Abstract
Background
Bleeding remains a common occurrence in surgery. Data describing the burden of difficult-to-control bleeding and topical absorbable hemostat use are sparse. This study was conducted to estimate the clinical and economic impact that remains associated with uncontrolled surgical bleeding, even when hemostats are used during surgery.
Methods
This US retrospective analysis used the Premier Perspectives Database. Hospital discharges from 2012 were used to identify patients treated with hemostats during eight surgery types. Patients were included if they were ≥18 years, had an inpatient hospitalization with one of the eight surgeries, and received a hemostat on the day of surgery. Patients were stratified by procedure and presence or absence of major bleeding (uncontrolled) despite hemostat use. Outcomes were all-cause hospitalization costs, hemostat costs, length of stay, reoperation, and surgery-related complications (eg, mortality). Statistical significance was tested through chi-square or t-tests. Multivariate analyses were conducted for all-cause costs and length of stay using analysis of covariance.
Results
Among 25,048 procedures, major bleeding events occurred in 14,251 cases. Despite treatment with hemostats, major bleeding occurred in 32%–68% of cases. All-cause costs were significantly higher in patients with uncontrolled bleeding despite hemostat use versus controlled bleeding (US$24,203–$61,323 [uncontrolled], US$14,420–$45,593 [controlled]; P<0.001). Hemostat costs were significantly greater in the uncontrolled bleeding cohort for all surgery types except cystectomy and pancreatic surgery. Reoperation and mortality rates were significantly higher in the uncontrolled bleeding cohort in all surgical procedures except cystectomy and radical hysterectomy.
Conclusion
Uncontrolled intraoperative bleeding despite hemostat use is prevalent and associated with significantly higher hospital costs and worse clinical outcomes across several surgical procedures compared to controlled bleeding. There is an unmet need for newer hemostats that can more effectively control bleeding, improve outcomes, and reduce hospital resource use.
Background
Intraoperative and postoperative bleeding remains a common major complication of surgery.Citation1–Citation5 An aging population with growing comorbidities and high anticoagulant use are important factors that contribute to high surgical bleeding risks.Citation6–Citation8 Surgical bleeding can range from mild or moderate in intensity to severe or traumatic. There are a number of conventional surgical methods (eg, suture, ligature, compression, and cautery) and topical absorbable hemostats (TAHs) available to achieve hemostasis in mild to moderate bleeding scenarios.Citation9–Citation13 Hemostatic agents in particular have become a growing treatment option over the past couple of decades, and have been associated with improved surgical and clinical outcomes.Citation14
Mild or moderate surgical bleeding may be straightforward to manage; however, bleeding may also be problematic or difficult to control, depending on several factors including bleeding severity, visibility and access to the bleeding source, anatomic location of the bleeding, patient coagulation status, and surgical skill.Citation12 These types of bleeding scenarios are often referred to in the literature using several common bleeding terms including severe,Citation1 major,Citation5 or excessive.Citation15 For example, diffuse bleeding from broad surface areas in patients who are coagulopathic may be particularly difficult to manage which may lead to additional procedures such as blood transfusion.Citation9,Citation12 Traumatic bleeding may be placed at the top of this spectrum where patients have severe bleeding from injured tissues and often traditional methods of hemostasis are ineffective, necessitating multiple units of transfused blood.Citation16,Citation17
In more problematic and difficult bleeding, there is often no single solution that can allow surgeons to rapidly stop bleeding.Citation18–Citation20 As a result, these situations often involve combinational use of hemostatic products in addition to conventional methods, which may be cumbersome, time-consuming, and costly.Citation12,Citation21 Furthermore, several studies describe the substantial clinical and economic burden with such bleeding.Citation15,Citation16,Citation22–Citation24 Bleeding can lengthen, interrupt, or complicate the surgery as well as increase likelihood of transfusion, reoperation, and associated complications.Citation22,Citation25–Citation28 Furthermore, it has been reported that severe, excessive, or uncontrolled bleeding during surgery can increase mortality rates to 20%.Citation1,Citation3 It has also been estimated that uncontrollable bleeding accounts for approximately 40% of trauma-related deaths.Citation29
Despite available data describing the burden of difficult or uncontrollable bleeding, there is still a need to understand how hemostat use impacts the incidence of such bleeding, and the risk of associated complications. Currently, no studies have explicitly assessed the burden of surgical bleeding in relation to hemostat use. Consequently, this retrospective analysis of the Premier database was conducted to estimate the hospital resources and costs that remain associated with uncontrolled surgical bleeding, even when hemostatic agents are used during surgery.
Methods
Study design and data source
A retrospective analysis was conducted using data from the Premier Perspectives Database (PPD). Information contained within the PPD is de-identified making it fully compliant with the Health Insurance Portability and Accountability Act (HIPAA). The PPD includes data on more than 600 participating hospitals and 47 million hospital discharges in the US. Participating hospitals submit data on patient demographic and payer information as captured on the hospital billing record. Before the information is added to the database, all data go through quality assurance and validation checks. Available data include all billed items by the cost-accounting department, including medications; laboratory, diagnostic, and therapeutic services; and primary and secondary diagnoses for each patient. Further, hospital information, such as geographical location, bed size, and teaching hospital status, is also included within the PPD.
Patient population
All hospital discharges with admission dates in 2012 were used to identify patients who were treated with hemostatic agents during select surgeries. Eight major surgeries were selected that were deemed by surgeons to be commonly associated with major bleeding and included cardiac revascularization, cardiac valve surgery, cholecystectomy, cystectomy, pancreatic, partial hepatic resection, pulmonary, and radical abdominal hysterectomy. Surgeries of interest were identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure codes (). Specific hemostatic agents used in surgery included mechanical, thrombin, flowable, and fibrin sealant agents (). Patients were identified for inclusion if they were admitted to a hospital in 2012, underwent an inpatient surgery of interest as the primary procedure, and received a hemostatic agent on the day of the surgery. Patients were excluded if they were less than 18 years old or had received an additional major surgical procedure on a different body system on the same day as the index procedure. For patients with multiple hospitalizations, only the first was included for analysis.
Major bleeding events
Within each of the eight surgery subgroups, patients were further stratified by the presence or absence of a major bleeding (ie, uncontrolled bleeding) event despite hemostat use. Major bleeding events were identified by following the ICD-9-CM diagnosis and procedure codes: hemorrhage or hematoma complicating a procedure (998.11 and 998.12); interventions to control bleeding (34.09, 39.98, 44.44, 44.49, 54.19, 39.41, 34.03, 54.12, 57.93); charges billed for use of hemovac drainage devices; charges billed for use of erythropoietin; blood product transfusions (99.00–99.09); and charges billed for cryoprecipitates, fresh frozen plasma, red blood cells, plasma, platelets, and whole blood. A detailed listing of these major bleeding events is outlined in .
Study outcomes
The main study outcomes included in the study were the all-cause costs incurred during hospitalization, the cost of hemostatic agents, length of stay (LOS) between surgery and discharge, intensive care unit (ICU) stay, operation time, reoperation, and potential surgery-related complications (eg, mortality, infection, transfusions, ventilator use). Total all-cause costs included room and board, surgery, professional fees, supplies, pharmacy services, and laboratory services. Reoperation was defined as procedures on the same body system as the original procedure, performed during the same hospitalization. Additionally, both infections and transfusions were defined according to specific ICD-9-CM codes, which are summarized in and , respectively. Other study measures included were patient demographics, payment source, admitting hospital characteristics, type of hemostatic agents used (eg, mechanical, active, flowable, fibrin sealant), and the all payer refined-diagnosis related groups (APR-DRGs). The APR-DRG simultaneously evaluates the interactions of multiple comorbidities, age, and primary and secondary discharge diagnoses.
Statistical analyses
All data transformations and statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA). Patient demographics and hospital characteristics were evaluated for all surgical subgroups combined. Descriptive statistics (eg, means, patient counts) were stratified by the presence or absence of major (ie, uncontrolled) bleeding events. All statistical analyses on outcome measures were conducted separately for each surgical subgroup. Chi-square or t-tests were used to test for statistical significance whenever applicable; all tests were two-sided with a significance level of 0.05. Multivariate analyses were conducted to compare all-cause costs and LOS between patients with and without uncontrolled bleeding. Patient demographics and admitting hospital characteristics thought to have an impact on costs and LOS were included into the multivariate analysis, including age, race, sex, payment source, hospital geographic region, hospital location (rural vs urban), surgical admission type (elective vs emergent), teaching hospital status, and bed size. Analysis of covariance (ANCOVA) was used to adjust for these baseline characteristics.
Results
A total of 50,696 patients were identified within the Premier database that underwent a selected surgery in 2012, of which 25,155 were excluded because no hemostatic agent was used during surgery (). Of the remaining 25,541 patients, 125 were excluded as they were younger than 18 years, and 368 were further excluded because they required additional surgery on a different body system on the same day. Thus, 25,048 patients were included in the analysis (cardiac revascularization: 12,799; cardiac valve surgery: 8,016; cholecystectomy: 1,576; cystectomy: 423; pancreatic: 464; partial hepatic: 620; pulmonary: 954; radical abdominal hysterectomy: 196).
Patient demographics and admitting hospital characteristics are presented in and . There were some notable differences between controlled and uncontrolled bleeding patients. In particular, there was a larger percentage of urgent cases in the uncontrolled versus controlled bleeding group (ie, 52% vs 40%), as well as a higher proportion of extreme APR-DRG disease severity in uncontrolled versus controlled bleeding (ie, 28% vs 8.4%).
Table 1 Baseline patient demographics and admitting hospital characteristics
Table 2 Hospital characteristics
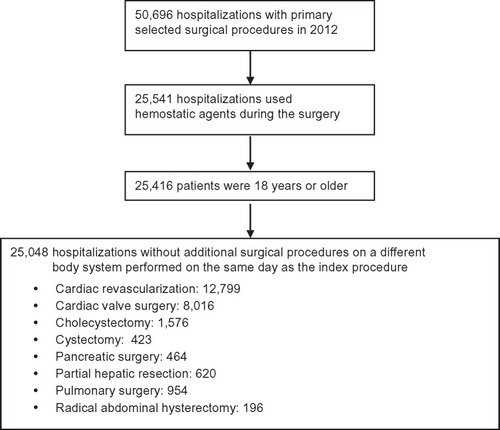
Among 25,048 procedures, 14,251 uncontrolled bleeding events were recorded. The prevalence of uncontrolled bleeding events within each surgical subgroup is presented in . Despite the use of hemostatic agents, uncontrolled bleeding events occurred in 32%–68% of patients, depending on the type of procedure. The most common type of event was use of a blood product, which occurred in 49.0% of all patients. Within the uncontrolled bleeding cohort, 25%–71% of patients required transfusions, with 5.8%–32.8% of patients receiving platelets, and up to 3.2% receiving coagulation factors. By definition, patients in the controlled bleeding cohort did not require transfusions.
Figure 2 Percentage of patients with a major bleeding event despite hemostat use, stratified by surgery group.
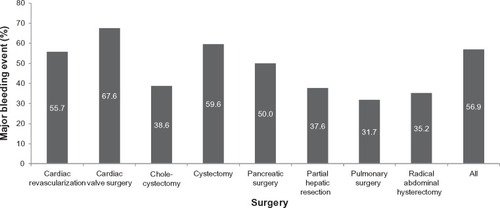
Mortality for each surgical subgroup, stratified by the presence of uncontrolled bleeding despite hemostat use, is presented in . Mortality was statistically significantly higher in the uncontrolled versus controlled bleeding cohort in all surgical subgroups except cystectomy and radical hysterectomy. Mortality rates ranged from 1.2% to 7.3% for uncontrolled bleeding and 0% to 1.2% for controlled bleeding cohorts.
Figure 3 Patient mortality, stratified by surgery type and presence or absence of uncontrolled bleeding despite hemostat use.
Results pertaining to hospital resource use and costs are presented in and for each surgical group, stratified by the presence or absence of uncontrolled bleeding despite hemostat use. All-cause costs were statistically significantly greater in patients with uncontrolled bleeding versus controlled bleeding for all surgery subgroups (uncontrolled bleeding: US$24,203–$61,323 vs controlled bleeding: US$14,420–$45,593; P<0.001). Similarly, LOS was also statistically significantly greater with uncontrolled bleeding patients for all subgroups (uncontrolled bleeding: 7.1–17 days vs controlled bleeding: 4.1–10 days; P<0.001). After adjusting for baseline differences, results for all-cause costs and LOS were consistent with unadjusted values ().
Table 3 Unadjusted mean (SD) costs and resource use, stratified by surgical procedure and presence or absence of uncontrolled bleeding despite hemostat use
Table 4 Mean adjusted all-cause costs (95% CI) and mean adjusted hospital LOS (95% CI) for controlled versus uncontrolled bleeding in patients treated with hemostatic agents, stratified by surgical procedure
The cost of hemostatic agents was also statistically significantly greater in the uncontrolled bleeding cohort for all surgical groups, except pancreatic surgery and cystectomy (uncontrolled bleeding: US$287–$799 vs controlled bleeding: US$203–$451) (). Furthermore, ICU stay and infection were always statistically significantly greater in the uncontrolled versus controlled bleeding cohorts, across surgery subgroups. Reported infections included urinary tract infections, septicemia, fever, and pneumonia. Reoperation rates were also statistically significantly greater in uncontrolled bleeding patients, with the exception of radical abdominal hysterectomy. Ventilator use was also more common in uncontrolled bleeding in all surgery cohorts except cystectomy. Finally, operating time was typically higher in uncontrolled versus controlled bleeding cohorts by 13.3–37.6 minutes, but differences were only statistically significant for cardiac revascularization, cardiac valve surgery, pulmonary surgery, and radical hysterectomy ().
Discussion
Using a sample of over 25,000 patients, we found that a substantial proportion of patients have uncontrolled surgical bleeding despite current hemostat use, with rates ranging from 32% to 68% depending on the procedure. Both infection rate and mortality were statistically significantly higher for uncontrolled versus controlled bleeding cohorts for all surgery types. Resource use, including length of hospital stay, ICU stay, ventilator use, operation time, and reoperation were often higher in patients with uncontrolled bleeding. These results were consistent with adjusted all-cause costs, which were always significantly greater in uncontrolled versus controlled bleeding cohorts.
Several studies have reported on the risk of surgical bleeding; however, reported rates span a wide range, which may be due to varying definitions of bleeding, differences in study design and geographic location, as well as variations in surgical procedures studied.Citation1,Citation5,Citation15,Citation24 For example, a recent study by Dyke et alCitation1 reported a major (ie, moderate or severe/massive) bleeding rate of 33.8% in cardiac surgery. Classification of major bleeding in this study depended on the amount of total blood loss, transfusion units, need for surgical re-exploration, and whether there was delayed sterna closure. This rate is reportedly lower than the observed rate of 56%–68% in cardiac revascularization or valve surgery in this study. Another study by Stone et alCitation5 reported a major bleeding rate in the US cardiac surgery patients of 52.9% where the bleeding definition encompassed decrease in hemoglobin levels, reoperation for bleeding, access site hemorrhage requiring intervention, ≥5 cm hematoma, or transfusion. Other studies reported major or excessive bleeding rates of lower than 10%; however, those studies used a more restrictive definition, which specified the number of transfusion units needed to qualify under the bleeding definitionCitation24 or the amount of postoperative bleeding drainage in cardiac surgery.Citation15 Our study included more liberal definitions of uncontrolled bleeding as well as several additional surgery types relative to these latter studies. Also, our study included eight surgery types deemed by surgeons to be commonly associated with major bleeding. Furthermore, unlike our study which focused solely on surgeries involving hemostat use, it is unclear to what extent hemostats were used in most of these published studies reporting bleeding risk.
These current study findings are aligned with studies that have quantified resource use and costs associated with surgical bleeding. An earlier 2011 US study by Stokes et alCitation23 reported that patients with bleeding-related complications (eg, transfusions) across different surgery types had significantly greater hospital costs and longer LOS. Our current study adds additional granularity in the types of resources comprising greater hospital costs in uncontrolled bleeding patients, such as reoperation, infection treatment, and ICU stay. Further, our study uniquely shows that these additional resources and costs are still high despite single or multiple hemostat product use. From the European perspective, Christensen et alCitation15 demonstrated that hospital costs and resources including ICU stay, ventilator, and reoperation were significantly higher in patients with excessive postoperative bleeding compared to patients without.
The uptake of hemostats has been rapid over the last several years. A study by Wright et alCitation14 showed that hemostat use continues to rise even for surgical procedures that are associated with very low bleeding complication and transfusion risk. Reviews of randomized trials demonstrate that hemostats can improve hemostasis and certain resource outcomes (eg, transfusions); however, benefits may vary by patient population type and hemostat product used.Citation30–Citation34 In surgical situations where bleeding is more difficult to control, combined use of multiple hemostats is sometimes undertaken to try to achieve hemostasis.Citation12,Citation21 In our study, hemostat costs have been observed to be significantly higher in patients with uncontrolled bleeding, which may be partially explained by more combination hemostat use. Despite these additional hemostat costs, uncontrolled bleeding rates and associated resource use remained high, signifying the suboptimal benefit that some currently approved hemostats may have. Limitations with such hemostats, including insufficient adhesion strength, lack of efficacy in a wet field, and inability to withstand forces of brisk hemorrhage, may explain the continued risk of uncontrolled bleeding in many surgery types.Citation9,Citation12,Citation18–Citation20
To address the prevalent problem of difficult-to-control surgical bleeding, a multifaceted approach is required. Essentially, methods to better assess appropriateness of operation technique and use of the various surgical methods for hemostasis are needed. Optimizing the use of right hemostatic technique (or product) with the right procedure can be an important goal for continuing education. Furthermore, new hemostats becoming available on the market that are targeted to problematic bleeding situations may help to alleviate this burden. The EVARREST® fibrin sealant patch is one novel bioabsorbable combination product composed of human fibrinogen and thrombin along with a flexible composite patch that provides mechanical integrity and supports clot formation.Citation35 EVARREST® is supported by several clinical studies across challenging bleeding populations demonstrating rapid onset of action with high hemostasis efficacy.Citation27,Citation36 A recent economic evaluation also showed that this new fibrin sealant patch was predicted to be cost saving in problematic surgical bleeding for hospital stakeholders due to hospital resources averted, such as transfusions and bleeding retreatment, versus standard of care.Citation37 Such results are particularly relevant in light of the findings of the current study showing significantly greater hemostat costs in uncontrolled bleeding cohorts. Several additional new hemostatic agents have also been developed that are currently undergoing clinical trials. Examples of these products include Veriset™ hemostatic patch (Covidien Inc., Mansfield, MA, USA), Fibrocaps™ (ProFibrix, Leiden, the Netherlands), and Hemopatch Sealing Hemostat (Baxter International, Deerfield, IL, USA). These products have numerous ongoing trials for the treatment of surgical bleeding across a wide range of surgery types with demonstrated effectiveness in some trials.Citation38–Citation41 No economic evaluations have been published to date with these products.
Limitations
This study is not without limitations. First, it was retrospective; therefore, it was not possible to control for all potential confounding variables as can be done within a randomized controlled trial. Second, limitations of this study include those common to all claims-based studies. Specifically, the data for this study were derived from hospital discharge records designed to be used for billing rather than research. There is some degree of miscoding that is common in these records, and the records were not independently validated. Furthermore, data such as these miss clinical details that ideally would be used to further explain study results. For example, there are no disease-specific measures of severity, no clinical assessments of preoperative risk (eg, hematocrit levels), and no data on surgeon’s skill level and techniques used. This information could not be captured and could not be evaluated or controlled for, as this was a retrospective database analysis. However, the potential impact of several patient and hospital characteristics was controlled for in adjusted multivariate analyses for the all-cause hospital costs as well as length of hospital stay, with adjustment having little impact on overall conclusions. Third, data are limited to the index hospitalization, so pre-existing comorbidities are not well captured. Fourth, data were only collected on hemostat class (eg, active, fibrin sealant, mechanical), and therefore it was not possible to conduct analyses on the association between specific hemostat products and bleeding control. Such information would have been useful for assessing the extent to which multiple product use or more expensive products contributed to the significantly higher total hemostat cost per patient in the uncontrolled bleeding cohort.
Conclusion
Despite the use of hemostatic agents, uncontrolled bleeding is common and is associated with significantly higher costs; longer hospitalization; and higher rates of reoperation and mortality in multiple major surgical procedures compared to controlled bleeding. There is an unmet need for newer hemostats that can improve clinical outcomes in surgery and minimize the economic burden to hospitals and payers. Future studies need to assess the clinical and economic impact of newer, highly efficacious hemostats in real-world, difficult-to-control bleeding populations.
Author contributions
MC was involved in the study protocol design and development, data acquisition, and critical review of the manuscript. NF and SH were involved in data analysis/interpretation and development of the manuscript draft. MSB was involved in study protocol design and development, data analysis/interpretation, and critical review of the manuscript. EC was involved in study protocol design and development, data analysis/interpretation, and critical review of the manuscript. All authors have given final approval for the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
Cornerstone Research Group (SH, NF) received funding from Ethicon Inc., to conduct the study and prepare the manuscript. The Partnership for Health Analytic Research (PHAR) (MSB, EC) also received funding from Ethicon Inc., to conduct this study. MC is an employee of Ethicon Inc. The authors would like to acknowledge Gordon Sun for assisting in the study design and results interpretation, and Bryanna Tibensky for assisting with the drafting of the manuscript.
Supplementary materials
Table S1 Selected primary surgical procedures
Table S2 Hemostatic agents
Table S3 Major bleeding events
Table S4 Infections
Table S5 Transfusion coding descriptions
Disclosure
The authors report no conflicts of interest in this work.
References
- DykeCAronsonSDietrichWUniversal definition of perioperative bleeding in adult cardiac surgeryJ Thorac Cardiovasc Surg2014147514581463.e124332097
- ErcanMBostanciEBOzerIPostoperative hemorrhagic complications after elective laparoscopic cholecystectomy in patients receiving long-term anticoagulant therapyLangenbecks Arch Surg2010395324725319294412
- MariettaMFacchiniLPedrazziPBusaniSTorelliGPathophysiology of bleeding in surgeryTransplant Proc200638381281416647479
- ShanderAFinancial and clinical outcomes associated with surgical bleeding complicationsSurgery20071424 SupplS20S2518019945
- StoneGWClaytonTCMehranRImpact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trialAm Heart J2012163352252922424026
- LevyJHDuttonRPHemphillJC3rdMultidisciplinary approach to the challenge of hemostasisAnesth Analg2010110235436420007735
- ParekhAKBartonMBThe challenge of multiple comorbidity for the US health care systemJAMA2010303131303130420371790
- United NationsWorld Population Ageing 1950–2050Division DoEaSA-PNew YorkUnited Nations Publications2001145
- BoucherBATraubOAchieving hemostasis in the surgical fieldPharmacotherapy2009297 Pt 22S7S19558278
- GabayMAbsorbable hemostatic agentsAm J Health Syst Pharm200663131244125316790576
- KulkarniRAlternative and topical approaches to treating the massively bleeding patientClin Adv Hematol Ooncol200427428431
- SamudralaSTopical hemostatic agents in surgery: a surgeon’s perspectiveAORN J2008883S2S1118790097
- VoilsSPharmacologic interventions for the management of critical bleedingPharmacotherapy2007279 Pt 269S84S17723110
- WrightJDAnanthCVLewinSNPatterns of use of hemostatic agents in patients undergoing major surgeryJ Surg Res2014186145846623993203
- ChristensenMCKrapfSKempelAvon HeymannCCosts of excessive postoperative hemorrhage in cardiac surgeryJ Thorac Cardiovasc Surg2009138368769319698857
- MariettaMPedrazziPGirardisMLuppiMMassive bleeding: are we doing our best?Transfus Apher Sci201145328729022071225
- RossaintRCernyVCoatsTJKey issues in advanced bleeding care in traumaShock200626432233116980877
- SchreiberMANeveleffDJAchieving hemostasis with topical hemostats: making clinically and economically appropriate decisions in the surgical and trauma settingsAORN J2011945S1S2022035823
- SpotnitzWDEfficacy and safety of fibrin sealant for tissue adherence in facial rhytidectomyClin Cosmet Investig Dermatol201254351
- SpotnitzWDBurksSHemostats, sealants, and adhesives: components of the surgical toolboxTransfusion20084871502151618422855
- RossaintRBouillonBCernyVManagement of bleeding following major trauma: an updated European guidelineCrit Care (London, England)2010142R52
- ClaridgeJASawyerRGSchulmanAMMcLemoreECYoungJSBlood transfusions correlate with infections in trauma patients in a dose-dependent mannerAm Surg200268756657212132734
- StokesMEYeXShahMImpact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patientsBMC Health Serv Res20111113521627788
- LauzierFArnoldDMRabbatCRisk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxisIntensive Care Med201339122135214323942857
- BochicchioGVNapolitanoLJoshiMBochicchioKMeyerWScaleaTMOutcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted studyWorld J Surg200832102185218918575931
- DoussauAPerezPPuntousMFresh-frozen plasma transfusion did not reduce 30-day mortality in patients undergoing cardiopulmonary bypass cardiac surgery with excessive bleeding: the PLASMACARD multicenter cohort studyTransfusion20145441114112424117772
- FischerCPBochicchioGShenJPatelBBatillerJHartJCA prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgeryJ Am Coll Surg2013217338539323969113
- SaifRJacobMRobinsonSUse of fibrin-based sealants and gelatin-matrix hemostats in laparoscopic liver surgerySurg Laparosc Endosc Percutan Tech201121313114121654294
- SpahnDRRossaintRCoagulopathy and blood component transfusion in traumaBrJ Anaesth200595213013915964891
- AubourgRPutzoluJBoucheSSurgical hemostatic agents: assessment of drugs and medical devicesJ Visc Surg20111486e405e40822136914
- CarlessPAHenryDAAnthonyDMFibrin sealant use for minimising peri-operative allogeneic blood transfusionCochrane Database Syst Rev20032CD00417112804501
- RousouJAUse of fibrin sealants in cardiovascular surgery: a systematic reviewJ Card Surg201328323824723578196
- SanjayPWattDGWigmoreSJSystematic review and meta-analysis of haemostatic and biliostatic efficacy of fibrin sealants in elective liver surgeryJ Gastrointest Surg201317482983623086450
- WangHShanLZengHSunMHuaYCaiZIs fibrin sealant effective and safe in total knee arthroplasty? A meta-analysis of randomized trialsJ Orthop Surg Res20141693624884626
- HuntBJBleeding and coagulopathies in critical careN Engl J Med2014370984785924571757
- KoeaJBBatillerJPatelBA phase III, randomized, controlled, superiority trial evaluating the fibrin pad versus standard of care in controlling parenchymal bleeding during elective hepatic surgeryHPB (Oxford)2013151617023216780
- CorralMFNHollmannSCost analysis of a fibrin sealant patch for mild, moderate, and problematic soft tissue surgical bleeding: a hospital perspectiveValue in Health2013A380.PSY315
- OllingerRMihaljevicALSchuhmacherCA multicentre, randomized clinical trial comparing the Veriset haemostatic patch with fibrin sealant for the management of bleeding during hepatic surgeryHPB (Oxford)201315754855823458162
- VerhoefCSinglaNMonetaGFibrocaps for surgical hemostasis: two randomized, controlled phase II trialsJ Surg Res Epub12102014
- BochicchioGVGuptaNPorteRJThe FINISH-3 trial: a phase 3, international, randomized, single-blind, controlled trial of topical fibrocaps in intraoperative surgical hemostasisJ Am Coll Surg20152201708125458801
- FingerhutAUranuesSEttorreGMEuropean Initial Hands-On Experience with HEMOPATCH, a Novel Sealing Hemostatic Patch: Application in General, Gastrointestinal, Biliopancreatic, Cardiac, and Urologic SurgerySurg Technol Int201425293525433173