Abstract
Background:
Lung cancer is the leading cause of cancer deaths worldwide (1.38 million cancer deaths, 18.2% of the total) and of cancer morbidity (1.61 million new cases, 12.7% of all new cancers). Currently only three second-line non-small-cell lung cancer (NSCLC) pharmacotherapies are licensed in the European Union: the chemotherapies pemetrexed and docetaxel and the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib. These therapy alternatives have shown a comparable efficacy (survival benefit). In the past, cost comparisons showed that erlotinib was less costly compared to docetaxel, which in turn is cheaper than pemetrexed. Nowadays erlotinib (and docetaxel) are still less expensive than pemetrexed; but docetaxel lost patent protection (basic compound patent) at the end of 2010, so docetaxel drug costs have decreased rapidly and the question remains whether erlotinib is still the least costly therapy alternative in second-line NSCLC.
Material and methods:
Italy was selected for base case analysis to compare the total therapy costs, estimated by combining country-specific drug costs, administration costs, and adverse event costs of erlotinib and generic docetaxel in second-line NSCLC therapy. Sensitivity analyses on central input parameters have been performed.
Results:
The total costs of treating one patient with erlotinib therapy of €5121 are lower than the docetaxel costs of €6699 for the Italian health care setting. Although the drug costs of erlotinib are higher than generic docetaxel (incremental €3770): the costs of intravenous chemotherapy administration (incremental −€4510), and the costs of adverse event therapy (incremental −€837) lead to higher total therapy costs for docetaxel compared to the epidermal growth factor receptor tyrosine kinase inhibitor therapy erlotinib.
Conclusion:
The cost comparison findings for Italy show that erlotinib is still the less costly therapy alternative in second-line NSCLC. These results were robust to changes of central input parameters and robust to further potential price decreases for docetaxel.
Background
Lung cancer is the leading cause of cancer deaths worldwide (1.38 million cancer deaths, 18.2% of the total) and also of cancer morbidity (1.61 million new cases, 12.7% of all new cancers).Citation1
Approximately 80%–85% of lung cancer patients have non-small-cell lung cancer (NSCLC), which is categorized in three major histological subtypes: squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma.Citation2 Around 70% of NSCLC patients present with advanced or metastatic disease (tumor node metastasis [TNM] stages IIIB/IV) at the time of initial diagnosis.Citation3–Citation5 These patients with late stage NSCLC have a very poor prognosis with just about 7% of patients with stage IIIB and about 2% of those with stage IV surviving beyond 5 years.Citation6
Once first-line therapy or first-line maintenance therapy have failed, NSCLC patients are often treated with second-line agents, as recommended by major practice guidelines,Citation2,Citation7,Citation8 aiming at a palliation of symptoms, a benefit in quality of life, and a prolongation of survival.Citation9
In Europe, there are currently only three licensed second-line pharmacotherapy options available in nonsquamous NSCLC: the oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib, and the intravenous chemotherapies docetaxel and pemetrexed. In patients with squamous cell histology, only erlotinib and docetaxel are available.Citation10–Citation12
Hence, the second-line NSCLC therapy options for physicians and patients are considerably limited.Citation9,Citation13
All these therapy alternatives have shown a comparable survival benefit. In the past this finding of “comparable efficacy” was supported by indirect treatment comparisonsCitation14 of the pivotal trial data;Citation15–Citation18 head-to-head evidenceCitation19,Citation20 confirms these indirect estimates ().
Table 1 Survival outcomes of pivotal trials and head-to-head trials of erlotinib, docetaxel and pemetrexed in 2L NSCLC therapy
Although efficacy is comparable, erlotinib is the only second-line therapy that has shown a statistically significant improvement of patients’ quality of life and of lung cancer symptoms,Citation21 whereas docetaxel (75 mg/m2)Citation22,Citation23 and pemetrexedCitation18 have failed to demonstrate comparable benefits. These findings might be due to the nonchemotherapeutic nature of erlotinib, which shows comparable efficacy to chemotherapeutics coupled with a more favorable tolerability profile (see also ).
Table 2 Overview of serious adverse events (≥grade 3 in %) observed in the pivotal trials and head-to-head trials of erlotinib, docetaxel and pemetrexed in 2L NSCLC therapy
As the three second-line therapies show similar survival (efficacy) outcomes a cost minimization approach is the health economic standard analysis of choice for comparing these alternatives.Citation24,Citation25 Previous cost comparisons (including a review) published prior to loss of patent for docetaxel, showed that erlotinib was less costly than docetaxel, which in turn was less costly than pemetrexed, across different health care settings and different years of publication.Citation26–Citation32
Nowadays erlotinib and docetaxel are still less expensive than pemetrexed; but docetaxel lost patent protection (basic compound patent) in most European countries at the end of 2010, since then the docetaxel drug costs have decreased.
As erlotinib is now facing a generic competitor, the question arises as to whether erlotinib is still the least costly therapeutic alternative in second-line NSCLC therapy.
Material and methods
In order to answer the research question the Italian health care system has been selected for base case analysis to compare the total therapy costs of erlotinib and generic docetaxel in second-line NSCLC therapy from a health care payer perspective. Total therapy costs have been calculated by summing the country-specific drug costs, administration costs and adverse event costs for erlotinib and docetaxel. The Italian cost data applied are shown in .
Table 3 Italian cost data applied in the cost comparison of erlotinib and docetaxel in 2L NSCLC therapy
The drug costs have been assessed on the basis of Italian ex-factory prices using the recommended dosing schemes (erlotinib 150 mg/day; docetaxel 75 mg/body surface area in m2 once [on day 1] every 3 weeks). The proxy treatment duration of 2.5 months has been used for both drugs, according to the pivotal trials’ median progression-free survival outcomes shown in .
According to the European marketing authorization, the injection of docetaxel requires a health care unit specialized for the injection of cytotoxic chemotherapy and the supervision of an oncologist.Citation11 In the analysis this charge is assumed the same whether the oncologist attends throughout the infusion or at the inception only. Hence, administration costs of intravenously injected docetaxel were estimated based on the official Italian diagnosis-related groups cost values for an inpatient (50%) and a hospital-based outpatient (50%) administration of cytotoxic chemotherapeutics. The diagnosis related groups tariffs for inpatient and outpatient administration exclude drug costs in most Italian regions and this assumption is held in the analysis. As erlotinib is an oral medication requiring neither the supervision of an oncologist, nor a specialized oncology unit (in contrast to the chemotherapy docetaxel), no administration costs have been applied for erlotinib.
The incidence of adverse events is based on published evidence shown in .
The cost per adverse event is based on published Italian cost estimates and have been applied to all relevant erlotinib or docetaxel serious adverse events (≥grade 3) as per . The total adverse event cost has been applied as a one-off as the total of the products of adverse event frequencies and respective costs. In cases where more than one incidence value was published (incidence reported in different clinical trials) a conservative approach was applied by taking into account the highest published value for erlotinib and docetaxel to avoid underestimating costs (this occurred more often for docetaxel).
By summarizing the drug costs, administration costs, and adverse event costs the total costs have been calculated for each therapy approach. These total costs of erlotinib and generic docetaxel have been incrementally compared to determine the actual total cost difference between both second-line NSCLC therapy approaches.
In order to investigate the robustness of results the underlying key input data have been changed in one-way deterministic sensitivity analyses. Additional analyses have been performed using a longer (3.0 months; five docetaxel injections) and a shorter (2.0 months; three docetaxel administrations) therapy time horizon, using varying estimates for the proportion of inpatient administration (high 75%; low 25%) and higher (+25%) and lower (−25%) cost estimates for each adverse event. In order to take into account potential further price decreases of generic docetaxel in the future, simulations have been performed applying a further 25% and a further 50% price reduction of generic docetaxel.
Results
As shown in , the average total costs of erlotinib therapy of €5121 are lower than the docetaxel costs of €6699, in the Italian health care setting under the central assumptions of the analysis.
Figure 1 Cost comparison results of erlotinib vs generic docetaxel as second-line NSCLC therapy in Italy.
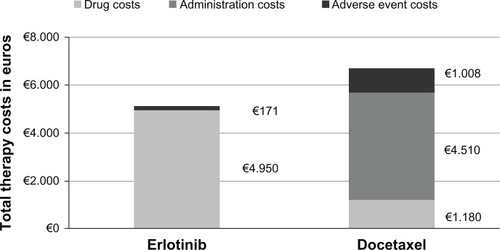
The erlotinib drug costs of €4950 are higher than the docetaxel drug costs of €1180. However, the erlotinib adverse event costs of €171 are lower than the docetaxel adverse event costs of €1008. Furthermore, the hospital-based intravenous docetaxel injection costs of €4510 compare unfavorably to oral erlotinib that requires no administration effort.
According to these base case analyses, the incremental total cost difference of erlotinib vs docetaxel amounts to −€1577. The robustness of the results has been investigated with sensitivity analyses.
As shown in , the sensitivity analysis results confirm the robustness of the “base case” analyses results. Erlotinib was less costly than generic docetaxel, which is shown in the negative incremental total costs for the range of sensitivity analyses undertaken.
Figure 2 Sensitivity analyses on the incremental total costs of erlotinib vs generic docetaxel as second-line NSCLC (non-small-cell lung cancer) therapy in Italy.
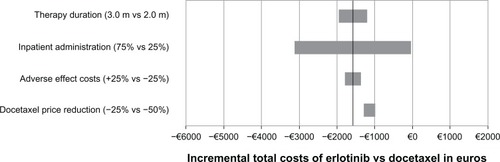
The incremental total costs were most sensitive to changes in the distribution of inpatient administrations followed by changes in the therapy duration. The changes in adverse event costs and the simulated further reduction of docetaxel costs of up to 50% had a limited impact on the incremental total cost results.
Discussion
The presented health economic analysis investigates the cost impact of the patent protection loss of docetaxel, which has led to a rapid drug cost decrease, in comparison to the patent protected EGFR TKI erlotinib. In the past there was consensus that erlotinib was the least costly pharmacotherapy in second-line NSCLC but the consequences of the price decrease of docetaxel generics has raised doubts as to whether this point of view is still valid and is the rationale for performing this assessment.
The Italian health care system has been used for the base case analysis based on the availability of data. One key limitation is that the cost analysis results presented in this paper are highly dependent on the drug prices, the costs of treating specific adverse events, and especially on the reimbursement rates for the intravenous administration of chemotherapy that may vary from country to country. Hence, the results presented have to be regarded as specific to the Italian health care setting and potential similar findings in other countries and health care settings would need to be confirmed in separate analyses. The reader is cautioned not to apply these results to other health care settings. It is also noted that there is variation of these costs across different regions in Italy.
The research findings for the Italian health care setting show that erlotinib still is the least costly therapy alternative in second-line NSCLC. Although the drug costs of generic docetaxel are lower than the drug costs of erlotinib there are other therapy-related costs that counteract this advantage.
The costs for the intravenous chemotherapy administration have been identified as the key docetaxel cost driver. The docetaxel label indicates that injection of the drug requires a unit specialized for the injection of cytotoxic chemotherapy and the supervision of an oncologist, which results in high additional efforts for the health care payers. In contrast, such administration costs are not required for the oral EGFR TKI erlotinib. One limitation related to the simulation of the administration costs is the lack of information related to the proportion of inpatient administrations performed for docetaxel. The inpatient administrations are more costly than the outpatient administrations () which may have an impact on the cost comparison results. Hence sensitivity analyses were performed investigating a range from 25% up to 75% of inpatient administrations, without having a major impact on the cost comparison results (in each scenario erlotinib was less costly compared to docetaxel).
Median progression free survival has been used as a proxy for treatment duration due to the availability of data. As shown in , progression-free survival/time to progression varies for docetaxel from 2.0 to 2.5 months and for erlotinib from 1.5 to 3.6 months. Mean progression-free times have not been used as they require further survival analysis.
Furthermore, as erlotinib shows a more favorable tolerability profile the costs of treating adverse events are lower for erlotinib than for the cytotoxic chemotherapy, which again results in additional effort for health care payers due to docetaxel. In addition, the influence of this cost driver has been investigated in sensitivity analyses, by varying the underlying costs of single adverse events (±25%), without having a major impact on the cost comparison results.
In specific sensitivity analyses, it was assumed that docetaxel costs might decrease further in the future. Hence an additional 25% and a 50% drug cost reduction was simulated for generic docetaxel, in order to test whether the research findings are robust to such possible changes in the future, without having a major impact on the cost comparison results.
Conclusion
In summary, the presented assessment focusing on the Italian health care system has found that docetaxel therapy costs, apart from drug costs, consist of two additional major cost components: namely the intravenous chemotherapy administration costs and the adverse event therapy costs. These “hidden costs” lead to higher total therapy costs of (generic) docetaxel compared to the EGFR TKI therapy erlotinib, that largely consist of drug costs which are transparent and easier to predict for the health care payers.
The findings for Italy show that under the central assumptions of the analysis, erlotinib is the least costly therapy alternative in second-line NSCLC, considering generization of docetaxel. These results were robust to changes of central input parameters and robust to potential further price decreases of docetaxel.
Acknowledgements
This work was funded by Roche SpA, Italy. Roche was involved in gathering the country-specific input data, in reviewing the analysis results and in reviewing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- D’AddarioGFrühMReckMBaumannPKlepetkoWFelipEfor ESMO Guidelines Working GroupMetastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201021Suppl 5116119
- BlanchonFGrivauxMCollonTEpidémiologie du cancer bronchique primitif pris en charge dans les centres hospitaliers généraux françaisRev Mal Respir200219672773412524492
- YangPAllenMSAubryMCClinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003Chest2005128145246216002972
- HowlanderNNooneAKrapchoM SEER Cancer Statistics Review, 1975–2008 [updated 2011]. Available from: http://seer.cancer.gov/csr/1975_2008/. Accessed September 15, 2011.
- GoldstrawPCrowleyJChanskyKThe IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumoursJ Thorac Oncol20072870671417762336
- AzzoliCGBakerSJrTeminSAmerican Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancerJ Clin Oncol200927366251626619917871
- NCCN Practice Guidelines in Oncology – Non-Small Cell Lung Cancer [updated 2011]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed September 15, 2011.
- MaionePRossiABareschinoMAFactors driving the choice of the best second-line treatment of advanced NSCLCRev Recent Clin Trials201161445120868346
- Erlotinib – Summary of Product Characteristics [updated June 7, 2011]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000618/WC500033994.pdf. Accessed September 15, 2011.
- Docetaxel – Summary of Product Characteristics [updated February 3, 2011]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002032/WC500101758.pdf. Accessed September 15, 2011.
- Pemetrexed – Summary of Product Characteristics [updated February 23, 2011]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000564/WC500025611.pdf. Accessed September 15, 2011.
- ScagliottiGVBeyond first-line treatment: expanding the optionsProceedings of the 2nd International Thoracic Oncology Congress Dresden (ITOCD), published in Session: Treatment strategies to extend survival in advanced NSCLC2010 Sep 16–18Dresden, Germany ITOCD 2010. http://www.itocd.com/.
- HawkinsNScottDAWoodsBSThatcherNNo study left behind: a network meta-analysis in non-small-cell lung cancer demonstrating the importance of considering all relevant dataValue Health2009126996100319402854
- ShepherdFARodriguesPJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- ShepherdFADanceyJRamlauRProspective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapyJ Clin Oncol200018102095210310811675
- FossellaFVDeVoreRKerrRNRandomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study GroupJ Clin Oncol200018122354236210856094
- HannaNShepherdFAFossellaFVRandomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapyJ Clin Oncol20042291589159715117980
- VamvakasLAgelakiSKentepozidisNKPemetrexed (MTA) compared with erlotinib (ERL) in pretreated patients with advanced non-small-cell lung cancer (NSCLC): Results of a randomized phase III Hellenic Oncology Research Group trialProceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting2010 June 4–8Chicago, IL, USA American Society of Clinical Oncology; J Clin Oncol. 2010;28(15S):7519.
- CiuleanuTStelmakhLCicenasSEstebanEErlotinib versus docetaxel or pemetrexed as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC) and poor prognosis: efficacy and safety results from the phase III TITAN studyProceedings of the Chicago Multidisciplinary Symposium in Thoracic Oncology2010 Dec 9–11Chicago, IL, USA American Society for Radiation Oncology; J Thorac Oncol. 2010;5(12):LBOA5.
- BezjakATuDSeymourLSymptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21J Clin Oncol200624243831383716921034
- DanceyJShepherdFAGrallaRJKimYSQuality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trialLung Cancer200443218319414739039
- MillerVFossellaFVDe VoreRDocetaxel Benefits Lung Cancer Symptoms and Quality of Life in a Randomized Phase III Study of Non-Small Cell Lung Cancer Patients Previously Treated with Platinum-Based TherapyProceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting1999 May 15–191999Atlanta, GA, USA American Society of Clinical Oncology, 1999
- NewbyDHillSUse of pharmacoeconomics in prescribing research. Part 2: cost-minimization analysis – when are two therapies equal?J Clin Pharm Ther200328214515012713612
- BriggsAHO’BrienBJThe death of cost-minimization analysis?Health Econ200110217918411252048
- Lyseng-WilliamsonKAErlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancerPharmacoeconomics2010281759220014878
- AraújoAParenteBSotto-MayorRAn economic analysis of erlotinib, docetaxel, pemetrexed and best supportive care as second or third line treatment of non-small cell lung cancerRev Port Pneumol200814680382719023496
- CapriSOrabitoARillioGEconomic evaluation of erlotinib, docetaxel and pemetrexed as second line therapy in non-small cell lung cancer [in Italian]Pharmacoeconomics Ital Res Articles201092113124
- CarlsonJJReyesCOestreicherNLubeckDRamseySDVeenstraDLComparative clinical and economic outcomes of treatments for refractory non-small cell lung cancer (NSCLC)Lung Cancer200861340541518295368
- Doral StefaniSGiorgio SaggiaMVicino dos SantosEACost-minimisation analysis of erlotinib in the second-line treatment of non-small-cell lung cancer: a Brazilian perspectiveJ Med Econ200811338339619450094
- GatzemeierUPirkOGabrielAKotowaWSecond-Line-Therapy for Non-Small Cell Lung Cancer (NSCLC) – a Retrospective Cost AnalysisTumor Diagnostik und Therapie2008294211217
- KotowaWGatzemeierUPirkOGabrielAHeigenerDA comparison of the estimated costs of erlotinib, docetaxel and pemetrexed for the second-line treatment of non-small cell lung cancer from the German health care perspectiveJ Med Econ2007103255271
- Italian ex-factory costs of erlotinib and docetaxel [updated 2011]. Available from: http://www.informatorefarmaceutico.it/. Accessed August 6, 2011.
- Chemotherapy administration costs in Italy (Tariffa unica convenzionale per le prestazioni di assistenza ospedaliera regole e tarife valide per l’anno 2009; Inpatient DRG 17 M 410; Daycare DRG 17 M 410) [updated 2010]. Available from: http://www.regioni.it/mhonarc/details_confpres.aspx?id=172630. Accessed August 18, 2011.
- BanzKBischoffHBrunnerMComparison of treatment costs of grade 3/4 adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and SpainLung Cancer201174352953421592611
- MickischGGoreMEscudierBProcopioGWalzerSNuijtenMCosts of managing adverse events in the treatment of first-line metastatic renal cell carcinoma: bevacizumab in combination with interferon-alpha2a compared with sunitinibBr J Cancer20101021808619920817
- BrownBDiamantopoulosABernierJAn economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United KingdomValue Health200811579179918194407