Abstract
Background
Vitamin D levels play a pivotal role in most biological processes and differ according to age. A deficiency of vitamin D in chronic hepatitis C (CHC) patients has been shown to be linked with the severity of liver fibrosis, but little is known about the mechanism of this association.
Objective
In this study, we evaluate the potential interrelation between vitamin D levels, oxidative stress, and apoptosis, based on liver fibrosis in geriatric patients infected with hepatitis C virus (HCV) genotype 4.
Subjects and methods
A total of 120 adult individuals aged 30–68 years were recruited in this study. Of these, 20 healthy subjects (15 men and five women) with a mean age of 48.3±6.1 years were selected as controls, and 100 patients with a mean age of 47.8±4.9 years with chronic HCV (CHC) who had undergone liver biopsy (80 men and 20 women) were included in this study. Based on liver radiographic (computed tomography, magnetic resonance imaging) and histological Metavir system analyses, the CHC patients were classified into three groups: asymptomatic CHC carriers (n=30), fibrosis (n=25), and cirrhosis (n=45). HCV RNA, HCV genotypes, inflammatory cytokines AFP and TNFα, 25-hydroxyvitamin D (25[OH]D) levels, apoptotic markers single-stranded DNA (ssDNA) and soluble Fas (sFas), and oxidative stress markers nitric oxide (NO) and total antioxidant capacity (TAC) were estimated by using molecular, immunoassay, and colorimetric techniques.
Results
Approximately 30% of the study population (n=30) were diagnosed as asymptomatic CHC carriers, and 70% of the study population (n=70) had severe fibrosis; these were classified into fibrosis and cirrhosis. There was a significant reduction in 25(OH)D levels and TAC activity, along with an increase in levels of NO, AFP, TNFα, ssDNA, and sFas in fibrosis and cirrhosis subjects compared with those of asymptomatic CHC carriers and health controls. The deficiency in 25(OH)D levels correlated positively with sFas, ssDNA, AFP, TNFα, NO, and TAC, and negatively with age, sex, liver function, body mass index, homeostatic model assessment – insulin resistance, HCV RNA, and viral load. Significant intercorrelation was reported between serum 25(OH)D concentrations and apoptotic and oxidative markers, which suggested progression of liver pathogenesis and fibrogenesis via oxidative and apoptotic mechanisms.
Conclusion
The data showed that vitamin D status was significantly correlated with pathogenesis and fibrogenesis of the liver in geriatric patients infected with HCV genotype 4. The deficiency in 25(OH)D levels was shown to have a pivotal role in the pathogenesis of liver via apoptotic, oxidative stress, and inflammatory mechanistic pathways. The data point to adequate vitamin D levels being recommended for a good response to treatment strategies, especially in older CHC patients.
Introduction
Hepatitis C virus (HCV) is considered one of the major causes of liver injury worldwide.Citation1 Globally, >170 million individuals suffer from different HCV health problems, ranging from liver injury and cirrhosis to hepatocellular carcinoma (HCC).Citation2–Citation4 Acute and chronic stages are the most reported stages of HCV infection, with significant appearance of HCV RNA, elevation of serum ALT levels, jaundice, and consequently appearance of HCV antibodies (anti-HCV). In the chronic hepatitis stage, progressive expression of hepatic fibrosis has been reported, ultimately leading to the progression of cirrhosis and HCC.Citation5
In HCV-infected patients, oxidant–antioxidant imbalance status has been reported, with a significant increase in reactive oxygen species (ROS).Citation6 These oxidative free radicals result in more free radical initiation via liver cell-damage mechanisms.Citation7,Citation8 Most studies have reported that the HCV core protein and nonstructural proteins are responsible for the biological changes in human liver cells, such as an increase in ROS production, inhibition of the electron-transport chain, and altering apoptosis, the transcription process, and cell signaling.Citation9 Also, these viral proteins provide substantial alterations in endogenous antioxidants and antioxidant enzymes within the human body:Citation10 an excessive amount of ROS was produced from mitochondria, inflammatory cells, and peroxisomes of the infected liver cells. Besides their role in the promotion of HCV RNA replication and suppression of gene regulation of liver cells, these oxidative free radicals produce more free radicals from the fat and protein constituents of the cell wall and the cell’s genetic materials via lipid peroxidation.Citation11,Citation12 These biologically active oxidative radicals promote the progression of liver-cell fibrosis,Citation13 and the excessive generation of ROS by HCV infection leads to activation of oncogenic transcription factors and mutagenesis that ultimately ends in a state of HCC.Citation14
It has been reported that the integrity of liver tissues depends on homeostatic balance between liver-cell proliferation and apoptosis, and that any disruption of this balance by core proteins of HCV may lead to hepatic carcinogenesis, whereas viral proteins, along with activating transcription of viral and cellular genes, coordinate the balance between proliferation and death processes of liver cells by inducing or blocking apoptosis.Citation15 In patients with acute and chronic hepatitis (CH), the expression of apoptosis-related proteins was shown to be linked with intrahepatic bile-duct development.Citation16 The change in 25-hydroxyvitamin D (25[OH]D) levels in the serum of patients with CH is significantly associated with the expression of apoptosis-related proteins, especially in HCC subjects.Citation17
Besides its role in the regulation of bones and calcium homeostasis, 25(OH)D is involved in many biological processes.Citation18 25(OH)D deficiencies have been reported in hepatitis B/C patients.Citation19,Citation20 Recent studies suggest a relation of vitamin D status with fibrosis progression and response to the IFN-based therapy.Citation21 It was reported that during the treatment of CHC with polyethylene glycol–IFNα, considerable amounts of vitamin D administration were shown to increase sustained viral response.Citation22–Citation24
Most studies have reported that the response to therapy depends mainly on HCV genotypes,Citation25 targeting of apoptosis,Citation26 and 25(OH)D levels in CH patients.Citation17,Citation27,Citation28 Considerable benefits of therapeutic treatments have been achieved in patients with HCV genotype 2 and 3 infections than genotype 1 or 4, which had significantly lower response rates.Citation29 Also, vitamin D deficits were significantly reported in HCV genotype 4-infected subjects compared with healthy controls.Citation30,Citation31
It has been shown that the hydroxylated form of 25(OH)D controls a variety of genes that regulate more biological processes, including apoptosis and angiogenesis.Citation32 There was a significant correlation among HCV genotypes, hepatic fibrosis scores, and 25(OH)D deficiency in treated and untreated CHC patients.Citation33,Citation34
With regard to age, elderly patients with CHC showed significant decreases in serum vitamin D concentration compared with young patients, especially in elderly women,Citation21 and young patients were shown to be at lower risk of developing HCC than elderly patients.Citation35,Citation36 However, little is known about the link between severity of fibrosis, higher 25(OH)D deficiency, and apoptosis as a target for therapeutic treatments in genotype 4 CHC patients. Therefore, in this study, we evaluate the potential interrelation between vitamin D levels, oxidative stress, and apoptosis based upon liver fibrosis in geriatric patients infected with HCV genotype 4.
Subjects and methods
Patients
A total of 120 adult individuals aged 30–68 admitted to the outpatient department of the Gastroenterology Surgical Centre, Faculty of Medicine, Mansoura University, Mansoura, Egypt, were recruited in this retrospective study. Of these, 20 healthy subjects (15 men and five women) with a mean age of 48.3±6.1 years were selected as controls from a population undergoing routine medical investigations for medical insurance, and 100 patients with CHC who had undergone liver biopsy (80 men and 20 women) with a mean age of 47.8±4.9 years were included in the study. Full reported informed consent was obtained from all participants prior to liver biopsy.
Patient selection
Patients with proven HCV viremia, HCV RNA positivity, and genotype determinations were selected. Liver biopsy was taken from patients prior to antiviral therapy or any other antifibrotic therapy. Serum marker levels (such as AST, ALT, and AFP) were performed on the day of biopsy or within 5 days after liver biopsy.
Exclusion criteria were presence of HIV and/or HBV coinfection, other causes of chronic liver diseases, HCC, and prior liver transplantation. Also, subjects taking iron supplementation, the overweight and obese (body mass index [BMI] ≥25 and ≥30 kg/m2, respectively), with previous IFN therapy, and insufficient liver biopsy were excluded from this study.
The study protocol conformed with the ethical guidelines of the 1975 Declaration of Helsinki and was reviewed and approved by the ethical committee of the Gastroenterology Surgical Centre, Faculty of Medicine, Mansoura University. Also, the study was conducted after obtaining clearance from the institutional ethics committee and written informed consent from each participant. All subjects completed a structured questionnaire with questions regarding demographics and daily medication use. Venous blood samples from each patient were collected either before the administration of preoperative drugs on the day of biopsy or within 5 days after biopsy. Samples were given a coded study identification number and were shipped frozen at −80°C for analysis.
Virology
Patients with CHC were diagnosed by elevated levels of ALT and higher titers of anti-HCV, established by third-generation enzyme immunoassay (AxSym HCV 3.0; Abbott Laboratories, Abbott Park, IL, USA). Also, HCV RNA as a measure of diagnosis was reported qualitatively using a nested polymerase chain-reaction Qiagen RNA-extraction kit (Thermo Fisher Scientific, Waltham, MA, USA) and quantitatively using Smart Cycler II real-time polymerase chain reaction (Cepheid, Sunnyvale, CA, USA) with HCV RNA-quantification kits (Sacace Biotechnologies, Como, Italy) for estimation of HCV RNA-positive subjects, as previously described.Citation37,Citation38 In addition, reverse hybridization was performed to identify HCV genotypes using a line-probe assay (Inno-LiPA HCV II kit; Innogenetics, Ghent, Belgium).Citation39
Laboratory determinations
A previously validated questionnaireCitation40 was used to collect demographic and medical information. Laboratory test results used in this study were serum ALT, AST, and blood platelet counts and fasting blood glucose, using standard methods. Serum AFP and 25(OH)D levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) for and immunoassay kits (IDS, Boldon, UK), respectively. Also, TNFα (Orgenium Laboratories, Helsinki, Finland) and insulin (Monobind Inc, Lake Forest, CA, USA) were estimated for all participants using ELISA kits. Insulin resistance was determined by homeostatic model assessment (HOMA)Citation41 using the formula: fasting insulin (μIU/mL) × fasting blood glucose (mg/dL)/405. Serum total antioxidant capacity (TAC) was measured using a colorimetric assay kit (K274-100; BioVision, Milpitas, CA, USA). The data were measured and calculated as previously reported.Citation42 Plasma NO concentration as measure of oxidative stress parameter was estimated as nitrate and nitrite using a Griess reagent. The concentration of nitrite was measured at 540 nm using high-performance liquid chromatography technology as previously reported.Citation43 Plasma soluble Fas (sFas; ng/mL) and single-stranded DNA (ssDNA) concentrations were determined as early markers for liver-cell apoptosis using a commercial quantitative enzyme immunoassay kit (Quantikine®; R&D Systems) for Fas antigens and an ssDNA ELISA kit (EMD Millipore, Billerica, MA, USA) for ssDNA.
Histologic examination
After informed consent documents had been submitted, hepatic biopsies were obtained from all cases by a surgeon after computed tomography or magnetic resonance imaging scans. A preoperative clinical diagnosis of primary liver cancer was made on the basis of an elevated serum AFP level (≥400 ng/mL) and characteristic features of the disease that were visible in the scans. Histological diagnosis of cirrhosis and HCC was based on international criteria. For histological examination, liver biopsies were obtained using an automatic 16-gauge Trucut needle (biopsy gun), which provides adequate specimens for evaluation and fewer cases with tissue fragmentations. Liver biopsy specimens analyzed were at least 15–25 mm long with complete portal tracts (>10). Formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin and Masson’s trichrome. Slides were labeled with patient-identification numbers and then reviewed and graded blindly by a senior pathologist. The mean length of liver biopsies and the number of portal tracts were assessed (including only the complete, intact portal tracts). The degree of fibrosis was scored according to the Metavir system,Citation44 and no fibrosis was defined as F0, mild fibrosis as F1, moderate fibrosis as F2, severe fibrosis as F3, and cirrhosis as F4. Significant fibrosis was also defined as F2–F4. Hepatic inflammatory activity was also scored.
Statistical analysis
SPSS version 16 was used for statistical analysis. Data are expressed as mean ± standard deviation or number (percentage) of patients with a condition. Correlation analysis between the studied variables was done by Student’s t-test, one-way analysis of variance, and Mann–Whitney U-test for continuous variables.
Results
A total of 120 subjects aged 18–68 years with a mean age of 48.3±6.1 years were recruited in this study. Based on clinical investigations, Anti-HCV titers, HCV RNA, and pathological examination, the subjects were classified into control healthy subjects (n=20) and CHC patients (n=100), as shown in . Approximately 70% of CHC patients showed severe pathological symptoms: they were classified according to fibrosis scores into 25% with fibrosis (n=25) and 45% with cirrhosis (n=45), and only 30% (n=30) of patients were diagnosed as asymptomatic CHC (ASC) carriers. There were significant changes in BMI, increases in serum levels of AST, ALT, and bilirubin, and decreases in albumin in CH patients compared with the control group.
Table 1 Demographics, parameters studied, and clinical characteristics of geriatric CHC and control subjects
Also, significant changes in serum inflammatory markers, such as AFP and TNFα, along with glucose and HOMA – insulin resistance (IR) were reported in CHC patients compared to control subjects (). Also, in HCV patients with cirrhosis, there were significant increases in serum levels of AST, ALT, bilirubin, AFP, TNFα, glucose, and HOMA-IR, along with decreases in albumin compared with ASC patients (). However, significant reductions in levels of AST, ALT, albumin, and HOMA-IR were reported in cirrhotic patients compared with those with fibrosis ().
Table 2 Biochemical profiles of geriatric CHC patients according to liver-fibrosis scores
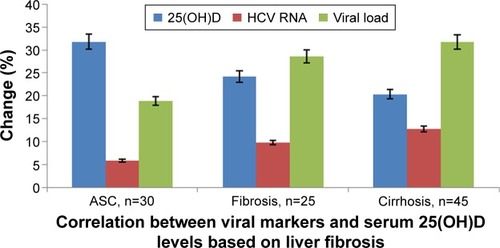
With regard to the association between 25(OH)D levels and severity of liver fibrosis, this was significantly lower in CHC patients compared to the control group (P≤0.05). Also, in cirrhotic patients, the reduction in 25(OH)D levels was shown to be significantly correlated (P=0.001) with ASC and fibrosis cases. HCV patients with fibrosis showed lower 25(OH)D levels when compared with either controls or ASC cases. There was significant correlation between lower levels of 25(OH)D and severity of liver fibrosis. HCV-related disease progression was associated with lower median values of 25(OH)D levels compared to nondiseased control patients, while those with cirrhosis had the lowest 25(OH)D concentrations among those with liver disease (). Also, there was significant association between severity of HCV infection and the deficiency in 25(OH)D levels. In CHC patients with fibrosis and cirrhosis, there were significant increases in viral load, HCV RNA-expression rates, and depletion in 25(OH)D levels compared with the ASC group ().
Table 3 Change in 25(OH)D levels, oxidative stress, and apoptosis-related biomarkers of geriatric CHC patients based on liver-fibrosis scores
Figure 1 Correlation between viral markers and serum 25(OH)D levels based on liver fibrosis.
To study the role of inflammation in the pathogenesis of CHC, the cytokines/inflammatory markers AFP and TNFα were estimated in CHC patients. There were significant increases in AFP and TNFα levels among CHC patients with cirrhosis compared with ASC and control subjects, as shown in .
To study the relationship between oxidative stress status and severity of liver fibrosis, NO and TAC as biomarkers of oxidative free radicals were measured in all participants. All CH patients showed significant increases in NO levels and depletion in TAC activity when compared with control subjects. However, patients with cirrhosis showed significantly higher increases in NO free radical levels and decreases in TAC activity when compared with patients of ASC and fibrosis groups, as shown in .
In order to estimate the possible association between apoptosis and liver fibrosis, sFas and ssDNA were estimated as apoptosis-related biomarkers in the plasma of all participants. HCV patients with fibrosis and cirrhosis showed significant increases in plasma levels of sFas and ssDNA when compared with ASC patients and healthy controls, as shown in ; however, significant increases in sFas and ssDNA as apoptosis markers were reported in cirrhotic patients compared with ASC and fibrosis (P=0.001) patients. Expression of the apoptosis-related markers ssDNA and sFas showed positive significant correlations with the increase in HCV RNA, viral load, AFP, TNFα, and NO, and decreases in levels of TAC activity ().
Table 4 Correlation coefficients (r) of apoptosis-related markers ssDNA and sFas with other parameters in geriatric patients with CHC (n=100)
In all CHC patients, deficient 25(OH)D levels showed significant correlations with the parameters studied in all groups. Lower 25(OH)D levels correlated positively with increases in sFas, ssDNA, AFP, TNFα, NO, and decreases in TAC activity, and negatively with age, sex, liver function, BMI, HOMA-IR, HCV RNA, and viral load in all patients with varying liver fibrosis, as shown in . Such a relation appeared to have higher significance in cirrhosis patients (P=0.001) compared to ASC or fibrosis patients (P=0.01).
Table 5 Correlation coefficients (r) of 25(OH)D deficiency with other parameters in geriatric patients with CHC (n=100)
Discussion
CH is one of the most pathological disorders caused by HCV worldwide. During progression of the disease, there is a significant increase in HCV RNA expression, elevated ratios of viral loads, and significant fibrotic protein deposits.Citation1,Citation4,Citation5 Vitamin D deficits have been shown to be commonly linked with chronic liver diseases. While 93% of these patients reported insufficient vitamin D levels,Citation45 almost a third of these showed severe deficiency.Citation46 The concentration of 25(OH)D in plasma is the most reliable indicator of vitamin D status. It reflects the total amount of vitamin provided from all natural sources, including conversion from fatty deposits of the liver.Citation47 Therefore, we evaluate the association between 25(OH)D levels, apoptosis, and liver fibrosis in genotype 4 CHC patients.
In the current study, based up on Metavir fibrosis-scoring system, only 70% of CHC patients were diagnosed with severe progression of fibrosis: they are classified into 25% with fibrosis (n=25) and 45% with cirrhosis (n=45), and only 30% (n=30) of patients were diagnosed as ASC carriers. There were significant changes in BMI, increases in serum levels of AST, ALT, and bilirubin, and decreases in albumin in CH patients compared with the control group. Our results are in line with other research, where the progression of hepatic fibrosis is significantly associated with higher rates of obesity-related factors, such as BMI.Citation48 Abnormal liver functions are expected to be associated with the progression of hepatic fibrosis. The obtained elevated serum levels of AST, ALT, and bilirubin and decreased albumin in CHC patients in our study were in accordance with most of the published data.Citation21,Citation49,Citation50 Other important inflammatory biomarkers released during CHC infection are AFP and TNFα levels in plasma. The elevated levels of AFP and TNFα in plasma of CHC patients compared to the controls were in agreement with other reports.Citation51,Citation52
The clinical data of our patients were correlative and supportive of previous reports that showed significant decreases in platelet counts and increases in glucose and HOMA-IR compared to controls.Citation53,Citation54 Also, the results of our study showed that activity and progression of chronic liver disease measured by Metavir fibrosis stages (F0–F4), necroinflammatory activity grades (A0–A3), and viral load (38.7±6.2) were similar to those previously reported.Citation55,Citation56
Significant 25(OH)D deficits have been reported in almost half of the healthy population of developed countries.Citation51 Besides its effects in bone health, 25(OH)D deficiency has been reported in the progression of many diseases, such as several types of cancers and cardiovascular and infection-related diseases, including CHC.Citation57–Citation60
Correlations between 25(OH)D levels and the progression of liver fibrosis were estimated in CHC patients under this study. Lower levels of 25(OH)D were significantly reported in CHC patients with fibrosis, cirrhosis, and ASC compared with control subjects. The data showed that 25(OH)D deficits correlated negatively with abnormal liver function, viral load, and HCV RNA expression. The deficiency in 25(OH)D levels among our patients was shown to be associated with liver severity, as reported in most of the literature, whereas vitamin D deficiency has been estimated among CHC cases with higher rates of mortality,Citation61–Citation63 portal hypertension,Citation64 and fibrosis severity.Citation65 25(OH)D deficits were proposed to be linked with poor hepatic hydroxylation of vitamin D to 25(OH)D or calcidiol,Citation47 lower albumin, and decreased expression of DBP, which is synthesized mainly in the liver, and in normal liver cells ~88% of serum 25(OH) D is attached to a DBP.Citation66 It was reported that only 5% of DBP-binding sites were required for the binding of vitamin D metabolites,Citation67 and this confirms that liver dysfunction should be severe enough to decrease DBP levels and ultimately produce vitamin D deficiency.Citation68 Therefore, these previous reports explained the variability in deficiency rates of vitamin D levels among our CHC patients, especially those with cirrhosis.
Also, other studies also showed a close association between 25(OH)D deficiencies, degree of fibrosis and necrosis in CHC patients, and decrease in sustained viral response factor toward HCV viral treatments, especially IFN/ribavirin (RBV)-based therapies.Citation5,Citation21,Citation50,Citation69
Vitamin D defects in CHC patients with fibrosis were shown to be inversely correlated with age, sex, HOMA-IR, and obesity (BMI). Negative correlations between 25(OH)D levels and obesity, glucose intolerance, IR, and BMI have been reported in many diseases, especially liver disorders.Citation70,Citation71 In addition, previous studies have shown an increase in the accumulation of vitamin D in adipose tissue of obese patients, which might relate to vitamin D deficiency.Citation72,Citation73 In contrary, although vitamin D levels are inversely correlated with fibrosis and necroinflammation of liver tissues, this correlation is independent of age, sex, BMI, HOMA-IR score, and presence of metabolic syndrome,Citation74–Citation76 whereas normal vitamin D levels have been shown to enhance HCV response to IFN and RBV therapyCitation77 via improving sensitivity to insulinCitation78 and prevent the development and progression of fatty liver by modulation of lipid metabolism.Citation79
In the present study, the deficiency in vitamin D levels also showed significant associations with inflammation and fibrosis among CHC patients. There were positive correlations among vitamin D defects and the release of both AFP and TNFα as inflammatory markers in patients with severe fibrosis scores. Previous evidence that matches our results suggests that during inflammatory diseases of the liver, the production of active vitamin D is affected due to significant decreases in the expression of vitamin D receptors. These receptors were significantly expressed in tissue macrophages, whereas it comprises of ~90% in the liver, non-parenchymal cells, and biliary epithelial cells.Citation80,Citation81 Vitamin D in its active form is involved in the decrease of inflammation.Citation21,Citation76,Citation82 Also, it has been reported that excessive inflammatory response was regulated by proinflammatory signals present in monocytes and macrophages. These signals may regulate the local metabolism of vitamin D through autoexpression of CYP27B1 and the local production of 1α, 25(OH)2D. It has been proposed that antifibrotic and anti-inflammatory activity of vitamin D decreases the persistence of HCV against IFN/RBV therapy. This occurs via minimizing the induction of proinflammatory cytokines.Citation20,Citation83
In various liver diseases, pathogenesis and progression attributed to initiation of higher amounts of oxidative stress free radicals, such as ROS. Mitochondria, cytochrome P450 enzymes, Kupffer cells, and neutrophils were shown to be the main sources of ROS.Citation9,Citation84
The excessive production of ROS produces significant damage to cellular proteins, the cell wall, and DNA. These harmful effects can be nullified with both enzymatic (superoxide dismutase, catalase, glutathione peroxidase), and non-enzymatic antioxidants, such as vitamins (A, C, E, D), and reduced glutathione.Citation85 Therefore, CHC patients included in this study were subjected to estimation of the nitric oxide free radical (NO) and TAC as markers of oxidative stress.
The consequence increase in the levels of NO and decrease in the levels of TAC activity among patients with cirrhosis and fibrosis were reported as Oxidative stress parameters compared with ASC subjects. These data were in line with other studies that reported the role of oxidative stress in potentiating the pathogenesis and progression of liver diseases.Citation9,Citation20,Citation78–Citation84,Citation86,Citation87 Also, the decrease in vitamin D levels of the studied subjects was positively correlated with changes in both NO and TAC in CHC patients with varying pathological lesions.
Previous studies have reported that low vitamin D levels were estimated in >92% of HCV patients, with 25% of these patients suffering from severe vitamin D deficiency.Citation21,Citation88 It was proposed that HCV infection may affect direct or indirect 25-hydroxylation of vitamin D through cytokine induction or oxidative stress,Citation89 or suppression of 25(OH)D levels via a disruption in lipid metabolism.Citation90
On a molecular basis, little is known about events leading to cellular damage and progression of liver diseases among CHC patients; however, host cells and viral and environmental factors may have a significant role in the pathogenesis of liver. Apoptosis is one of the molecular processes that occurs in both normal and pathological organs to maintain tissue development and homeostasis.Citation91 Previous research has reported that hepatocyte apoptosis plays a role in liver fibrogenesis, persistent liver inflammation, and subsequent severe pathogenesis of the liver among patients with CHC.Citation92–Citation94
In the present study, higher rates of liver cell apoptosis were reported in CHC patients. There was a significant increase in the expression levels of ssDNA and sFas as apoptotic markers in patients with cirrhosis and fibrosis compared with ASC and healthy control subjects. The data were in line with many previous studies that reported significant increases in levels of ssDNA and sFas and their association with fibrosis.Citation95–Citation97 Similarly, a significant positive correlation was reported between increased ssDNA, sFas, and fibrosis levels in CHC patients,Citation95,Citation98 which suggests that increased levels of these markers may reflect the degree of liver fibrosis.
The overexpression of apoptotic markers was shown to be positively correlated with oxidative stress markers: NO, TAC, inflammatory cytokines, TNFα, AFP, viral load, and HCV RNA. The data were matched with previous reports that supported the association of apoptosis with the expression of oxidative stress,Citation87 inflammatory cytokines,Citation93 and HCV-infection parameters.Citation99–Citation101 Also, deficits in vitamin D levels showed positive significant correlation with apoptosis among our CHC patients under investigation. Our data are in agreement with previous studies supporting the association of vitamin D-deficient status and excessive expression of Fas and ssDNA apoptotic markers,Citation17,Citation95,Citation102 whereas long-term vitamin D deficiency can activate chronic inflammation and subsequently higher oxidative stress that can induce apoptosis.
Finally, HCV genotype 4 is the most prevalent genotype in North Africa, the Middle East, and central and east sub-Saharan Africa, and prevalence has been increasing in Europe.Citation103–Citation106 Our study is considered the first to evaluate the potential role of 25(OH)D status in the progression of liver fibrosis via apoptotic and oxidative mechanisms in geriatric patients with HCV genotype 4.
Conclusion
The data showed that vitamin D status was significantly correlated with the pathogenesis and fibrogenesis of the liver in geriatric patients infected with HCV genotype 4. The deficiency in vitamin D levels was shown to have severe pathological effects on the liver via apoptosis, oxidative stress, and inflammatory mechanistic pathways. The data suggest that adequate vitamin D levels be recommended for good response to treatment strategies, especially in older CHC patients.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project RGP-VPP-240.
Disclosure
The authors report no conflicts of interest in this work.
References
- AliISiddiqueLRehmanLUPrevalence of HCV among the high risk groups in Khyber PakhtunkhwaVirol J2011829621663685
- AshfaqUAJavedTRehmanSNawazZRiazuddinSAn overview of HCV molecular biology, replication and immune responsesVirol J2011816121477382
- AttaullahSKhanSAliIHepatitis C virus genotype in Pakistan: a systemic reviewVirol J2011843321902822
- AverhoffFMGlassNHoltzmanDGlobal burden of hepatitis C: considerations for healthcare providers in the United StatesClin Infect Dis201255Suppl 1S101S105
- HoofnagleJHCourse and outcome of hepatitis CHepatology200236S21S2912407573
- RobinsonWSHepatitis B virus and hepatitis D virusMandelGLBennettJEDolinRPrinciples and Practice of Infectious Diseases5th edNew YorkChurchill Livingston200016521684
- MottolaGCardinaliACeccacciCHepatitis C virus nonstructural proteins are localized in a modified endoplasmic reticulum of cells expressing viral subgenomic repliconsVirology2002293314311853397
- ZhangCHXuGLJiaWDLiJSMaJLGeYSEffects of interferon treatment on development and progression of hepatocellular carcinoma in patients with chronic virus infection: a meta-analysis of randomized controlled trialsInt J Cancer20111291254126421710498
- LoguercioCFedericoAOxidative stress in viral and alcoholic hepatitisFree Radic Biol Med20033411012498974
- Abd EllahMRThe role of liver biopsy in detection of hepatic oxidative stressVet Med Int20111417
- OkudaMLiKBeardMRMitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core proteinGastroenterology200212236637511832451
- HalliwellBThe wanderings of free radicalsFree Radic Biol Med20094653154219111608
- TakagiHKakizakiSSoharaNPilot clinical trial of the use of alpha-tocopherol for the prevention of hepatocellular carcinoma in patients with liver cirrhosisInt J Vitam Nutr Res20037341141514743544
- WarisGAhsanHReactive oxygen species: role in the development of cancer and various chronic conditionsJ Carcinog200651416689993
- PatelTApoptosis in hepatic pathophysiologyClin Liver Dis2000429531711232194
- TsamandasACThomopoulosKGogosCExpression of bcl-2 oncoprotein in cases of acute and chronic viral hepatitis type B and type C: a clinicopathologic studyDig Dis Sci2002471618162412141825
- FingasCDAltinbasASchlattjanMExpression of apoptosis- and vitamin D pathway-related genes in hepatocellular carcinomaDigestion20138717618123635474
- RefaatBAshourTHEl-ShemiAGRibavirin induced anaemia: the effect of vitamin D supplementation on erythropoietin and erythrocyte indices in normal Wistar ratInt J Clin Exp Med201472667267625356124
- HuangYWLiaoYTChenWVitamin D receptor gene polymorphisms and distinct clinical phenotypes of hepatitis B carriers in TaiwanGenes Immun201011879319693091
- Abu-MouchSFiremanZJarchovskyJZeinaARAssyNVitamin D supplement improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patientsWorld J Gastroenterol2011175184519022215943
- PettaCCammàCScazzoneCLow vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis CHepatology2010511158116720162613
- AtsukawaMTsubotaAShimadaNEfficacy of alfacalcidol on PEG-IFN/ribavirin combination therapy for elderly patients with chronic hepatitis C: a pilot studyHepat Mon201313e1487224403915
- AtsukawaMTsubotaAShimadaNSerum 25(OH)D3 levels affect treatment outcomes for telaprevir/PEG-interferon/ribavirin combination therapy in genotype 1B chronic hepatitis CDig Liver Dis20144673874324880716
- AtsukawaMTsubotaAShimadaNSerum 25-hydroxyvitamin D levels affect treatment outcome in PEGylated interferon/ribavirin combination therapy for compensated cirrhotic patients with hepatitis C virus genotype 1B and high viral loadHepatol Res2014441277128524417888
- LeeSSFerenciPOptimizing outcomes in patients with hepatitis C virus genotype 1 or 4Antivir Ther200813Suppl 191618432158
- MuntanéJTargeting cell death and survival receptors in hepatocellular carcinomaAnticancer Agents Med Chem20111157658421554206
- BluttSEMcDonnellTJPolekTCWeigelNLCalcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2Endocrinology2000141101710614618
- KwonHJWonYSSuhHWVitamin D3 upregulated protein 1 suppresses TNF-α-induced NF-κB activation in hepatocarcinogenesisJ Immunol20101853980398920826751
- DahlanYAtherHMAl-AhmadiMBatwaFAl-HamoudiWSustained virological response in a predominantly hepatitis C virus genotype 4 infected populationWorld J Gastroenterol2009154429443319764095
- El HusseinyNMFahmyHMMohamedWAAminHHRelationship between vitamin D and IL-23, IL-17 and macrophage chemoattractant protein-1 as markers of fibrosis in hepatitis C virus EgyptiansWorld J Hepatol2012424224722993666
- SchaalanMFMohamedWAAminHHVitamin D deficiency: correlation to interleukin-17, interleukin-23 and PIIINP in hepatitis C virus genotype 4World J Gastroenterol2012183738374422851868
- NagpalSNaSRathnachalamRNoncalcemic actions of vitamin D receptor ligandsEndocr Rev20052666268715798098
- PettaSGrimaudoSMarcoVDAssociation of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patientsJ Viral Hepat20132048649323730842
- MohamedAASabryNAAbbassiMMIbrahimWAAli-EldinZAVitamin D levels in Egyptian HCV patients (genotype 4) treated with PEGylated interferonActa Gastroenterol Belg201376384423650781
- AsahinaYTsuchiyaKTamakiNEffect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infectionHepatology20105251852720683951
- IkedaKSaitohSAraseYEffect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysisHepatology1999291124113010094956
- ZhangQCaoLYChengSJZhangAMJinXSLiYP53-induced microRNA-1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIBOncol Rep2015331335134125591821
- Abdel-HamidMEl-DalyMMolnegrenVGenetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinomaJ Gen Virol2007881526153117412982
- AllamAGabrSAjaremJAbdel-MaksoudMBcl-2 and p53 expression in hepatic tissues of Egyptian patients with chronic hepatitis CJ Pak Med Assoc2015651186119226564290
- KaziAMKhalidWQuestionnaire designing and validationJ Pak Med Assoc20126251451622755326
- MatthewsDRHoskerJPRudenskiASNaylorBATreacherDFTurnerRCHomeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia1985284124193899825
- AlghadirAHGabrSAAnwerSAl-EisaEFatigue and oxidative stress response to physical activity in type 2 diabetic patientsInt J Diabetes Dev Ctries2016365964
- TsuchiyaMAsadaAKasaharaESmoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasmaCirculation20021051155115711889006
- BedossaPIntraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis CHepatology19942015208020885
- HolickMFVitamin D: evolutionary, physiological and health perspectivesCurr Drug Targets20111241820795941
- FisherLFisherAVitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver diseaseClin Gastroenterol Hepatol2007551352017222588
- HeaneyRPThe vitamin D requirement in health and diseaseJ Steroid Biochem Mol Biol200597131916026981
- ThomopoulosKCArvanitiVTsamantasACPrevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosisEur J Gastroenterol Hepatol20061823323716462535
- PoortahmasebiVAlavianSMKeyvaniHNorouziMMahmoodiMJazayeriSMHepatic steatosis: prevalence and host/viral risk factors in Iranian patients with chronic hepatitis B infectionAsian Pac J Cancer Prev2014153879388424935567
- BaurKMertensJCSchmittJCombined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patientsLiver Int20123263564322151003
- ArouchaDCdo CarmoRFMouraPHigh tumor necrosis factor-α/interleukin-10 ratio is associated with hepatocellular carcinoma in patients with chronic hepatitis CCytokine20136242142523602201
- NeumanMGSchmilovitz-WeissHHilzenratNMarkers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis CInt J Hepatol2012201223121022530132
- El RazikyMFathalahWFEl-AkelWAThe effect of peginterferon alpha-2a vs. peginterferon alpha-2b in treatment of naive chronic HCV genotype-4 patients: a single centre Egyptian studyHepat Mon201313e1006923922556
- KnoblerHSchattnerATNF-α, chronic hepatitis C and diabetes: a novel triadQJM2005981615625348
- EvansJLMadduxBAGoldfineIDThe molecular basis for oxidative stress-induced insulin resistanceAntioxid Redox Signal200571040105215998259
- AwadMDShihaGESallamFAMohamedAEl TawabAEvaluation of liver stiffness measurement by fibroscan as compared to liver biopsy for assessment of hepatic fibrosis in children with chronic hepatitis CJ Egypt Soc Parasitol20134380581924640880
- HolickMFVitamin D deficiencyN Engl J Med200735726628117634462
- Pérez-LópezFRVitamin D and its implications for musculoskeletal health in women: an updateMaturitas20075811713717604580
- LookerACMussolinoMESerum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adultsJ Bone Miner Res20082314315017907920
- PeterlikMCrossHSVitamin D and calcium deficits predispose for multiple chronic diseasesEur J Clin Invest20053529030415860041
- TrépoEOuzielRPradatPMarked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver diseaseJ Hepatol20135934435023557869
- Putz-BankutiCPilzSStojakovicTAssociation of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver diseaseLiver Int20123284585122222013
- GerovaDIGalunskaBTIvanovaIIPrevalence of vitamin D deficiency and insufficiency in Bulgarian patients with chronic hepatitis C viral infectionScand J Clin Lab Invest20147466567225005344
- MalhamMJørgensenSPOttPVitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiologyWorld J Gastroenterol20111792292521412501
- BarchettaICarottiSLabbadiaGLiver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virusHepatology2012562180218722753133
- YamamotoNHommaSVitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytesProc Natl Acad Sci U S A199188853985431924312
- WhitePCookeNThe multifunctional properties and characteristics of vitamin D-binding proteinTrends Endocrinol Metab20001132032710996527
- MasudaSOkanoTOsawaKShinjoMSuematsuTKobayashiTConcentrations of vitamin D-binding protein and vitamin D metabolites in plasma of patients with liver cirrhosisJ Nutr Sci Vitaminol (Tokyo)1989352252342585144
- García-ÁlvarezMPineda-TenorDJiménez-SousaMAFernández-RodríguezAGuzmán-FulgencioMResinoSRelationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: a meta-analysisHepatology2014601541155024975775
- AfzalSBrøndum-JacobsenPBojesenSENordestgaardBGVitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation studyLancet Diabetes Endocrinol2014229830624703048
- LiuEMeigsJBPittasAGPlasma 25 hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adultsJ Nutr200913932933419106328
- LimJSMietus-SnyderMValenteASchwarzJMLustigRHThe role of fructose in the pathogenesis of NAFLD and the metabolic syndromeNat Rev Gastroenterol Hepatol2010725126420368739
- GoldnerWSStonerJAThompsonJPrevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controlsObes Surg20081814515018175194
- WeintraubSJFleckensteinJFMarionTNMadeyMAMahmoudiTMSchechtmanKBVitamin D and the racial difference in the genotype 1 chronic hepatitis C treatment responseAm J Clin Nutr2012961025103123015322
- DasarathyJPeriyalwarPAllampatiSHypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver diseaseLiver Int201434e118e12724118743
- TargherGBertoliniLScalaLAssociations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver diseaseNutr Metab Cardiovasc Dis20071751752416928437
- GrassoAMalfattiFDe LeoPInsulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirinJ Hepatol20095198499019695729
- AlvarezJAAshrafARole of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasisInt J Endocrinol2010201035138520011094
- YinYYuZXiaMLuoXLuXLingWVitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolismEur J Clin Invest2012421189119622958216
- BilzerMRoggelFGerbesALRole of Kupffer cells in host defense and liver diseaseLiver Int2006261175118617105582
- Gascon-BarréMDemersCMirshahiANéronSZalzalSNanciAThe normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cellsHepatology2003371034104212717384
- AdamsJSHewisonMUnexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunityNat Clin Pract Endocrinol Metab20084809018212810
- NgTIMoHPilot-MatiasTIdentification of host genes involved in hepatitis C virus replication by small interfering RNA technologyHepatology2007451413142117518369
- MoustafaAHAliEMMohamedTMAbdouHIOxidative stress and thyroid hormones in patients with liver diseasesEur J Intern Med20092070370819818291
- ParolaMRobinoGOxidative stress related molecules and liver fibrosisJ Hepatol20013529730611580156
- GabrSAAlghadirAHPrediction of fibrosis in hepatitis C patients: assessment using hydroxyproline and oxidative stress biomarkersIndian J Virol20142591100
- OsmanHGGabrOMLotfySGabrSSerum levels of bcl-2 and cellular oxidative stress in patients with viral hepatitisIndian J Med Microbiol20072532332918087079
- LangeCMBibertSKutalikZA genetic validation study reveals a role of vitamin D metabolism in the response to interferon-alfa-based therapy of chronic hepatitis CPLoS One20127e4015922808108
- BellecavePSarasin-FilipowiczMDonzéOCleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon systemHepatology2010511127113620044805
- ClarkPJThompsonAJVockDMHepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific mannerHepatology201256495622318926
- ThompsonCBApoptosis in the pathogenesis and treatment of diseaseScience1995267145614627878464
- FischerRBaumertTBlumHHepatitis C virus infection and apoptosisWorld J Gastroenterol2007134865487217828818
- ValvaPDe MatteoEGaloppoMCGismondiMIPreciadoMVApoptosis markers related to pathogenesis of pediatric chronic hepatitis C virus infection: M30 mirrors the severity of steatosisJ Med Virol20108294995720419808
- ZuckermanEZuckermanTSaharDBcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infectionBr J Haematol200111236436911167830
- LapinskiTWPanasiukAJaroszewiczJKowalczukOFlisiakRRogalskaMSpecific ssDNA concentration in liver tissue as an index of apoptosis in hepatitis C virus-infected patientsWorld J Gastroenterol2005116130613316273639
- HahnCSChoYGKangBSLesterIMHahnYSThe HCV core protein acts as a positive regulator of Fas-mediated apoptosis in a human lymphoblastoid T cell lineVirology200027612713711022001
- ZhuNWareCFLaiMMHepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptorVirology200128317818711336543
- ToyodaMKakizakiSHoriguchiNRole of serum soluble Fas/soluble Fas ligand and TNF-α on response to interferon-α therapy in chronic hepatitis CLiver20002030531110959809
- CaponeFCostantiniSGuerrieroESerum cytokine levels in patients with hepatocellular carcinomaEur Cytokine Netw2010219910420478763
- PiankoSPatellaSOstapowiczGDesmondPSievertWFas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infectionJ Viral Hepat2001840641311703571
- Di MartinoVBrenotCSamuelDInfluence of liver hepatitis C virus RNA and hepatitis C virus genotype on Fas-mediated apoptosis after liver transplantation for hepatitis CTransplantation2000701390139611087158
- ZhuLKongMHanYPSpontaneous liver fibrosis induced by long term dietary vitamin D deficiency in adult mice is related to chronic inflammation and enhanced apoptosisCan J Physiol Pharmacol20159338539425894394
- SchnellGTripathiRBeyerJHepatitis C virus genotype 4 resistance and subtype demographic characterization of patients treated with ombitasvir plus paritaprevir/ritonavirAntimicrob Agents Chemother2015596807681526282418
- GowerEEstesCBlachSRazavi-ShearerKRazaviHGlobal epidemiology and genotype distribution of the hepatitis C virus infectionJ Hepatol201461S45S5725086286
- KamalSMImproving outcome in patients with hepatitis C virus genotype 4Am J Gastroenterol20071022582258817900328
- KamalSMNasserIAHepatitis C genotype 4: what we know and what we don’t yet knowHepatology2008471371138318240152
