancer Res20041051670167715014018
- DavisKLMitraDKotapatiSIbrahimRWolchokJDDirect economic burden of high-risk and metastatic melanoma in the elderly: evidence from the SEER-Medicare linked databaseAppl Health Econ Health Policy200971314119558193
- KotapatiSDavisKLMitraDTreatment patterns in high risk and metastatic melanoma: Evidence from linked electronic medical records and administrative claims dataJ Clin Oncol200826Suppl Abstr 17540
- RaySTunceliAGanguliSEconomic burden of metastatic melanoma in a commercially insured US populationJ Clin Oncol2010Suppl Abstr e19000
- HansenLGChangSHealth research data for the real worldThe Thomson Reuters MarketScan® DatabasesAnn Arbor, MIThomson Reuters2010
- US Bureau of Labor StatisticsConsumer price index, 2011 Available at: http://www.bls.gov/cpi/Accessed May 8, 2012
- KirkwoodJMStrawdermanMHErnstoffMSSmithTJBordenECBlumRHInterferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684J Clin Oncol19961417178558223
- AgarwalaSSLeeSJFlahertyLESmylieMRandomized Phase III trial of high-dose interferon alfa-2b (HDI) for 4 weeks induction only in patients with intermediate- and high-risk melanoma (Intergroup trial E1697)Abstract presented at the American Society of Clinical Oncology annual meetingChicago, ILJune 3–7, 2011
- KirkwoodJMBenderCAgarwalaSMechanisms and management of toxicities associated with high-dose interferon alfa-2b therapyJ Clin Oncol200220173703371812202672
- EggermontAMSuciuSSantinamiMAdjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised Phase III trialLancet2008372963311712618620949
- TsaoHRogersGSSoberAJAn estimate of the annual direct cost of treating cutaneous melanomaJ Am Acad Dermatol1998385 Pt 16696809591809
- AlexandrescuDTMelanoma costs: a dynamic model comparing estimated overall costs of various clinical stagesDermatol Online J200915111