Abstract
Background
The purpose of this study was to investigate the savings accrued using bevacizumab-based treatment for non-small-cell lung cancer from the societal perspective, taking only public costs into account, in France, Germany, Italy, and Spain.
Methods
Societal costs were estimated by collecting and analyzing labor costs, carer costs, sickness benefits, disability benefits, and home care benefits. Cost inputs were derived from publicly available databases or from the published literature. Expert opinion was only used if no other source was available. Efficacy data from two randomized clinical trials were used. The time horizon in the health economic model was lifetime. Efficacy and costs were discounted by 3.5%. All main model parameters were tested in deterministic and probabilistic sensitivity analyses.
Results
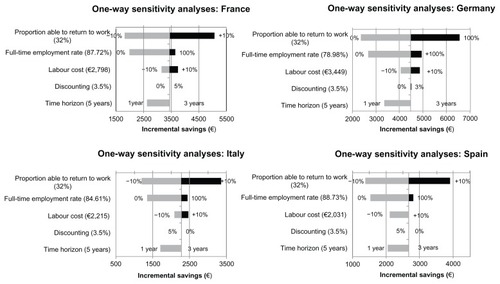
Mean incremental savings to society per patient ranged from €2277 in Italy to €4461 in Germany. The results were most sensitive to the change in proportion of patients working fulltime and the proportion of patients who were able to return to work.
Conclusion
This analysis shows that bevacizumab-based treatment in non-small-cell lung cancer is associated with more savings to society compared to standard chemotherapy in terms of increased productivity and decreased social benefits paid to patients who are able to work in France, Germany, Italy, and Spain.
Introduction
There are about 200,000 new cases of lung cancer and 140,000 lung cancer deaths each year in the European Union.Citation1 Patients with lung cancer have a poor prognosis. The disease accounts for approximately 20% of all cancer-related deaths in Europe, and accounts for more deaths than any other malignancy.Citation2 The majority of lung cancer cases are diagnosed when the disease is at an advanced stage.Citation3 The age-adjusted 5-year survival rate for lung cancer is only around 10%.Citation1 Non-small-cell lung cancer (NSCLC) accounts for around 80% of lung cancer cases.Citation3
Patients with advanced lung cancer experience a high symptom burden.Citation4–Citation12 The significant burden on lung cancer patients is due to the large number of symptoms experienced and their severity, which increase as the disease progresses, and health-related quality of life decreases accordingly.Citation13 In addition to improving overall survival of advanced NSCLC patients, extending progression-free survival is a clinically meaningful treatment goal for patients. Prolonging the time before symptoms worsen (disease progression) can delay the negative physical and emotional consequences associated with disease progression.
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor and indicated for first-line treatment of unresectable, locally advanced, recurrent, or metastatic non-squamous NSCLC in combination with platinum-based chemotherapy in Europe.Citation14 It has been shown to delay tumor progression significantly compared with standard platinum chemotherapy alone ().Citation15,Citation16 The efficacy of the various chemotherapies available has been shown to be similar.Citation17,Citation18 It has been reported that the efficacy of bevacizumab is generally consistent across chemotherapies, including carboplatin and cisplatin doublets.Citation19
Table 1 Efficacy of randomized clinical trials
Research has demonstrated that improvements in health care have considerable value to society.Citation20 The value is created by investments in medical research and through the experiences using these technologies in clinical practice. Increasingly, questions are being asked about the value of new health technologies in respect to their costs. Although benefits are commonly considered in their widest possible sense; costs in economic evaluations are typically limited to the “payer” perspective.Citation21,Citation22 Some health technology assessment and reimbursement agencies recommend considering the societal perspective.Citation23 The societal perspective is defined as incorporating all costs and benefits, no matter who pays the costs and who receives the benefits.Citation24 It has been argued that abandoning the societal perspective may lead to suboptimal decisions about allocation of resources.Citation25 In this analysis, the societal perspective is defined as public health service and government.
Governments face high costs related to the treatment of lung cancer. Treatment of advanced lung cancer is associated with frequent hospital visits, surgery, radiation therapy, chemotherapy, and targeted therapies. There are further losses arising from lost productivity due to sick leave, disability, and premature deaths linked to lung cancer. Patients with advanced NSCLC who can no longer work do not pay into social contribution schemes like health insurance funds, pension funds, or nursing care funds. Depending on the country, these payments will be made on behalf of the patient by the health insurance fund or out of tax funds. The productivity losses double when an employed family member becomes a carer for the patient with lung cancer. Additionally, patients who are no longer able to look after themselves will require formal care.
An observational cost-of-illness study, ie, ALCEA (Advanced Lung Cancer Economic Assessment), reports on considerable societal burden associated with the disease in patients with advanced NSCLC. A high level of health care resource utilization and a significant productivity loss were observed.Citation26 Another study found that bevacizumab-based treatment can result in substantial cost savings in patients with advanced NSCLC who are progression-free.Citation27 The aim of this study was to explore the incremental societal savings attributable to bevacizumab-based treatment compared with chemotherapy in France, Germany, Italy, and Spain.
Materials and methods
This study estimates the potential savings from the societal perspective resulting from reduced productivity losses in patients with advanced NSCLC in Germany, France, Italy and Spain.
A health economic model was developed to assess the societal savings associated with bevacizumab-based treatment when compared with chemotherapy. A Markov model was selected because it allows assessment of patient transition between different health states separately in both treatment arms. Patient level data from two randomized, controlled, double-blind Phase III trials of first-line therapy for advanced NSCLC were used to derive Markov model cycle-specific numbers of progression-free patients as well as those patients whose disease progressed, who died, or who were censored.Citation15,Citation16,Citation28 These data were fitted into Weibull distributions that were used to estimate the numbers of patients in each health state for the duration of the model. Health outcomes as well as associated costs were modeled in monthly cycles. The analysis time horizon was lifetime. The model has two states, ie, progression-free survival and disease progression or death. The progression-free survival state is divided into proportions of patients who can work after induction therapy and those who cannot do so. Each patient has a probability of having an informal carer, a formal carer, or not having a carer. Patients were given bevacizumab + standard chemotherapy or only standard chemotherapy. The chemotherapy was either cisplatin-gemcitabine or carboplatin-paclitaxel. More details of the studies and treatment regimes have been described elsewhere.Citation15,Citation16,Citation28 Clinical outcomes and costs were discounted at a rate of 3.5%.
All patients were assumed to be off work during the treatment induction phase, when bevacizumab + chemotherapy or chemotherapy is administered for six cycles. Each treatment cycle lasts 21 days for both therapies. The proportion of patients assumed to return to work after initial induction therapy was 32% for the bevacizumab-based treatment arm and 19% for the chemotherapy arm.Citation29
Incident lung cancer patients in the working age population were estimated from the Globocan database.Citation2 The working age was defined as 15–55 years at the time of diagnosis. A normal distribution was assumed around the mean age to derive the cohort of patients who would be aged 55 years or under at diagnosis. Of these patients, clinical experts estimated that 36% were patients with advanced and/or metastatic NSCLC. The proportion of patients treated with various therapies was assumed to be equally split, eg, 25% each treated with bevacizumab + cisplatin-gemcitabine, bevacizumab + carboplatin-paclitaxel, cisplatin-gemcitabine, or carboplatin-paclitaxel.
Only patients who were employed prior to their diagnosis are considered in the study. The employment rates for France, Germany, Italy, and Spain are 64%, 71%, 57%, and 59%, respectively.Citation30 The proportions of patients assumed to be working full-time and part-time are shown in . These proportions were applied to both patients and informal carers. For simplicity, it was assumed that patients who worked full-time prior to their diagnosis would return to work full-time. The same assumption was made for part-time workers, ie, if they were part-time workers they returned to work part-time. The informal carer’s return to work was not dependent on their working status prior to diagnosis, but on whether the patient returned to work, and whether it was full-time or part-time. Thirteen percent of the patients were assumed to have informal carers.Citation29
Table 2 Model input data
The patients who were estimated to return to work continued to work until disease progression or death, whichever occurred first. Only patients who were aged 55 years or under at the time of diagnosis were assumed to return to work. This estimate is based on expert opinion because no published evidence was identified.
The labor costs applied are shown in . The cost of sick leave and a possible disability pension were also taken into consideration. The details of the figures included are shown in . Informal carers were assumed to be on unpaid leave during the time that they were unable to work. No long-term benefits were considered in this study due to the complexity of including them. No home care benefits were assumed for Italy, because there was no home care benefit scheme in place during the study period.
Results
Our analysis shows that gains in productivity and associated reductions in societal expenditure are higher in the bevacizumab treatment arm (€5216, €6739, €3455, and €4046 per person in France, Germany, Italy, and Spain, respectively) than in the chemotherapy arm (€1774, €2278, €1178, and €1377, respectively). These societal gains are due to the time spent in progression-free survival by patients who were estimated to be able to return to work in each treatment arm. A comparison of the two treatments arms is shown in .
Figure 1 Comparison of Bevacizumab plus chemotherapy treatment and chemotherapy treatment savings (5 year cumulative savings).
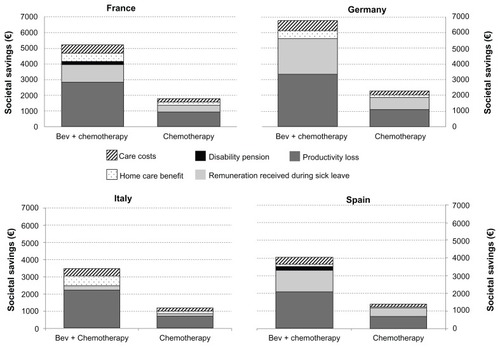
Most of the societal savings were accrued from reduced losses in labor costs. These savings represented 55%, 50%, 64%, and 52% of all savings in France, Germany, Italy, and Spain, respectively. The rest of the societal savings were due to not having to file a claim sickness benefit, disability benefit, or home care benefit, and having reduced formal care costs. Additionally, if the patient had an informal carer, savings were increased because the carer was able to return to work. The breakdown of these savings can be seen in .
To assess for uncertainty in the model structure and inputs, two types of sensitivity analysis techniques were used, ie, one-way and probabilistic. In one-way sensitivity analysis, the varied inputs were discounting rate (0% and 5%), time horizon (one year and 3 years), labor cost (±10%), proportion of patients in full-time employment (of those who are employed) prior to diagnosis (0% and 100%), and proportion able to return to work (±10%). The results of the one-way sensitivity analyses are shown in .
Probabilistic sensitivity analyses are often used to assess how uncertainty in model outputs can be apportioned to imperfect knowledge of the parameter input values. In our probabilistic sensitivity analysis, we used a cohort simulation technique to assess for parameter uncertainty. Cohort simulation is often used to test the robustness of the results of random variation in the values of the key parameters. The number of iterations run was 1000. shows the results.
Table 3 Probabilistic sensitivity analysis results
Discussion
For most patients, a diagnosis with advanced cancer is a terminal prognosis. The impact of advanced NSCLC extends beyond the individual, affecting both the emotional and financial well-being of patients and their families.
There are only a few studies published on the cost of illness in lung cancer, especially ones taking a societal perspective. Country cancer registries have been used to assess the overall morbidity from lung cancer.Citation31,Citation32 A study by Stanisic et al assessed the economic burden of metastatic NSCLC from a societal perspective,Citation27 and found that the mean cost savings after one year were €21,667, €21,171, €17,578, and €12,401 in France, Germany, Italy, and Spain, respectively. The study results suggest that there are reduced productivity losses due to improved progression-free survival in patients treated with bevacizumab-based therapy compared with patients treated with chemotherapy alone. Another study found that the productivity losses associated with advanced lung cancer were around €11,390,000 in Italy, making the average loss per patient approximately €60,263.Citation26 The study by Perrone et al is a cost-of-illness study in Italy, assessing the direct and indirect costs associated with patients having lung cancer in Italy. Although these studies are not directly comparable with our analysis, our findings are in line with these studies. The previous work by Stanisic et al only took labor costs into consideration, whereas this analysis has taken a wider perspective by including productivity losses associated with informal carers, cost of formal carers, sick leave remuneration, disability pension, and home care benefits.Citation27 The outcomes from the Phase III trials used in our analysis are consistent with outcomes from the Phase IV trial.Citation19
Our approach only took into account public expenditure, but excluded any similar private expenditure. For example, in Germany, the employer pays part of the sick leave remuneration, and after that it is paid by health insurance. The remuneration paid by the employer has not been included in our analysis. Including this expenditure would have increased the societal savings from bevacizumab-based therapy. Taking a wider societal perspective would have enabled inclusion of all changes in resources as measured by changes in service production, changes in resources used by patients and their carers, and changes in the gross domestic product,Citation33 but would have resulted in a less conservative estimate of societal savings.
A real-life study was used to assess the proportion of patients who return to work after induction therapy.Citation34 The study reported that 32% of bevacizumab-based and 19% of chemotherapy patients returned to work. Other studies in various countries report that the proportion of NSCLC patients returning to work was between 13% and 40%.Citation29,Citation35–Citation38 Hence, the real-life study results can be considered to be in line with previous findings.
Standard average OECD tax and contribution rates have been used in this study, and the average income has been used from the same source. However, this average income is available for single households only. There are reasons why this average might be considered an overestimation or underestimation. There is an inverse association between the highest level of education and lung cancer.Citation39,Citation40 On the other hand, patients with lung cancer tend to be older and there is a higher proportion of males than in the general population, and hence they have a higher income than the general population. Rather than trying to adjust for these variables, we opted to use simply the average income to maintain transparency in our analysis.
As with any analysis, some assumptions had to be made due to no published data being available. In this analysis, only patients who were employed prior to their diagnosis were considered. It was also assumed that patients who worked full-time prior to their diagnosis would return to work full-time. The same assumption was made for part-time workers, ie, if they were part-time workers they returned to work part-time. These assumptions applied equally to the bevacizumab-based treatment arm and chemotherapy arm. Nevertheless, they could overestimate the savings.
Deterministic sensitivity analyses showed that changes in time horizon had very little impact on overall societal savings. Full-time employment and the proportion able to return to work seemed to have the most impact on savings in all other countries, except France. In France, the labor costs represented the most influential impact, followed by the proportion working full-time. The results of the probabilistic sensitivity analysis are in line with the deterministic findings.
Currently, there are not many studies assessing savings related to treatments in advanced NSCLC from the societal perspective. This study has adopted the societal perspective because this is the classic approach to assessing the profitability of societal investments. Using a perspective other than the societal increases the risk that maximal health is not produced due to inefficiencies in the use of resources for health.Citation25 To allow for a more realistic estimate of the benefit to society, bevacizumab-based combination therapy should be examined from a wider societal perspective as well.
This study suggests that societal savings can be made in France, Germany, Italy, and Spain in patients who are treated with bevacizumab-based therapies when compared with standard chemotherapy alone. By extending the progression-free survival in patients with advanced NSCLC, bevacizumab-based therapy is favorable from the perspective of society by reducing the societal losses associated with this devastating disease.
Acknowledgment
Elaine Wright, who is employed by F. Hoffmann-La Roche, provided assistance in running the statistical analyses. The authors confirm an independent control over the study design, methods, analysis, and interpretation of data as well as having independence over the decision to submit the manuscript.
Disclosure
JL, SS, DG, KK, and CH have received consultancy payments from F La-Hoffmann Roche. The study was sponsored by F Hoffmann-La Roche. SW was an employee of La-Hoffmann Roche at the time work for the manuscript was conducted.
References
- SantMAareleidTBerrinoFEUROCARE-3: survival of cancer patients diagnosed 1990–1994 – results and commentaryAnn Oncol200314 Suppl 5v61v11814684501
- FerlayJSinHRBrayFFormanDMathersCParkinDMGLOBOCAN 2008 v1.2, Cancer incidence and mortality worldwideIARC CancerBase No 10Lyon, FranceInternational Agency for Research on Cancer2010 Available from: http://globocan.iarc.frAccessed on August 7, 2012
- WilkingNJönssonBA Pan-European Comparison Regarding Patient Access to Cancer DrugsStockholm, SwedenKarolinska Institutet2005
- GrallaRJThatcherNQuality-of-life assessment in advanced lung cancer: considerations for evaluation in patients receiving chemotherapyLung Cancer200446 Suppl 2S41S4715698531
- HeyesAClinical trial experience with functional assessment of cancer therapy-lung in conventional and targeted non-small-cell lung cancer therapySemin Oncol200431162215206078
- JohnLDQuality of life in patients receiving radiation therapy for non-small-cell lung cancerOncol Nurs Forum20012880781311421140
- MontazeriAGillisCRMcEwenJQuality of life in patients with lung cancer: a review of literature from 1970 to 1995Chest19981134674819498968
- PaccagnellaAFavarettoAOnigaFCisplatin versus carboplatin in combination with mitomycin and vinblastine in advanced non small cell lung cancer. A multicenter, randomized phase III trialLung Cancer200443839114698542
- StoutRBarberPBurtPClinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs external beam radiotherapy in the palliative treatment of inoperable non-small-cell lung cancerRadiother Oncol20005632332710974381
- Van PuttenJWBaasPCodringtonHActivity of single-agent gemcitabine as second-line treatment after previous chemotherapy or radiotherapy in advanced non-small-cell lung cancerLung Cancer20013328929811551424
- WachtersFMVan PuttenJWKramerHFirst-line gemcitabine with cisplatin or epirubicin in advanced non-small-cell lung cancer: a Phase III trialBr J Cancer2003891192119914520444
- WagnerLRueMFischMNeuropsychiatric symptoms during cancer treatment: an examination of quality of life data from two ECOG lung cancer trialsProc Am Soc Clin Oncol200221Abstract 1426
- CollinsLGHainesCPerkelREnckRELung cancer: diagnosis and managementAm Fam Physician200775566317225705
- European Medicines AgencyEuropean Public Assessment Report (EPAR) summary for the public – Avastin® (bevacizumab) Available from: http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000582/WC500029260.pdfAccessed on August 7, 2011
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAilJ Clin Oncol2009271227123419188680
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med20063552542255017167137
- National Institute for Health and Clinical ExcellenceThe diagnosis and treatment of lung cancer: methods evidence and guidance. 2005CG24 Lung cancer: Full guideline2005 Available from: http://www.nice.org.uk/nicemedia/pdf/cg024fullguideline.pdfAccessed August 8, 2012
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med2002346929811784875
- CrinoLDansinEGarridoPSafety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 studyLancet Oncol20101173374020650686
- LuceBRMauskopfJSloanFAOstermannJParamoreLCThe return on investment in health care: from 1980 to 2000Value Health2006914615616689708
- Canadian Agency for Drugs and Technologies in HealthGuidelines for the Economic Evaluation of Health Technologies3rd ed2006 Available from: http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdfAccessed August 7, 2012
- National Institute for Health and Clinical ExcellenceGuide to the methods of technology appraisal2008 Available from: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdfAccessed August 7, 2012
- Swedish Pharmaceutical Benefits BoardGeneral guidelines for economic evaluation from the Pharmaceutical Benefits Board2003 Available from: http://www.tlv.se/Accessed August 7, 2012
- GoldMRSiegelJERussellLBWeinsteinMCCost-Effectiveness in Health and MedicineOxford, UKOxford University Press1996
- JohannessonMJönssonBJönssonLKobeltGZethraeusLWhy Should Economic Evaluations of Medical Innovations Have a Societal Perspective? Report No 51London, UKOffice of Health Economics2009
- PerroneFBenelliGLopatrielloSCost of non-small-cell lung cancer in Italy. Results of the longitudinal study ALCEA (Advanced Lung Cancer Economic Assessment)J Clin Oncol200422Suppl 14SAbstr 8265
- StanisicSBischoffHGHeigenerDFSocietal cost savings through bevacizumab-based treatment in non-small-cell lung cancer (NSCLC)Lung Cancer201069 Suppl 1S24S3020727459
- SandlerAYiJDahlbergSTreatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small-cell lung cancerJ Thorac Oncol201051416142320686429
- RocheReal life data in metastatic non-small-cell lung cancer (final pooled results of a pilot study in Germany and France focusing on Avastin)2011 (Data on file)
- EurostatEurostat – tables, graphs and maps interface – employment rate by gender (total)Eurostat2011 Available from: http://epp.eurostat.ec.europa.eu/tgm/refreshTableAction.do;jsessionid=9ea7d07d30e638c532abb5a84095bdbcc9c4e5677df0.e34OaN8PchaTby0Lc3aNchuMb3qNe0?tab=table&plugin=1&pcode=tsiem010&language=enAccessed September 29, 2011
- Institute National du CancerAnalyse Économique des Coûts du Cancer en France: Impact sur la qualié de la vie, prévention, dépistage, soins, rechercheNational Cancer Institute: Economic Analysis of Cost of Cancer in France: Impact on the quality of life, prevention, screening, cancer care and researchBoulogne-Billancourt, FranceInstitut National du Cancer2007 French
- TanAFreemanDHFreemanJLZhangDDDayalHPhilipsBUThe cost of cancer in Texas, 2007Publication No 10-13121Austin, TXTexas Cancer Registry, Texas Department of Health Care Service2009
- WilliamsAWelfare economics and health status measurementvan der GaagJPerlmanMHealth, Economics and Health EconomicsAmsterdam, The NetherlandsNorth Holland Publishing1981
- BischoffHGChouaidCVergnenegreAPCN8 real life outcomes in 1st line non-squamous non-small-cell lung cancer (NSCLC): a pilot study in France and Germany analysing bevacizumab-based versus non-bevacizumab-based treatmentsValue Health201114A156
- BradleyCJBednarekHLEmployment patterns of long-term cancer survivorsPsychooncology20021118819812112479
- GridelliCFerraraCGuerrieroCInformal caregiving burden in advanced non-small-cell lung cancer: the HABIT studyJ Thorac Oncol2007247548017545841
- JacouletPDepierreAMoroDLong-term survivors of small-cell lung cancer (SCLC): a French multicenter study. Groupe d’Oncologie de Langue FrancaiseAnn Oncol19978100910149402175
- Taskila-BrandtTMartikainenRVirtanenSVPukkalaEHietanenPLindbohmMLThe impact of education and occupation on the employment status of cancer survivorsEur J Cancer2004402488249315519524
- van LoonAJBrugJGoldbohmRAvan den BrandtPABurgJDifferences in cancer incidence and mortality among socio-economic groupsScand J Soc Med1995231101207676217
- van LoonAJGoldbohmRAKantIJSwaenGMKremerAMvan den BrandtPASocioeconomic status and lung cancer incidence in men in The Netherlands: is there a role for occupational exposure?J Epidemiol Community Health19975124299135784