Abstract
The World Health Organization (WHO) recommends dolutegravir (DTG), a human immunodeficiency virus (HIV) medicine, as the first- and second-line treatment for all populations because, when compared to an efavirenz (EFV) regimen, plus two nucleoside reverse transcriptase inhibitors (NRTIs) has demonstrated significant effectiveness in HIV suppression in persons. This study aims to review evidence of the cost-effectiveness of DTG in combination with tenofovir and lamivudine compared with the standard of care for HIV therapy. The systematic review involved searching electronic databases for articles published between January 2018 and May 2022. Electronic database sources include PubMed, ScienceDirect, and EBSCO for articles on DTG in combination with tenofovir and lamivudine as subjects with cost-effectiveness outcomes. The inclusion criteria in this systematic review were studies about the cost-effectiveness analysis (CEA) of DTG in combination with tenofovir and lamivudine, written in English. A total of 145 articles were identified from three databases. After removing nine duplicates, 142 articles were screened by title and abstract, excluding 123 articles. After a full-text screening of 19 articles, five articles were selected for further analysis. Five articles reviewed in sub-Saharan Africa, India, and China implemented different modelling methods for CEA but produced similar results. The results of these studies demonstrate that it is more cost-effective than standard care for HIV treatment. The study conducted in sub-Saharan Africa from 2018 to 2020 showed a cost-effective result with disability-adjusted life years averted (DALY averted) by 83%; in India, it resulted in incremental cost-effectiveness ratio (ICER) $130 per year of live-saved (YLS); and a study in China found that dolutegravir plus tenofovir and lamivudine led to 0.006 incremental quality-adjusted life years (QALYs) with cost savings of $64. The DTG regimen is cost-effective and recommended for HIV therapy in all studies that provide results.
Introduction
Antiretroviral therapy (ART) has played a significant role in HIV control, and integrase strand transfer inhibitors (INSTIs), such as dolutegravir (DTG), are becoming more widely used.Citation1 In 2016, the World Health Organization issued guidelines for the use of antiretroviral drugs for the treatment and prevention of HIV infection. Since 2018, WHO has recommended a combination of tenofovir disoproxil fumarate and lamivudine or emtricitabine plus DTG as the preferred first-line regimen for HIV therapy and updated this guidance in 2021.Citation2,Citation3 This guideline provides a more comprehensive view of DTG as an ARV in the first-line due to the significant risk of neural tube defects risk and observed efficacy.Citation4
DTG shows excellent efficacy and tolerability with a low risk of toxicities.Citation5,Citation6 DTG with two Nucleoside Reverse Transcriptase Inhibitors (NRTIs) has shown significant efficacy in HIV suppression in individuals.Citation7,Citation8 DTG-based regimens may be more effective for CD4 recovery and virologic suppression than EFV-based regimens, making them a preferred treatment option for initial HIV treatment.Citation9 DTG also has fewer drug interactions than EFV, a better genetic barrier to developing drug resistance, and is particularly effective against HIV-2 infection, which is inherently resistant to EFV. The efficacy or effectiveness of health-care interventions has been assessed in clinical trials by measuring outcomes.
The availability of DTG as a once-daily generic fixed-dose formulation at lower prices in most low- and middle-income countries (LMICs) further supports the recommended use of DTG.Citation4 However, it must be determined whether the intervention is cost-effective and feasible to implement.Citation10 Cost-effectiveness analysis (CEA) is used to improve resource allocation efficiency and assess the relative costs and health benefits of various competing health therapies.Citation11 Comparing studies and interventions using cost-effectiveness analyses can assist stakeholders in making evidence-based health policies.Citation12
Furthermore, initial regimens recommended for most people living with HIV are expenses over $36,000 per patient per year, with an average 6% increase since 2012, ART costs outpaced overall inflation rates.Citation13 The cost of antiretroviral therapy should be one of many factors considered in regimen selection because it can affect adherence and overall costs to the healthcare system, insurers, and society.Citation7 According to previous studies, DTG may result in lower costs for HIV treatment and be the most efficacious core agents belonging to integrase strand transfer inhibitors (INSTIs).Citation14,Citation15 This study aims to review evidence of the cost-effectiveness of DTG in combination with tenofovir and lamivudine compared with the standard of care for HIV therapy.
Methods
Study Design and Search Strategies
This study was focused on a systematic review of CEA assessing DTG in combination with tenofovir and lamivudine for HIV treatment. A literature search was conducted in several electronic databases, such as PubMed, ScienceDirect, and EBSCO, by two principal investigators (SA and AMU). The time frame for the research database was set to run from January 2018 to May 2023. The research question was developed using the population, intervention, comparator, and outcome (PICO) format to conduct a systematic review most suitable to answer the research question (see ). The search term strategies were used in combination with the problem or disease keyword “HIV” or “human immunodeficiency virus” the keyword intervention was “dolutegravir” or “combination of dolutegravir” the keyword comparison was “efavirenz regimen” and the keyword outcome was “cost-effectiveness.” The authors used Mendeley Reference Manager version 1.19.8 to extract articles and check for duplicates, and the related articles were manually screened by two researchers based on the title and abstract. In particular, full-text screening was used to determine the studies potentially eligible according to the established inclusion criteria. As a result, the final articles collected were referred to the Consolidated Health Economic Evaluation Reporting Standard (CHEERS) checklist.Citation16 The quality rating of eligible studies was scored as excellent (100%), good (76–99%), moderate (51–75%) or low (<50%).Citation17,Citation18
Table 1 PICO for Search Term of Systematic Review
Inclusion and Exclusion Criteria
The inclusion criteria were articles that studied cost-effectiveness of combination of tenofovir-lamivudine-DTG compared to tenofovir-lamivudine-efavirenz (TLE). The articles were published from January 2018 to May 2023, and the full-text articles are available in English. Articles that only examined clinical efficacy and cost-effectiveness studies were not compared with the EFV regimen; design studies were undertaken as review articles or systematic reviews; and not full-text articles were excluded.
Results
Selected Studies
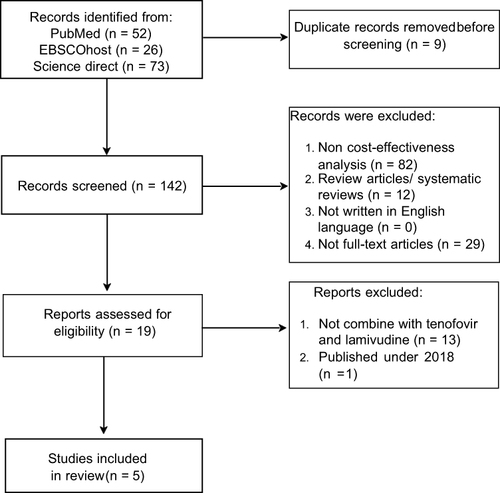
The search strategy identified 145 potential publications in PubMed, ScienceDirect, and EBSCO databases. After removing duplicates, 142 articles were manually screened for inclusion in the review based on title and abstract by two reviewers. A total of 123 articles were excluded after pre-assessing the title and abstract. From 19 articles included in the full-text assessment, 14 articles were excluded because the studies only discussed clinical efficacy, not compared with efavirenz regimen, not full-text articles, and one article was published before 2018. Only five articles met the inclusion criteria following the evaluation of full-text articles (see ). The article selection process is presented in .
Table 2 Study Characteristics
Modelling Design
provides a description of the study’s characteristics. The selected studies were conducted in sub-Saharan Africa, India, and China. Cost-effectiveness analyses of tenofovir-lamivudine-DTG combinations conducted between 2018 and 2020 were also discussed. Three of the five studies used an individual-based simulation model of sexual HIV transmission, progression, and ART’s effect on adults.Citation20,Citation21,Citation23 The cost-effectiveness of preventing AIDS complications international modelCitation19 and the dynamic Markov modelCitation22 have been implemented in other studies (see ).
Table 3 Modelling Method
The studies discussed the following outcomes: disability-adjusted life-years (DALYs), quality-adjustable life-years (QALYs), and incremental cost-effectiveness ratio (ICER). Most studies have compared the cost-effectiveness of DTG in combination with tenofovir and lamivudine to that of existing standard treatments, such as tenofovir, lamivudine, and EFV.Citation19–23 Punekar et al used EFV and ritonavir-boosted lopinavir, both of which combined with two of the NRTI classes of ART in China.
A CEA should use time horizons extending beyond the present time to accurately assess the value of medical interventions.Citation24 The model’s time horizon should be long enough to capture relevant differences in outcomes across strategies.Citation25 McCreesh investigated the effect of different time horizons on the cost-effectiveness of the model and concluded that cost-efficiency would increase over time. In the first year, implementation of the intervention was highly unlikely, but it was cost-effective for more than 10 years.Citation26 Philips et al applied a 20-year time horizon in all studies to consider the future implications of current decisions. Punekar et al employed a short-term horizon at 5-year intervals according to the mean period of first-line ART in China, and Zheng et al employed multiple time horizons.
Discount rate selection significantly impacts the outcomes of economic evaluations of health interventions and policies.Citation27 Almost all studies applied the same discount rate for costs and health outcomes, which was 3%, following the general discount rate in global health.Citation27 There is a distinction in the discount Punekar et al use to comply with China’s inflation index, which was 2.3%.
Outcome Summary
Implementing DTG, tenofovir, and lamivudine was cost-effective and cost-saving (see ). The substitution of DTG with EFV in sub-Saharan Africa would likely impact public health owing to its efficacy, lower risk of side effects, and cost-effectiveness.Citation20,Citation21,Citation23 Phillips et al conducted a study in 2018 to describe the pretreatment HIV drug resistance of NNRTIs and provided a treatment option.
Table 4 Research Outcome
Over the 20 years, the results showed that 50,669 net DALYs were averaged for first-line DTG for all ART initiators, which was higher than that of the comparator.Citation20 Phillips et al continued those studies in 2019. They found that DTG-based regimens were the most cost-effective in 83% of setting scenarios, with net DALYs averted per year ranging from 30,000 to 85,000 compared with tenofovir, lamivudine, and EFV from a healthcare perspective. The last study was conducted by Phillips et al in 2020 to update the assessment of the risks and benefits of DTG-based regimens. In women planning pregnancy, initiation of tenofovir, lamivudine, and DTG averted more DALYs (83%), was cost-effective (87%), and showed net DALYs averted per year of 16,735.
According to Zheng et al DTG in combination with tenofovir and lamivudine is more cost-effective than the standard of care for HIV in India, with an ICER of $130/year of life-saved (YLS), which is less than 50% of India’s annual Gross Domestic Product (GDP). A study in China by Punekar et al showed that incremental cost-effectiveness analyses of DTG plus tenofovir and lamivudine resulted in 0.006 incremental QALYs with cost savings of RMB 467 compared with EFV plus tenofovir and lamivudine. The report provided ART costs in Renminbi (RMB), the People’s Republic of China’s official currency.Citation22 Another result of the study was that compared to ritonavir-boosted lopinavir (LPV/r) in first-line failure, LPV/r had higher QALYs (4.224 vs 4.221) and a lower cost (RMB 238,746 vs RMB 244,364); thus, DTG in combination with tenofovir plus lamivudine dominated in both settings.
Sensitivity Analyses
Each study conducted a sensitivity analysis, and the types of sensitivity analyses applied were one-way, multiway, and probabilistic sensitivity analyses. Sensitivity analysis helps measure and evaluates the uncertainty of economic evaluation outcomes.Citation28,Citation29 All models considered several critical parameters, such as clinical efficacy, prevalence of adverse events, cost, and utility varying within plausible ranges in the sensitivity analysis.Citation19–23 Zheng et al found two most essential parameters in one-way sensitivity analysis: the annual cost of the DTG regimen and the monthly probability of late virologic failure. Afterward, they conducted a multiway sensitivity analysis, simultaneously varying the annual cost of DTG and the monthly probability of virologic failure after 48 weeks on DTG, which remained cost-effective compared to standard of care (SoC). Regimen costing less than $102 per person per year was cost-effective, with an ICER less than 50% of per capita GDP. Despite costing more than twice that of SoC ($200), DTG is cost-effective with an ICER of less than 50% of GDP, as long as the late virologic failure probability is less than 0.21% per month. Punekar et al assessed the model’s robustness with multiple one-way deterministic sensitivity analyses by varying CD4+, adverse event prevalence, cost, and utilities combined with probabilistic sensitivity analyses to estimate the impact of these parameters. A one-way sensitivity analysis showed that improving CD4 and cost are critical drivers for cost-effectiveness and indicated that the cost-effectiveness of the DTG regimen was 98.2% in treatment-naive HIV.
presents the quality evaluation results of the included CEAs using the CHEERS checklist. According to quality appraisal, the reporting quality varied from 84.6% to 94.2%. About 17 of 28 items were the most compliant with the CHEERS checklist (100%). Some studies needed to meet a few items in the CHEERS checklist, such as the characterizing heterogeneity and distributional effects as an approach to engage with patients and others affected by the study. Details on the CHEERS checklist criteria are provided in Supplementary Table S1.
Table 5 Quality Appraisal of Included Studies
Discussion
In 2021, the global HIV program was making decisions regarding new antiretroviral therapy (ART) regimens with fewer side effects and higher resistance barriers, which may improve adherence and viral suppression.Citation14 A fixed-dose combination of DTG, lamivudine, and tenofovir was available for over 18 million adults and 100,000 children in 60 countries, as reported in 2022.Citation30 Affordable antiretrovirals have played a significant role in increasing global antiretroviral therapy coverage.Citation31
Research articles have been conducted in several countries to compare people living with HIV using a DTG-based regimen to a tenofovir-based regimen as therapy to assess its cost-effectiveness from a public health perspective. Based on the results of this review, the use of DTG in combination with tenofovir and lamivudine may be more effective for viral suppression in the treatment of HIV, and the transition to the DTG regimen was more cost-effective than first-line HIV therapy with tenofovir. Three studies were performed in sub-Saharan Africa, one of which was in India and China, where HIV prevalence was the highest in sub-Saharan Africa and India.Citation32 The WHO reported the situation and trends in 2022: the global number of people living with HIV was 39.0 million; the number of people living with HIV in the African Region and India was 25.6 and 2.5 million, respectively.Citation33
The modelling method was used to assess the cost-effectiveness of the antiretroviral HIV policy. The outcomes of each study were distinct, but they all point to the same conclusion. Net costs, health benefits expressed as life-years or QALYs gained, and ICERS are the most common outcomes.Citation34 The CEA method used ICER/YLS, which defines the net cost per unit of benefit gained from intervention per year saved by using the DTG regimen.Citation19 DALY is a measure of the overall disease burden, expressed as the number of healthy years of life lost due to illness, disability, or early death. The net DALY is calculated as the sum of DALYs plus the ratio of costs to the cost-effectiveness threshold.Citation20 The viral suppression rate is higher than the EFV-based regimen (>75%).Citation19–23
Even though this study is the first systematic review on the cost-effectiveness of DTG in combination with tenofovir and lamivudine for HIV therapy, it has several limitations. This study focused on specific interventions within specific patient characteristics that might lead to clinical variation in heterogeneity. Although an exhaustive search was performed, not all relevant studies were included. A search strategy that uses these terms results in irrelevant references. No additional relevant data in the search results discussed the cost-effectiveness of fixed-dose DTG with tenofovir and lamivudine in other countries. Nonetheless, policymakers in LMICs and high-income countries should consider using alternative therapies because of their cost-effectiveness. A DTG-based regimen is recommended as the preferred drug in antiretroviral initiators because of its population health benefits and cost-effectiveness, which aligns with the WHO recommendation. Overall, the reporting quality of the included studies varied from 84.6% to 94.2%, showing all the excellent quality.
Conclusion
This systematic review showed that the transition to HIV treatment using a combination of DTG, tenofovir, and lamivudine in several countries was potentially cost-effective or cost-saving because it can reduce the population burden of diseases. Since the HIV therapy guideline is a living document, further study is required when the guideline has been updated.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This study was supported by a grant from the Universitas Padjadjaran.
References
- Mbhele N, Chimukangara B, Gordon MHIV-1. Integrase strand transfer inhibitors: a review of current drugs, recent advances and drug resistance. Int J Antimicrob Agents. 2021;57(5):106343. doi:10.1016/j.ijantimicag.2021.106343
- World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. HIV treatment-interim guidance; 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/273632/WHO-CDS-HIV-18.18eng.pdf?ua=1.Accessed January 23, 2024.
- World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations; 2023. Available from: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations.Accessed. Accessed January 23, 2024.
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service, delivery and monitoring: recommendations for a public health approach; 2021. Available from: https://www.who.int/news/item/16-07-2021-who-publishes-new-consolidated-hiv-guidelines-for-prevention-treatment-service-delivery-monitoring. Accessed January 23, 2024.
- Zhao Y, Keene C, Griesel R, et al. AntiRetroviral therapy in second-line: investigating tenofovir-lamivudine-dolutegravir (ARTIST): protocol for a randomised controlled trial. Wellcome Open Res. 2021;6:1–13. doi:10.12688/WELLCOMEOPENRES.16597.1
- Keene CM, Griesel R, Zhao Y, et al. Virologic efficacy of tenofovir, lamivudine and dolutegravir as second-line antiretroviral therapy in adults failing a tenofovir-based first-line regimen. AIDS. 2021;35(9):1423–1432. doi:10.1097/QAD.0000000000002936
- HHS Panel on antiretroviral guidelines for adults and adolescents. guidelines for the use of antiretroviral agents in adults and adolescents with HIV; 2022. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.Accessed January 23, 2024.
- Dravid A, Morkar D, Prasad D, et al. A phase IV study on safety, tolerability and efficacy of dolutegravir, lamivudine, and tenofovir disoproxil fumarate in treatment naive adult Indian patients living with HIV-1. Pragmatic Obs Res. 2022;13:75–84. doi:10.2147/por.s361907
- Tongtong Y, Shenghua H, Yin W, et al. Effectiveness and safety of dolutegravir versus efavirenz-based antiviral regimen in people living with HIV-1 in Sichuan province of china: a real-world study. J Acquir Immune Defic Syndr. 2022;91(S1):S1–S7. doi:10.1097/QAI.0000000000003041
- Goodacre SW. An introduction to economic evaluation. Emerg Med J. 2002;19(3):198–201. doi:10.1136/emj.19.3.198
- Hutubessy R, Chisholm D, Tan-Torres Edejer T, et al. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):1–13. doi:10.1186/1478-7547-1-8
- Turner HC, Archer RA, Downey LE, et al. An introduction to the main types of economic evaluations used for informing priority setting and resource allocation in healthcare: key features, uses, and limitations. Front Public Health. 2021;9:1–17. doi:10.3389/fpubh.2021.722927
- McCann NC, Horn TH, Hyle EP, Walensky RP. Costs in the United States, 2012–2018. JAMA Intern Med. 2020;180(4):601–603. doi:10.1001/jamainternmed.2019.7108
- Jamieson L, Serenata C, Makhubele L, et al. Cost and cost-effectiveness of dolutegravir-based antiretroviral regimens: an economic evaluation of a clinical trial. AIDS. 2021;35(Supplement 2):S173–S182. doi:10.1097/QAD.0000000000003068
- Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Correction to: comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naive patients infected with HIV-1: an update on a systematic review and network meta-analysis (BMC Infectious Diseases. BMC Infect Dis. 2021;21(1):1–11. doi:10.1186/s12879-021-06016-8
- Husereau D, Drummond M, Petrou S, et al. CHEERS task force. consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi:10.1016/j.jval.2013.02.010
- Del Pino R, Díez-Cirarda M, Ustarroz-aguirre I, et al. Costs and effects of telerehabilitation in neurological and cardiological diseases: a systematic review. Front Med. 2022; 9: doi:10.3389/fmed.2022.832229
- Javan-Noughabi J, Rezapour A, Hajahmadi M, Alipour V. Cost-effectiveness of single-photon emission computed tomography for diagnosis of coronary artery disease: a systematic review of the key drivers and quality of published literature. Clin Epidemiol Glob Heal. 2019;7(3):389–395. doi:10.1016/j.cegh.2018.07.008
- Zheng A, Kumarasamy N, Huang M, et al. The cost-effectiveness and budgetary impact of a dolutegravir-based regimen as first-line treatment of HIV infection in India. J Int Aids Soc. 2018; (3):e25085
- Phillips AN, Cambiano V, Nakagawa F, et al. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. The Lancet HIV. 2018;5(3):e146–e154. doi:10.1016/S2352-3018(17)30190-X
- Phillips AN, Venter F, Havlir D, et al. Risks and benefit of dolutegravir-based antiretroviral drug regimen in sub-Saharan Africa: a modelling study. Lancet HIV. 2019; 6(2):e116
- Punekar YS, Guo N, Tremblay G, Piercy J, Holbrook T, Young B Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV. 2018;5(3): e146
- Phillips AN, Bansi-Matharu L, Venter F, et al. Updated assessment of risk and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. 2020; 7(3): e193–200.
- Kim DD, Wilkinson CL, Pope EF, Chambers JD, Cohen JT, Neumann PJ. The influence of time horizon on results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res. 2017;17(6):615–623. doi:10.1080/14737167.2017.1331432
- Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model: a report of the ISPOR-SMDM modeling good research practices task force-2. Value Heal. 2012;15(6):804–811. doi:10.1016/j.jval.2012.06.016
- McCreesh N, Andrianakis I, Nsubuga RN, et al. Choice of time horizon critical in estimating costs and effects of changes to HIV programmes. PLoS One. 2018;13(5):1–10. doi:10.1371/journal.pone.0196480
- Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–114. doi:10.1093/heapol/czz127
- Tonin FS, Aznar-Lou I, Pontinha VM, Pontarolo R, Fernandez-Llimos F. Principles of pharmacoeconomic analysis: the case of pharmacist-led interventions. Pharm Pract. 2021;19(1):1–10. doi:10.18549/PharmPract.2021.1.2302
- Jain R, Grabner M, Onukwugha E. Sensitivity analysis in cost-effectiveness studies: from guidelines to practice. Pharmacoeconomics. 2011;29(4):297–314. doi:10.2165/11584630-000000000-00000
- Clinton Health Access Initiative. ARV market report. The state of the HIV market in low- and middle-income countries.2022; Available from https://chai19.wpenginepowered.com/wp-content/uploads/2022/12/2022-CHAI-HIV-Market-Report-12.8.22.pdf. Accessed June 23, 2023.
- Sim J, Hill A. Is pricing of dolutegravir equitable? A comparative analysis of price and country income level in 52 countries. J Virus Erad. 2018;4(4):230–237. doi:10.1016/S2055-6640(20
- HIV. Rates by country; 2023.
- World Health Organization; 2023. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/estimated-number-of-people--living-with-hiv. Accessed November 2023.
- Ademi Z, Kim H, Zomer E, Reid CM, Hollingsworth B, Liew D. Overview of pharmacoeconomic modelling methods. Br J Clin Pharmacol. 2013;75(4):944–950. doi:10.1111/j.1365-2125.2012.04421.x