Abstract
Objective
To investigate the criteria for prescribing a combination pill for hypertensive patients, and whether the combination pill improves medication adherence.
Materials and methods
This was a retrospective cohort study, performed in three Italian local health units. We selected all adult subjects who received at least one prescription of antihypertensive drugs between September 1, 2011 and December 31, 2011 (the enrollment period). The date of the first antihypertensive claim was defined as the index date. For each patient, we documented the antihypertensive drug treatments and evaluated patients’ adherence to treatment, which was calculated, separately, as the proportion of days covered in the two 6-month periods preceding and following the index date. Only patients treated with olmesartan and/or amlodipine as a single therapy, or as a two-pill combination in the period prior the index date were included. Changes in adherence levels were compared in subjects who moved to the fixed combination of olmesartan/amlodipine after the index date and in subjects who did not.
Results
A cohort of 21,008 subjects with a 6-month history of a prescription of olmesartan and amlodipine as two pills in a combination treatment, or as single-pill treatment, was obtained. Subjects treated with the two-pill combination treatment moved to the olmesartan/amlodipine fixed combination treatment more frequently than did subjects with a single-pill treatment (P<0.001). Comparing the postindex date period to the preindex date period, adherence to treatment was found to be higher in the 239 subjects who moved to the olmesartan/amlodipine fixed combination therapy (from 59.0% to 78.7%; P<0.001), than in the 20,769 subjects who did not move to the olmesartan/amlodipine fixed combination therapy (from 56.3% to 63.0%, P<0.001).
Conclusion
The results of the present study show that the fixed combination of olmesartan/amlodipine contributes to increasing treatment adherence in subjects previously treated with a two-pill combination therapy or a single-pill therapy.
Introduction
Hypertension is a highly prevalent condition and an important risk factor for cardiovascular (CV) morbidity and mortality.Citation1,Citation2 Hypertension affects nearly one out of four adults in Italy, with a prevalence of about 60% in a population ≥65 years old.Citation3 Disappointingly, only two-thirds of treated hypertensive patients had controlled blood pressure (BP),Citation4 and most of them required more than one antihypertensive agent to achieve and maintain recommended BP goals.Citation5–Citation7 This lack of medical success and the need for multidrug therapy are some of the reasons why new antihypertensive drugs continue to be developed, and it also explains the interest in fixed-combination antihypertensive agents. The European Society of Hypertension–European Society of Cardiology guidelines recommend combination therapy as a first-line treatment option for hypertension that will likely not be controlled on monotherapy (systolic BP >20 mmHg or diastolic BP >10 mmHg above target BP) because of evidence showing that only a minority of patients will achieve and maintain BP goals on monotherapy.Citation7 Increasingly, a single-pill combination containing two antihypertensive drugs with complementary mechanisms of action offers potential advantages, including simplification of treatment regimens, more convenient drug administration, increased compliance, and reduced health care costs.Citation6,Citation8,Citation9 Several fixed-combination therapies consisting of two antihypertensive agents are available.Citation9 Preferred drug classes for combination regimens include angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and diuretics, with selection dependent on individual patient factors, including additional CV risk factors and comorbidities.Citation5,Citation7 Recently, a single-pill combination of olmesartan medoxomil/amlodipine has become available for clinical practice use in Italy. Hence, in the present study, we attempted to investigate the criteria for prescribing the combination pill for hypertensive patients in the context of clinical practice, and to determine whether the combination pill improves medication adherence. The study was conducted using data from the pharmaceutical services database.
Materials and methods
Data source
The study was based on administrative databases maintained by three local health units (LHU) located in three Italian regions: Lombardy; Emilia-Romagna; and Campania. The LHU Ethics Committees approved of the study. In the Medications Prescription Database, the LHU routinely measures the volume of expenditure generated by the dispensing of drugs to the enrollees. The data available in each prescription claim include the patient’s national health number, the prescribing physician’s number, the anatomical–therapeutic–chemical (ATC) code of the drug delivered, the number of packs, the number of units per pack, the dosage, the unit cost per pack, and the prescription date. Using the numeric code released to each citizen by the LHU as a unique identifier, this database was linked with the Beneficiaries’ Database, which listed some patients’ demographic characteristics such as their date of birth, sex, place of residence, physician’s license number, and the start and end registration dates. Moreover, the Hospital Discharge Database was also included, which provided hospitalization data, such as the discharge diagnosis codes that were classified according to the International Classification of Diseases, Ninth Revision, Classification Modified (ICD-9-CM). Finally, the Mortality Database was also used, where patients’ death data were recorded. It was not possible to retrieve the cause of death from death certificates. Universal health care coverage in Italy allows for the completeness and comprehensiveness of information contained in these databases, which have been used in previous epidemiological studies.Citation10,Citation11 The Italian Ministry of Health (Rome, Italy) reported that archives are 100% complete and 95% accurate.Citation12 In order to guarantee patient privacy, each subject was assigned an anonymous and unique alphanumeric code.
Cohort definition
This was a retrospective cohort study, which selected subjects aged 18 years and older, who received at least one prescription of antihypertensive drugs (diuretics [ATC code C03]; beta-blockers [ATC code C07]; calcium channel blockers [ATC code C08]; angiotensin-converting enzyme inhibitors [ATC code C09A/B]; angiotensin-receptor blockers [ATC code C09C/D]; and/or other antihypertensive drugs [ATC code C02] between September 1, 2011 and December 31, 2011 [enrollment period]). The date of the first antihypertensive claim was defined as the index date. We excluded records of subjects who died or moved to other LHU during the study period. For each subject, we compared the antihypertensive drug treatments (ADTs), as well as the patient’s adherence to treatment in the 6 months preceding and following the index date. For analysis purposes, we included only subjects who, in the previous 6-month period, received at least a prescription of olmesartan (ATC code C09CA08, C09DA08) and/or amlodipine (ATC code C08CA01) as a single therapy or in a two-pill combination. In order to define the patients’ clinical characteristics, in the 12 months before the index date, we evaluated prescriptions of hypoglycemic drugs (ATC code A10; at least two prescriptions) and hospital admissions for hypertension (ICD-9-CM code 401–405), myocardial infarction or other ischemic heart disease (ICD-9-CM code 410–414), heart failure (ICD-9-CM code 428), cerebrovascular disorders (ICD-9-CM code 430–438), or vascular diseases (ICD-9-CM code 440–442).Citation13
Adherence to ADT
The adherence to ADT was determined separately in the two 6-month study periods, grouping the enrolled subjects as follows: subjects treated with olmesartan or amlodipine in a single-pill treatment; or subjects treated with a free-drug combination in the preceding period, who either moved or did not move to a fixed combination of olmesartan/amlodipine in the following period. Adherence to ADT was estimated as the percentage of days a subject had tablets available (proportion of days covered [PDC]), from the first delivery of ADT until the last day of the 6-month follow-up period, regardless of any gap in therapy. Based on the method of Catalan and LeLorier,Citation14 the 6-month intervals were separated into treatment episodes of continuous ADT use. The PDC corresponded to the total of number of days’ supply of medication dispensed within each episode, divided by the 6-month follow-up period, and multiplied by 100. The period covered by a prescription was calculated by the number of tablets in the dispensed packs of drugs. We assumed a treatment schedule of one tablet per day regarding prescriptions of agents from the same drug class, considering a possible stockpiling of medication for future use, while we assumed one tablet per agent per day regarding prescriptions containing agents from antihypertensive different classes, identifying these as a combined therapy. The method we used avoided double counting in the case of overlapping refills. Any remaining tablets at the end of the study period were not accounted for. Consistent with data in the literature, subjects with a PDC >80% were defined as adherent to ADT.Citation15
Statistical analysis
Data were summarized as the mean ± standard deviation-for continuous variables, and as numbers (percentages) of subjects for categorical variables. Pearson’s chi-square and one-way analysis of variance tests were used to evaluate differences in baseline characteristics across the cohort of patients. McNemar’s test was used to assess the change in the proportion of adherent patients from the pre- to the postindex date period. Two-tailed P-values<0.05 were considered statistically significant. All statistical analyses were conducted using Stata software, version 12.1 (StataCorp LP, College Station, TX, USA).
Results
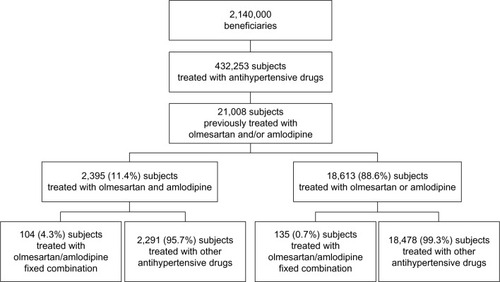
Out of a population of about 2,140,000 beneficiaries, 432,253 subjects had at least a prescription of antihypertensive drugs filled from September 1, 2011 to December 31, 2011. Of these, 2,395 were previously treated with olmesartan and amlodipine as a two-pill combination treatment and 18,613 were previously treated with olmesartan or amlodipine as a single-pill treatment (). Subjects treated with the two-pill combination treatment were significantly (P<0.001) more frequently diabetic and had significantly (P<0.001) more previous CV events than subjects treated with the single-pill treatment (). Subjects treated with the two-pill combination treatment moved to olmesartan/amlodipine fixed combination treatment more frequently than did subjects treated with a single-pill treatment (P<0.001) (), who were independent from the presence of diabetes and/or previous CV events (). Of the 21,008 enrolled patients (), 239 (1.1%) moved to olmesartan/amlodipine fixed combination therapy during the postindex date period, while 20,769 (98.9%) were mainly treated with olmesartan and amlodipine as a two-pill combination treatment (9.6%) or olmesartan or amlodipine as a single-pill treatment also combined with other antihypertensive drugs (87.9%) (). As shown in , the 239 patients who moved to olmesartan/amlodipine fixed combination therapy were more likely to have diabetes and a previous two-pill combination treatment. Among these patients, we compared the postindex date period to the preindex date period, and we observed an increase in the percentage of patients adherent to the treatment, both in the 104 subjects coming from the two-pill combination treatment, and in the 135 subjects coming from the single-pill treatment (from 61.5% [pre-index adherence level] to 76.0% [post-index adherence level], +24% increase; and from 57.0% to 80.7%, +42%, respectively) (). Also, among the 20,769 subjects who did not move to the olmesartan/amlodipine fixed combination therapy, when we compared the postindex date period to the preindex date period, we observed an increase in the percentage of patients who were adherent to the treatment both in the 2,291 subjects coming from the two-pill combination treatment, and in the 18,478 subjects coming from the single-pill treatment (from 66.7% to 70.8%, +6%; and from 55.0% to 62.0%, +13%, respectively) (). The percentage of adherent patients observed in the postindex period among the 239 subjects who moved to olmesartan/amlodipine fixed combination therapy compared with that observed among the 20,769 subjects who did not move to olmesartan/amlodipine fixed combination therapy resulted in significant differences (from 59.0% to 78.7%, +33%, P<0.001; and from 56.3% to 63.0%, +12%, P<0.001, respectively); the evaluated increase in treatment adherence in patients who moved to the olmesartan/amlodipine fixed combination therapy resulted in significantly higher differences than did the increase in treatment adherence among the patients treated with the other antihypertensive drugs (P<0.001).
Figure 2 Adherence to treatment in subjects who moved to olmesartan/amlodipine.
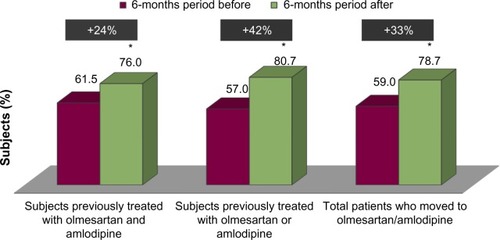
Figure 3 Adherence to treatment in subjects who did not move to olmesartan/amlodipine.
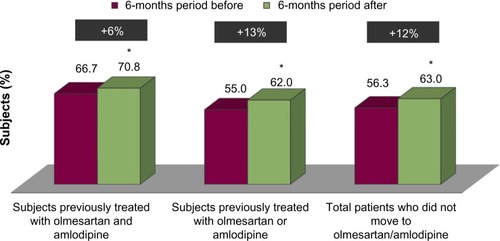
Table 1 Characteristics of the enrolled subjects according to the pretreatment index
Table 2 Characteristics of the subjects who moved to olmesartan/amlodipine fixed combination therapy, and those who did not move to olmesartan/amlodipine fixed combination therapy
Table 3 Postindex treatment of subjects who did not move to olmesartan/amlodipine fixed combination therapy
Discussion
Hypertension represents a significant economic burden, absorbing a large and growing share of health care resources. The economic burden of uncontrolled hypertension is primarily clinical, related to CV morbidity, including coronary heart and cerebrovascular diseases, and consequently economic due to the costs of managing cardiovascular events as well as the cost of medications and physician visits.Citation16 The high prevalence of hypertension in the real-world clinical setting is partly due to a lack of awareness of BP levels, but also to patients not achieving recommended treatment targets. Failure to achieve target BP levels may be related to physicians’ prescribing habits, and/or patients’ adherence and persistence to their antihypertensive medications.Citation16 Treatment adherence is an important issue for a chronic disease such as hypertension, with improvements in adherence expected to result in better long-term clinical outcomes, including reduced CV and renal morbidity/mortality and, consequently, containment of health care costs.Citation17 In fact, some studies have shown a direct correlation between noncompliance to antihypertensive therapy and increased health care expenditures.Citation17,Citation18 Treatment adherence to antihypertensive drugs has been previously estimated using administrative databases from drug reimbursement programs.Citation19–Citation22 A positive benefit of these studies is that they offer the opportunity to carry out clinical audits and evaluative research, they inform the planning and management of services, and they provide clinicians with accurate estimates of the outcomes of care.Citation23 They represent a complementary approach, rather an alternative to clinical trials, and can contribute to evaluate health care in everyday practice and how to improve the organization of services and individual pharmacological treatment.Citation24
In the present study, we have determined the adherence to antihypertensive agents. While treatment persistence reflects the duration over which a patient had not discontinued their drug therapy, treatment adherence refers to the intensity of drug use during the follow-up period.Citation25 Currently, the two most commonly used measures of medication adherence based on pharmacy data are the medication possession ratio and the PDC methods. The main difference between these two measures is that the maximum PDC is 1.0 (or 100%, if expressed as percentage), which indicates full adherence, whereas the medication possession ratio accounts for overprescription and can have a value >1.0.Citation25 We calculated adherence in terms of the percentage of days covered. This percentage was arbitrarily selected, but it was based on the assumption that patients who took at least 80% of their prescribed medications would benefit.Citation14 This dichotomous cutoff value was found to be reasonable for CV medications, even in the presence of cases of overprescription of the drug.Citation25 Hence, we believe our definition is relevant to clinical practice goals for treatment. Evidence from meta-analyses has shown that the use of an antihypertensive, single-pill combination therapies, when compared with corresponding free-drug combinations, is associated with significantly greater rates of treatment adherence to medications, as well as with potential advantages in terms of BP improvements and reductions in adverse effects.Citation26,Citation27 A large retrospective database study of an angiotensin II receptor blocker plus a calcium channel blocker in a two-drug, single-pill combination has also shown greater levels of adherence when compared with corresponding regimens consisting of a free-pill angiotensin II receptor blocker plus a calcium channel blocker.Citation28 Moreover, Chang et alCitation29 have shown that patients using a valsartan-based, single-pill combination were more adherent to pharmacological treatment, as well as more prone to achieve BP goals, than patients treated with an angiotensin II receptor blocker in free combination with other antihypertensive drugs.
The results of the present study performed in a clinical practice setting confirm that the rate of adherence on antihypertensive treatment is higher for hypertensive patients treated with a single-pill combination of olmesartan/amlodipine, as compared to patients treated with the two-pill combination or a single pill, or with other single or multipill ADTs. We suggest the possibility that the tolerability profile of the fixed combination of olmesartan/amlodipine might contribute to the patient’s persistence on treatment. Unfortunately, the occurrence of drug-related adverse effects was not reported in pharmaceutical databases; however, some evidence might be obtained by the analysis of data collected from clinical trials comparing olmesartan/amlodipine with their respective monotherapies. These studies described an incidence of drug-related adverse events in the range of 5.3%–7.7% among patients receiving approved doses of olmesartan/amlodipine, compared with the incidence rates of 7.4% and 8.9% among patients receiving amlodipine or olmesartan monotherapy, respectively.Citation30,Citation31 Our findings suggest that the combination pill improves medication adherence. Further analyses are needed to confirm whether a higher proportion of patients who persist on treatment are associated with greater BP decreases in response to antihypertensive drugs.
The point of this study is that the pharmaceutical databases were constructed to serve a billing role for the reimbursement of services provided; thus, information on patients’ BP levels was not available. However, a recent meta-analysis of 147 trials showed a BP-lowering benefit following the use of antihypertensive drugs, irrespective of what the patients’ BP levels were, thus avoiding the need to measure BP routinely.Citation32 This supports the hypothesis that the overall impact of the BP-lowering treatments in clinical practice may actually result from the treatments’ absolute BP-lowering effect and their capacity to positively promote patients’ persistence on treatment. From a public health perspective, studies based on administrative databases, like the present one, offer several advantages, including a prompt, easily updated, and representative picture of monitored cohorts with highly generalizable results. The ability for a health care system to be supported also depends on accurate and comprehensive data for good clinical management, administration, financial control, and general management.Citation33 Thus, government institutions have stressed the importance of implementing patient-oriented clinical information systems, and of using the administrative databases in monitoring clinical practice.Citation34
Conclusion
In conclusion, the results of the present study show that the rate of adherence to therapy can significantly differ among patients treated with several classes of antihypertensive drugs (even within the same pharmacological family), and among patients treated with the fixed combination of olmesartan/amlodipine. Additional studies are needed to assess whether these differences will be maintained in the following years, and whether the differences are associated with better health outcomes.
Acknowledgments
The development of this study was neither funded nor supported. The writing of this manuscript was supported with an unrestricted grant by Menarini, which played no role in the design of the study, analysis, or interpretation of the findings, nor in the preparation, review, and approval of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- EganBMZhaoYAxonRNUS trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008JAMA2010303202043205020501926
- EzzatiMOzaSDanaeiGMurrayCJTrends and cardiovascular mortality effects of state-level blood pressure and uncontrolled hypertension in the United StatesCirculation2008117790591418268146
- [No authors listed]Prevalence of chronic diseases in older Italians: comparing self-reported and clinical diagnoses. The Italian Longitudinal Study on Aging Working GroupInt J Epidemiol199726599510029363520
- De GiustiMDitoEPagliaroBA survey on blood pressure levels and hypertension control in a sample of the Italian general populationHigh Blood Press Cardiovasc Prev201219312913522994581
- KrauseTLovibondKCaulfieldMMcCormackTWilliamsBGuideline Development GroupManagement of hypertension: summary of NICE guidanceBMJ2011343d489121868454
- ChobanianAVBakrisGLBlackHRJoint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureNational Heart, Lung, and Blood InstituteNational High Blood Pressure Education Program Coordinating CommitteeSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension20034261206125214656957
- ManciaGDe BackerGDominiczakAManagement of Arterial Hypertension of the European Society of HypertensionEuropean Society of Cardiology2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- CheungBMLauCPKumanaCRCombination therapy for hypertensionHong Kong Med J20039322422612777663
- FrankJManaging hypertension using combination therapyAm Fam Physician20087791279128618540493
- Di BariMBalziDRobertsATPrognostic stratification of older persons based on simple administrative data: development and validation of the “Silver Code,” to be used in emergency department triageJ Gerontol A Biol Sci Med Sci201065215916419349591
- BroccoSVisentinCFedeliUMonitoring the occurrence of diabetes mellitus and its major complications: the combined use of different administrative databasesCardiovasc Diabetol20076517302977
- Ministero del lavoro, della Salute e delle Politiche SocialiRapporto annuale sulle attività di ricovero ospedaliero [Annual report on the activities of hospitalization]2009 Available from: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1491_allegato.pdfAccessed December 22, 2013 Italian
- Degli EspostiLDegli EspostiEValpianiGA retrospective, population-based analysis of persistence with antihypertensive drug therapy in primary care practice in ItalyClin Ther20022481347135712240784
- CatalanVSLeLorierJPredictors of long-term persistence on statins in a subsidized clinical populationValue Health20003641742616464201
- HoPMRumsfeldJSMasoudiFAEffect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitusArch Intern Med20061661836184117000939
- Degli EspostiLValpianiGPharmacoeconomic burden of undertreating hypertensionPharmacoeconomics2004221490792815362928
- ParamoreLCHalpernMTLapuertaPImpact of poorly controlled hypertension on healthcare resource utilization and costAm J Manag Care20017438939811310193
- McCombsJSNicholMBNewmanCMSclarDAThe costs of interrupting antihypertensive drug therapy in a Medicaid populationMed Care19943232142268145599
- BloomBSContinuation of initial antihypertensive medication after 1 year of therapyClin Ther19982046716819737827
- CaroJJSalasMSpeckmanJLRaggioGJacksonJDPersistence with treatment for hypertension in actual practiceCMAJ1999160131379934341
- Degli EspostiLSaragoniSBenemeiSAdherence to antihypertensive medications and health outcomes among newly treated hypertensive patientsClinicoecon Outcomes Res20113475421935332
- Degli EspostiLSaragoniSBatacchiPAdherence to statin treatment and health outcomes in an Italian cohort of newly treated patients: results from an administrative database analysisClin Ther201234119019922284998
- VirnigBAMcBeanMAdministrative data for public health surveillance and planningAnn Rev Public Health20012221323011274519
- BlackNWhy we need observational studies to evaluate the effectiveness of health careBMJ19963127040121512188634569
- HoPMBrysonCLRumsfeldJSMedication adherence: its importance in cardiovascular outcomesCirculation2009119233028303519528344
- BangaloreSKamalakkannanGParkarSMesserliFHFixed-dose combinations improve medication compliance: a meta-analysisAm J Med2007120871371917679131
- GuptaAKArshadSPoulterNRCompliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysisHypertension201055239940720026768
- ZengFPatelBVAndrewsLFrech-TamasFRudolphAEAdherence and persistence of single-pill ARB/CCB combination therapy compared to multiple-pill ARB/CCB regimensCurr Med Res Opin201026122877288721067459
- ChangJYangWFellersTChart review of patients on valsartan-based single-pill combinations vs ARB-based free combinations for BP goal achievementCurr Med Res Opin20102692203221220673201
- VolpeMBrommerPHaagUMieleCEfficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig20092911125
- BarriosVBrommerPHaagUCalderónAEscobarCOlmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig2009297427439
- LawMRMorrisJKWaldNJUse of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studiesBMJ2009338b166519454737
- RileyGFAdministrative and claims records as sources of health care cost dataMed Care200947S51S5519536019
- DickRSSteenEBThe Computer-Based Patient Record: An Essential Technology for Health CareWashington, DCNational Academy Press1991