Abstract
Background and Objectives
The 21-gene assay (the Oncotype DX Breast Recurrence Score® test) estimates the 10-year risk of distant recurrence in hormone receptor positive (HR+) and human epidermal growth factor receptor 2 negative (HER2-) early-stage breast cancer to inform adjuvant chemotherapy decisions. The cost-effectiveness of the 21-gene assay compared against standard clinical-pathological risk tools alone for HR+/HER2- early-stage breast cancer was assessed using an economic model informed by evidence from randomized controlled trials.
Materials and Methods
A cost-effectiveness model consisted of a decision-tree to stratify patients according to their Recurrence Score (RS) results and the use of adjuvant chemotherapy, followed by a Markov component to estimate the long-term costs and outcomes of the chosen treatment. Distributions of patients and distant recurrence probabilities were derived from the TAILORx (N0) and RxPONDER (N1) trials. The model was evaluated from a healthcare payer and societal perspective. Endocrine therapy and chemotherapy use were informed using clinical expert opinion to reflect US clinical practice and were combined with Medicare drug costs (2021) to estimate the cost of treatment. Societal costs included lost productivity and patient out-of-pocket costs obtained from literature.
Results
The Oncotype DX test generated more quality-adjusted life-years (QALYs) (N0: 0.25; N1: 0.08) at a lower cost (N0: -$13,395; N1: -$2526) compared to clinical-pathological risk alone from a societal cost perspective. The overall conclusions from the model did not change when considering a payer perspective. The main cost drivers were avoidance of distant recurrence for N0 (-$12,578), and the cost of adjuvant chemotherapy for N1 (-$2133). Lost productivity had a major impact in the societal perspective analysis (N0: -$4607; N1: -$1586).
Conclusion
Adjuvant chemotherapy decisions based on the RS result led to more life year gains and lower healthcare costs (dominant) compared to using clinical-pathological risk factors alone among patients with HR+/HER2- N0 and N1 early-stage breast cancer.
Introduction
Endocrine therapy or chemotherapy in combination with endocrine therapy is used as first-line treatment of early-stage hormone receptor positive (HR+) and human epidermal growth factor receptor 2 (HER2) negative breast cancer.Citation1,Citation2 Clinical assessment to inform decisions on the choice of treatment for early-stage breast cancer considers clinical-pathological factors, which as tumor grade, size, and nodal burden.Citation3 Breast cancer tumour gene expression profiling using multigene assays (MGAs) can contribute additional prognostic information to guide treatment decisions. Some MGAs can additionally predict the benefit of adjuvant chemotherapy, such as the 21-gene assay (the Oncotype DX Breast Recurrence Score® test, Exact Sciences, Madison, WI, USA). The 21-gene assay measures the expression of 21 genes using reverse-transcriptase polymerase chain reaction (RT-PCR). It is used to calculate a Recurrence Score result between 0 and 100, which can estimate risk of distant recurrence with hormone therapy alone (no chemotherapy) and the magnitude of reduction in risk from adding chemotherapy treatment for patients with HR+, HER2- early invasive breast cancer.Citation4,Citation5 The Recurrence Score result can be used by oncologists and patients to inform the use of adjuvant chemotherapy, in combination with information on prognostic clinical-pathological factors.
The clinical utility of the 21-gene assay to identify women with HR+/HER2- node-negative (N0) or node-positive (N1, 1–3 positive lymph nodes) early breast cancer who could safely forego chemotherapy treatment has been demonstrated in the TAILORx and RxPONDER Phase III randomized controlled trials (RCT).Citation6,Citation7 The TAILORx study recruited 10,273 patients with HR+/HER2 and node-negative early breast cancer and randomized patients with RS results 11–25 to be treated with chemo-endocrine therapy or endocrine therapy alone. It demonstrated that patients within this intermediate RS result range did not benefit from added chemotherapy in terms of distant recurrence-free survival, invasive disease-free survival or overall survival.Citation6 The RxPONDER study included 5083 patients with HR+/HER2- node-positive early breast cancer and RS results 0–25, randomized to be treated with chemo-endocrine or endocrine therapy alone. A statistically significant treatment effect of chemotherapy was reported in terms of overall survival, invasive disease-free survival, and distant recurrence-free interval, for premenopausal women only.Citation7
Previous economic evaluations from a US healthcare payer perspective have shown the 21-gene assay to be either cost-saving or cost-effective for patients identified as intermediate or high-risk based on clinical-pathological factors.Citation8,Citation9 These analyses relied on older data from a meta-analysis in the UK.Citation10 There is an unmet need to examine the cost-effectiveness of the 21-gene assay based on recently published evidence from the TAILORx and RxPONDER clinical trials, which have updated RS risk groups.
A diagnosis of breast cancer and concomitant chemotherapy utilization are both associated with substantial burden on the patient and society as a whole in terms of out-of-pocket costsCitation11 and lost productivity.Citation12,Citation13 Economic evaluations conducted solely from a healthcare payer perspective may be insufficient to capture the full economic impact of using multi-gene assays to guide chemotherapy decisions.
An economic evaluation estimated the cost-effectiveness of the 21-gene assay compared to using clinical-pathological risk factors alone to guide the use of adjuvant chemotherapy for HR+/HER2- early-stage breast cancer patients from a societal perspective in the base case.
Materials and Methods
Study Population, Intervention, and Comparators
A hypothetical patient cohort was divided into subgroups according to their RS result. It was assumed that the proportion of patients in each RS result category is identical for both the 21-gene assay and the comparator in the model, reflecting the same distribution of genomic risk whether or not the 21-gene assay was used. In other words, if we were to test those in the clinical-pathological risk alternative, there is no reason to believe that they would have a different genomic risk distribution compared to those tested with the 21-gene assay. The differences in costs and outcomes are determined by differences in chemotherapy assignment only.
The model-based case population included women with N0 and N1 HR+/HER2- early-stage invasive breast cancer. Additional subgroups included in the model that enabled stratification of results included age (≤50 and >50 years) and clinical risk (using the definition of low clinical risk from TAILORx: tumour size ≤3cm and grade 1, ≤2cm and grade 2, or ≤1cm and grade 3) or N0 patients, premenopausal and postmenopausal status for N1 patients, and patients with micrometastases (N1mi).
Model Structure
A cost-effectiveness model built in Microsoft Excel included a decision-tree to stratify patients according to genomic risk and assigned adjuvant treatment (), followed by a Markov model to simulate long-term costs and outcomes using 6-month model cycles (), which is a similar approach to that seen in other models evaluating the 21-gene assay.Citation8,Citation9 The analysis used a lifetime horizon and assumed a US societal perspective, which included Medicare costs, patient out-of-pocket costs, and indirect costs of lost employment associated with a diagnosis of breast cancer or its treatment. A scenario analysis was conducted from a narrower US Medicare perspective only. The model was designed to follow best practices for modelling and used an annual rate of 3% to discount lifetime costs and outcomes in accordance with US guidelines.Citation14
Figure 1 In the decision-tree component of the model, RS result subgroups were defined using cut-offs used in the TAILORx study for N0 (0–10, 11–25, 26–100) and RxPONDER for N1 (0–13, 14–25, 26–100). In the 21-gene assay alternative of the model, chemotherapy assignment was dependent on the subgroup. In the clinical-pathological risk alternative of the model, it differed according to patient age, clinical risk, and menopausal status. Once patients have been assigned their RS result and assigned adjuvant treatment, they proceed to the respective part of the Markov model.
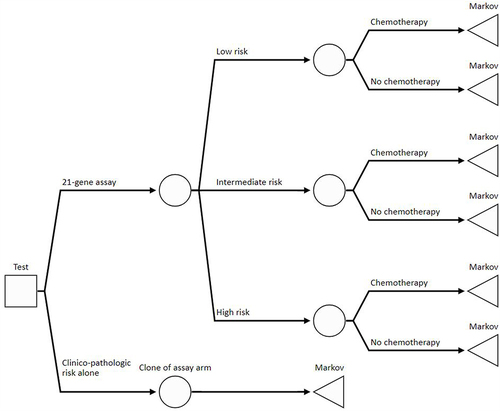
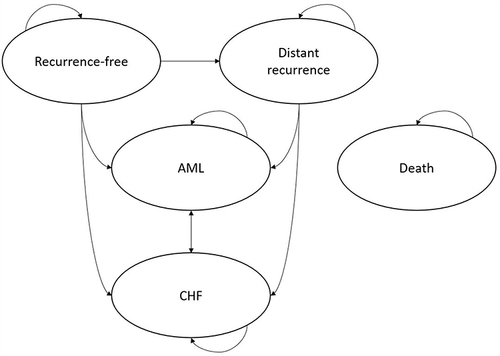
Figure 2 Markov model structure. The model included five health states; the arrows depict patient movement between health states in each model cycle. Patients can move to death from any health state. Patients enter the Markov portion in the “Recurrence-free” health state, and the probability of transition to distant recurrence, AML and CHF is conditional on the assigned adjuvant treatment, clinical risk, and RS category (if known).
Clinical Inputs
The assignment of patients to RS subgroups in the model was based on the TAILORx,Citation6 and RxPONDER studiesCitation7 for N0 and N1 patients, respectively. Clinical inputs were aligned with updated RS subgroup definitions used in TAILORx and RxPONDER (low: 0–10 (N0), 0–13 (N1); intermediate: 11–25 (N0), 14–25 (N1); high: ≥25). In some cases, older cut-points were used in the absence of recent studies, such as with the Surveillance, Epidemiology, and End Results (SEER) database used to inform the N1mi subgroup. A survey of nine breast oncologists informed chemotherapy allocation inputs (the methodology and results of the survey are described in the text and Table S1). The incidence and cost of short-term adverse events (AEs) of chemotherapy were taken from Wang et alCitation8 and are shown in .
Table 1 Chemotherapy-Related Adverse Events
The probabilities of distant recurrence with chemo-endocrine or endocrine therapy for the overall N0 patient group with RS results 11–25 were derived from 9-year distant recurrence-free interval (DRFI) data reported in TAILORxCitation6 and in the TAILORx exploratory analysis for subgroups by age and clinical risk.Citation15 In order to estimate the probability of distant recurrence for patients with RS>25 who were not randomized to endocrine therapy, baseline hazard rates from TAILORx were combined with hazard ratios reported in the NSABP B-20 study.Citation16 No chemotherapy benefit was assumed for patients with RS<11 across all N0 subgroups.
The probability of distant recurrence for N1 patients was derived from 5-year DRFI reported from RxPONDER in the 2021 SABCS presentation.Citation17 Considering that RxPONDER was restricted to patients with RS 0–25, distant recurrence outcomes with endocrine therapy for patients with RS>25 were derived from TransATACCitation18 and chemotherapy benefit for this subgroup was informed by SWOG-8814.Citation5 Inputs and assumptions for the N1mi subgroup are described in the Online supplement. All DRFI estimates were converted to 6-month transition probabilities and applied over the lifetime horizon in the model.
The estimated benefit of chemotherapy assumes that the treatment effect of endocrine therapy remains unchanged. The model did not account for possible addition of different types of endocrine therapy to the treatment regimen, or other treatments used as alternatives to chemotherapy, such as ovarian function suppression. The impact of these treatments is unknown.
Mortality in the recurrence-free health state was assumed to be in line with age-adjusted mortality for the general population based on US life tables (Table S2). Mortality after distant recurrence was informed by median survival from MONARCH 2 trial.Citation19 For the AML and CHF health states, mortality was informed using NICE technology appraisal TA552Citation20 and Wang et al,Citation8 respectively.
Health-Related Quality of Life (HRQoL) and Cost Inputs
The model estimated HRQoL using utility values attached to the Markov health states and decrements representing one-off decreases in utility associated with chemotherapy adverse events (AEs) and local recurrence. The sources of utility inputs in the model are described in the Online supplement.
The cost of the 21-gene assay was obtained based on the Medicare price in the US.Citation21 For the purposes of estimating drug costs, distribution of adjuvant treatments was obtained from NCCN guidelines to approximate real-life US clinical practice.Citation22 Inputs for health state costs were derived from literature and clinical expert opinion and are described in detail in the Online supplement. Inputs and calculations for treatment costs in the model are reported in Tables S3-S7. Societal costs have also been included in the model for patient out-of-pocket expenses associated with chemotherapy, and workdays lost secondary to chemotherapy administration and development of distant recurrence (Table S8). All costs were reported in 2021 US Dollars. The full set of model inputs and corresponding confidence intervals and distributions are reported in Table S9.
Analytical Approach
Cost-effectiveness analysis results were presented using incremental cost-effectiveness ratio (ICER), incremental cost, incremental quality-adjusted life-years (QALYs), life years (LYs), net monetary benefit (NMB), percentage of patients avoiding chemotherapy and percentage of patients avoiding distant recurrence. A societal perspective was used in the base case, with a narrower payer perspective presented as a scenario analysis. One-way and probabilistic sensitivity analyses (PSA) tested uncertainty in the model and scenario analyses conducted to examine key model assumptions. Decision uncertainty was illustrated using cost-effectiveness acceptability curves (CEAC). The external validity of the model was tested by comparing breast-cancer specific mortality (BCSM) and overall mortality estimated using the model against real-life data from the SEER registry. The reporting of the analysis was in line with the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) criteria,Citation23 reported in Table S30.
Results
Patient Characteristics in the Modelled Cohort
Patient characteristics were in line with the pivotal phase III trials used to inform the analysis: TAILORx for N0 and RxPONDER for N1. A full description of the cohort and composition according to prognostic clinical characteristics is reported in Table S10.
Base Case Cost-Effectiveness Analysis results
Use of the 21-gene assay generated was dominant compared to clinical-pathological risk alone (it generated more QALYs (N0: 0.25; N1: 0.08) with a lower cost (societal perspective: N0: -$13,395; N1: -$2526; healthcare payer perspective: N0: -$8842; N1: -$453) ( and ). A breakdown of the model results is shown in for N0 patients and for N1 patients. The estimates in the model compared well against outcomes in SEER data,Citation24,Citation25 as shown in Tables S28 and S29.
Table 2 Incremental Cost-Effectiveness of the 21-Gene Assay Compared to Clinical-Pathological Risk Alone N0 Population
Table 3 Incremental Cost-Effectiveness of the 21-Gene Assay Compared to Clinical-Pathological Risk Alone Combined N1 Population
Table 4 Breakdown of Cost for the 21-Gene Assay Compared to Clinical-Pathological Risk Alone N0 Population
Table 5 Breakdown of Cost for the 21-Gene Assay Compared to Clinical-Pathological Risk Alone Combined N1 Population
Uncertainty Analyses
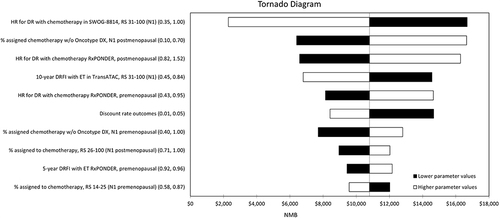
The one-way sensitivity analysis identified several model parameters, which had a substantial impact on the results of the cost-effectiveness analysis, as shown in the Tornado diagrams in and . The results of the probabilistic analysis were broadly in line with the deterministic base case. Based on the CEAC, the 21-gene assay was very likely to be considered cost-effective at a willingness-to-pay of $100,000 per QALY for both N0 and N1 subgroups (>95%). Additional interpretation of the sensitivity analysis results is included in the Online supplement. Results of the PSA are presented using scatterplots in Figure A1, A3 and A5, and CEACs in Figure A2, A4 and A6.
Figure 3 Tornado diagram reporting the results of one-way sensitivity analyses for the combined N0 population. The endpoint of interest for the one-way sensitivity analyses was net monetary benefit (NMB), which is the product of the threshold willingness-to-pay per QALY in the US ($100,000) and incremental QALYs gained, less incremental cost. NMB is a more appropriate endpoint to measure uncertainty in the presence of negative ICERs, which are difficult to interpret as they can represent both a dominant (higher incremental QALYs and lower incremental cost) and dominated (lower incremental QALYs and higher incremental cost) result.
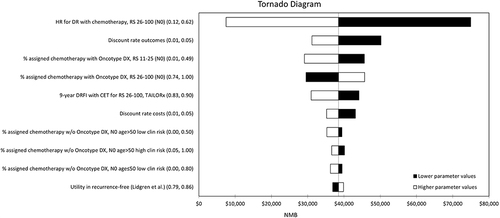
Scenario Analyses
Scenario analysis results were reported in Tables S11 and S12. The 21-gene assay remained dominant compared to clinical-pathological risk alone with alternative inputs for chemotherapy benefit, DRFI, and unit costs. The model was sensitive to the estimate used for chemotherapy benefit for the RS>25 group for both N0 and N1 patients, although the 21-gene assay was still expected to be a cost-effective option if the hazard ratio is set to be equal to the upper bound of the confidence interval reported in clinical studies. The model was also sensitive to changes in chemotherapy allocation with clinical-pathological risk alone in both N0 and N1 populations; however, the 21-gene assay was also still expected to be a cost-effective option if set to the upper and lower bound of the confidence interval obtained from the clinical expert responses.
Subgroup Analyses
A top-level summary of results for all subgroups is reported in Table S13 and detailed results by subgroup reported in Tables S14-S27. The results in all subgroups were consistent with the results in the main N0 and N1 analyses, with the exception of the N1 premenopausal subgroup where the 21-gene assay was not cost-effective in the base case.
Discussion
Interpretation of Results
This analysis demonstrated that the 21-gene assay is cost-saving and generated more QALYs (dominant) compared to clinical-pathological risk factors alone to guide adjuvant treatment decisions in both N0 and N1 early breast cancer. Despite no change in the proportion of patients allocated to chemotherapy in the N0 subgroup after testing, it was the targeted use of chemotherapy informed by RS result which led to reductions in the probability of local and distant recurrence, which ultimately resulted in both QALY gains and long-term cost savings. In the combined N1 group, the result was primarily driven by substantial reduction in estimated chemotherapy use for postmenopausal women with RS 0–25 (66% of the N1 cohort) who are treated with comparatively safer endocrine therapy without increasing the risk of recurrence. The analysis showed that the 21-gene assay was unlikely to be cost-effective in the premenopausal N1 subgroup. This result was driven by the findings from the RxPONDER study, which showed that premenopausal patients with RS≤25 benefit from chemotherapy irrespective of their RS result. Based on the clinical survey, the overall proportion of patients allocated to chemotherapy was expected to reduce from 76% to 65% with the 21-gene assay, which meant that the cost savings from chemotherapy sparing were offset by increased cost of treatment and decreased QALYs due to distant recurrence. The model did not consider alternative treatments, such as ovarian function suppression, which may be preferred by physicians and patients in order to avoid the risk of chemotherapy adverse events in this subgroup. The results were impacted by uncertainty in the input values obtained from different literature sources and the unknown benefit of ovarian suppression as an alternative to chemotherapy.
The model results were consistent from both a healthcare payer perspective and a societal perspective, with additional cost savings derived from avoided patient out-of-pocket expenditures and lost days at work associated with adjuvant chemotherapy and distant recurrence. Sensitivity analyses showed that inputs for the chemotherapy benefit and DRFI in the high-risk RS subgroup had the largest impact on the results and the probabilistic analysis demonstrated that the 21-gene assay had a high probability of cost-effectiveness assuming a threshold for willingness to pay of $100,000 per QALY. A large variation as observed in the chemotherapy allocation inputs obtained from clinical expert opinion, although scenario analyses showed that the model conclusions remained the same if alternative values were used.
Study Limitations
The cost-effectiveness analysis was based on an economic model with extrapolations informed using published data and assumptions, which inherently involves uncertainty. Although uncertainty associated with chosen parameter values and data sources was robustly analysed using one-way sensitivity analysis, probabilistic analysis, uncertainty emanating from the choice of model structure and type of model may remain in the form of structural uncertainty.
In the absence of clinical studies which included the full population and outcomes of interest, the cost-effectiveness analysis was informed using multiple studies for each patient subgroup. The benefit of chemotherapy in the RS>25 subgroup based on NSABP B-20 for N0 and SWOG-8814 for N1 was uncertain and the impact of this on model uncertainty was tested in scenario analysis showing no impact on study conclusions. For the N1 subgroup, DRFI estimates for the endocrine therapy arm were not reported for the pre-defined RS subgroups in RxPONDER: 0–13 and 14–25 (only the absolute chemotherapy benefit was reported for the strata). Thus, data for the overall 0–25 subgroup were applied to both, which may have underestimated the benefit of chemotherapy sparing for patients with the lowest risk of recurrence and can thus be considered a conservative case.
The investigators also had to rely on clinical expert opinion for key parameters, such as probability of chemotherapy based on RS, which had a substantial impact on the results. The estimated proportion of patients allocated to chemotherapy varied significantly across responses, which reflects variation in clinical practice across centers in the US. However, the SEER studies recruited patients diagnosed with chemotherapy prior to 2016, and therefore treatment decisions reported in these studies did not capture the changes in clinical practice triggered by the publication of results from pivotal phase III TAILORx and RxPONDER trials. In addition, the SEER database has previously documented issues with under-reporting of chemotherapy.Citation26 Despite the uncertainty associated with using clinical expert opinion, this was deemed to be the data source, which best reflects current clinical practice in the US. This uncertainty was explored in scenario analyses reported in the Online Supplement. Alternative inputs based on studies informed by SEER registry were tested in scenario analyses, with no changes to the conclusions for either N0 or N1 subgroups.
The applicability of the analysis results for certain patient groups is uncertain due to potential under-recruitment of racial and ethnic minorities that is common in clinical trials. A further description of the racial and ethnic representativeness of the TAILORx and RxPONDER studies is in the Online supplement. The impact of race and ethnicity on outcomes of treatment decisions guided by the 21-gene assay in the TAILORx and RxPONDER have been explored in recent studies, which showed no differences in the performance of the assay across race subgroups.Citation27,Citation28 Worse outcomes with endocrine therapy alone were observed for black patients, but no statistically significant differences in chemotherapy benefit were observed across RS result subgroups. The 21-gene assay remained prognostic and predictive of chemotherapy benefit across racial and ethnic groups. There is scope for future research to examine whether the cost-effectiveness of the 21-gene assay differs across race and ethnic subgroups.
Study Strengths
A systematic review of cost-effectiveness analyses of the 21-gene assay test by Wang et al criticized previously published analyses for ignoring the role of clinical-pathological factors in their evaluation of the multigene assays.Citation29 The analysis described in this article addressed this point by considering N0 and N1 populations separately, recognizing the importance of nodal status as a prognostic factor for distant recurrence and its impact on adjuvant treatment decisions. The population was stratified further based on age and clinical risk subgroups defined in the TAILORx exploratory analysis.Citation15 The model considered menopausal status for N1, which is a predictor of chemotherapy benefit based on the results of the RxPONDER trial. Stratification according to observed clinical risk factors allowed the investigators to assume equal chemotherapy allocation for all patients in the clinical-pathological alternative of the model within each subgroup. The assumption of the predictive ability of the 21-gene assay was highlighted as a source of uncertainty by Wang et al, with most of the studies which assumed a predictive ability concluding that the 21-gene assay is cost-effective. The analysis reported in this article considered chemotherapy benefit separately in each subgroup. Since the publication of the systematic review by Wang et al, TAILORx and RxPONDER randomized controlled trials demonstrated robust evidence of the absence of a chemotherapy benefit for patients with RS≤25 for all N0 and postmenopausal N1 patients, suggesting that the 21-gene assay is able to identify patients who can be safely spared chemotherapy and improve treatment decision-making in this setting. The degree of chemotherapy benefit for patients with RS>25 is uncertain in both N0 and N1 analyses, which was tested in scenario analyses. This is due to large confidence intervals for RS>25 in NSABP B-20 and SWOG-8814 and due to the design of TAILORx and RxPONDER, which did not include randomization to treatment among those with a RS>25. The 21-gene assay was still cost-effective in scenario analyses with reduced chemotherapy benefit for the RS>25 group.
Comparison to Published Evidence
Previous economic evaluations demonstrated the potential cost-effectiveness of the 21-gene assay to guide chemotherapy decisions for breast cancer patients in the US who were classified according to clinical-pathological risk using PREDICT.Citation8,Citation9 Both models were informed using registry data combined with assumptions for the treatment effect of chemotherapy for different clinical risk and RS result subgroups. The analysis described in this article took a different approach by presenting the analysis by nodal status informed by up-to-date evidence from randomized phase III trials to inform the long-term effectiveness of chemotherapy decisions. In terms of study design, the model described in this article more closely resembles the design in Kunst et al, which incorporated chemotherapy assignment probabilities from published literature reflecting clinical practice at the time of publication.Citation9 Kunst et al showed that the 21-gene assay is cost-effective for patients with intermediate and high clinical risk based on old RS cut-points but reported that the ICER is above the cost-effective range for patients with low clinical risk. Although all N0 subgroups were dominant in the analysis reported in this paper, a similar trend was observed, with substantially larger cost savings and QALY gains for patients with high clinical risk. The results of the analysis reported here was in line with cost-effectiveness analyses conducted from a UK NHS and personal social services perspective.Citation30,Citation31
Recommendations for Research and Policy
This model-based analysis incorporated the latest evidence from the TAILORx and RxPONDER trials, and has the potential to influence recommendations for the use of the 21-gene test in the US. The reduction in chemotherapy use resulting from MGA use for patients with a low risk of distant recurrence is likely to reduce pressure on resource-constrained oncology units. Moreover, this analysis showed that reduced chemotherapy use and avoidance of distant recurrence as a result of adjuvant treatment decisions utilizing the 21-gene assay substantially reduces patient out-of-pocket costs and lost productivity. Decision-makers need to consider patient and wider societal impacts in addition to healthcare costs when making their recommendation on the use of the 21-gene assay.
Due to key evidence gaps to inform the model, US-specific prospective or retrospective observational studies to examine the change in chemotherapy decisions resulting from the use of the 21-gene assay are needed to improve the specificity of these estimates by nodal burden, clinical-pathological risk, and menopausal status, particularly given the differential chemotherapy benefit across subgroups as demonstrated in the TAILORx exploratory analyses and the RxPONDER study. Further studies using real-world evidence to examine the distribution and cost of adjuvant chemotherapy could help further reduce uncertainty for cost estimates informing the model.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Vladislav Berdunov and Ewan Laws: employee of Putnam, who have received funding from Exact Sciences. Gebra Cuyun Carter, Christy Russell, Sara Campbell: employee and stockholder of Exact Sciences. Roger Luo has no conflicts of interest to declare. Yara Abdou and Jeremy Force: served as paid consultant for Exact Sciences.
Data Sharing Statement
Data parameters used in the model are specified in the article and appendices, alongside literature references to allow a reader to re-create the model if needed. For any questions related to the methodology or parameters used in the model, please contact the lead author.
Additional information
Funding
References
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the international society of geriatric oncology (siog) and European society of breast cancer specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–e160. doi:10.1016/S1470-2045(11)70383-7
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: Diagnosis and management.; 2018.
- National Comprehensive Cancer Network. NCCN Guidelines: Breast Cancer, Version 4.2022; 2022.
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi:10.1200/JCO.2005.04.7985
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi:10.1016/S1470-2045(09)70314-6
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi:10.1056/NEJMoa1804710
- Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385(25):2336–2347. doi:10.1056/NEJMOA2108873/SUPPL_FILE/NEJMOA2108873_DATA-SHARING.PDF
- Wang SY, Chen T, Dang W, Mougalian SS, Evans SB, Gross CP. Incorporating tumor characteristics to maximize 21-gene assay utility: a cost-effectiveness analysis. J Natl Compr Cancer Netw. 2019;17(1):39–46. doi:10.6004/JNCCN.2018.7077
- Kunst NR, Alarid-Escudero F, Paltiel AD, Wang SY. A value of information analysis of research on the 21-gene assay for breast cancer management. Value Heal. 2019;22(10):1102–1110. doi:10.1016/J.JVAL.2019.05.004/ATTACHMENT/C9092182-FB72-4552-BFB0-5A6BE5797ECC/MMC1.PDF
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
- Giordano SH, Niu J, Chavez-MacGregor M, et al. Estimating regimen-specific costs of chemotherapy for breast cancer: observational cohort study. Cancer. 2016;122(22):3447–3455. doi:10.1002/CNCR.30274
- Bradley CJ, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J Health Econ. 2005;24(1):137–160. doi:10.1016/J.JHEALECO.2004.07.003
- Meadows ES, Johnston SS, Cao Z, et al. Illness-associated productivity costs among women with employer-sponsored insurance and newly diagnosed breast cancer. J Occup Environ Med. 2010;52(4):415–420. doi:10.1097/JOM.0B013E3181D65DB7
- Institute for Clinical and Economic Review. ICER’s Reference Case for Economic Evaluations: Principles and Rationale; 2020.
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi:10.1056/NEJMOA1904819
- Geyer CE, Tang G, Mamounas EP, et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. Npj Breast Cancer. 2018;4(1). doi:10.1038/s41523-018-0090-6
- Kalinsky K Predicting chemotherapy benefit in node-positive breast cancer with the 21-gene test: the oncotype dx breast recurrence score® Test. In: San Antonio Breast Cancer Conference; 2021.
- National Institute for Health and Care Excellence. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer; 2018.
- Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine Therapy—MONARCH 2: A randomized clinical trial. JAMA Oncol. 2020;6(1):116. doi:10.1001/JAMAONCOL.2019.4782
- National Institute for Health and Care Excellence. Liposomal Cytarabine–Daunorubicin for Untreated Acute Myeloid Leukaemia.; 2018.
- Clinical Laboratory Fee Schedule Files | CMS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files. Accessed June 21, 2022.
- National Comprehensive Cancer Network. NCCN Guidelines: Breast Cancer, Version 2.2021; 2021.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20(1):1–8. doi:10.1186/s12916-021-02204-0
- Choi IS, Jung J, Kim BH, et al. The 21-gene recurrence score assay and prediction of chemotherapy benefit: A propensity score-matched analysis of the SEER database. Cancers. 2020;12(7):1829. doi:10.3390/cancers12071829
- Roberts MC, Miller DP, Shak S, Petkov VI. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database. Breast Canc Res Treat. 2017;163(2):303–310. doi:10.1007/s10549-017-4162-3
- Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55–e64. doi:10.1097/MLR.0000000000000073
- Albain KS, Gray RJ, Makower DF, et al. Race, ethnicity, and clinical outcomes in hormone receptor-Positive, HER2-Negative, node-negative breast cancer in the randomized tAILORx trial. J Natl Cancer Inst. 2021;113(4):390–399. doi:10.1093/JNCI/DJAA148
- Abdou Y, Barlow WE, Gralow JR, et al. Abstract GS1-01: race and clinical outcomes in the RxPONDER Trial (SWOG S1007). Cancer Res. 2023;83(5_Supplement):GS1–01. doi:10.1158/1538-7445.SABCS22-GS1-01
- Wang SY, Dang W, Richman I, Mougalian SS, Evans SB, Gross CP. Cost-Effectiveness analyses of the 21-Gene assay in breast cancer: systematic review and critical appraisal. J Clin Oncol. 2018;36(16):1619–1627. doi:10.1200/JCO.2017.76.5941
- Berdunov V, Millen S, Paramore A, et al. Cost-effectiveness analysis of the Oncotype DX Breast Recurrence Score test in node-positive early breast cancer. J Medl Eco. 2022;25(1):591–604. doi:10.1080/13696998.2022.2066399
- Berdunov V, Millen S, Paramore A, et al. Cost-Effectiveness Analysis of the Oncotype DX Breast Recurrence Score® Test in Node-Negative Early Breast Cancer. Clin Outcomes Res CEOR. 2022;14:619. doi:10.2147/CEOR.S360049