Abstract
Background
The prevalence of chronic pain and sleep disturbances substantially increases with age. Pharmacotherapy remains the primary treatment option for these health issues. However, side effects and drug interactions are difficult to control in elderly individuals.
Aims
The objective of this study was to assess the feasibility of conducting a randomized sham-controlled trial and to collect preliminary data on the efficacy of transcranial direct current stimulation (tDCS) to reduce pain and improve sleep in older adults suffering from chronic pain.
Methods
Fourteen elderly individuals (mean age 71±7 years) suffering from chronic pain and sleep complaints were randomized to receive either anodal tDCS, applied over the primary motor cortex (2 mA, 20 minutes), or sham tDCS, for 5 consecutive days. Pain was measured with visual analog scales, pain logbooks and questionnaires, while sleep was assessed with actigraphy, sleep diaries and questionnaires.
Results
There were no missing data for pain and sleep measures, except for actigraphy, that generated several missing data. Blinding was maintained throughout the study, for both the evaluator and participants. Active but not sham tDCS significantly reduced pain (P<0.05). No change was observed in sleep parameters, in both the active and sham tDCS groups (all P≥0.18).
Conclusion
The present study provides guidelines for the implementation of future tDCS studies in larger populations of elderly individuals. M1 anodal tDCS in this population appears to be effective to reduce pain, but not to improve sleep.
Introduction
Chronic pain and sleep disorders are recognized worldwide as health problems having a significant impact on quality of life and productivity.Citation1,Citation2 A vicious circle seems to govern the interaction between pain and sleep disorders, with chronic pain leading to sleep disturbances and poor sleep leading to enhanced pain perception.Citation3–Citation8 It is estimated that over 67% of people experiencing chronic pain also complain about their sleep.Citation9,Citation10
The prevalence of chronic pain and sleep disorders increases considerably with age.Citation11–Citation14 Pharmacotherapy remains the first line of treatment to alleviate sleep disorders and chronic pain symptoms.Citation14–Citation17 Despite the availability of many pharmacological approaches, sleep problems and pain often persist.Citation18 In addition, polypharmacy is an important challenge in elderly individuals, a population in which drug interactions are frequent, and often complex to manage.Citation19
Past studies have shown that noninvasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS), can improve sleep,Citation20 alleviate painCitation20–Citation24 and reduce medication consumption and related side effects.Citation25,Citation26 However, the majority of these studies were performed on young adults suffering from neurogenic pain syndromes, and it is still unknown if tDCS could be a valid treatment option for elderly individuals suffering from chronic musculoskeletal pain. Investigating the effect of tDCS on older populations is crucial given the age-related changes observed in brain function and anatomy.Citation27,Citation28 Compared to young adults, elderly individuals show reduced gray matter perfusion and cerebral oxygen metabolic rate.Citation27 Reduction in brain volume has also been documented with aging.Citation29 Reduction in brain volume increases skull–cortex distance, which can presumably affect the effect of tDCS.Citation29,Citation30 The aim of the present study was to assess the feasibility of conducting a randomized, double-blind, sham-controlled trial and to collect preliminary data on the efficacy of tDCS to reduce pain and improve sleep in older adults suffering from chronic musculoskeletal pain.
Materials and methods
Participants
Fourteen elderly individuals were included in the study. Individuals were regarded as suitable to participate if they fulfilled the following criteria: 1) aged 60 years or over, 2) reported stable musculoskeletal pain in the previous 3 months or more and 3) had a score higher than 7/28 at the Insomnia Severity Index.Citation31 Participants with tDCS contraindications, such as neurological or neuropsychiatric conditions (eg, stroke, traumatic brain injury), history of brain surgery or tumor, metallic implants, epilepsy or history of substance abuse or dependence, were excluded. Participants were asked to keep their medication and life habits stable for the duration of the study. Participants were also asked not to consume nicotine and caffeine at least 6 hours before each visit. The experiment took place at the Research Centre on Aging of CIUSSS de l’Estrie-CHUS (Sherbrooke, QC, Canada). Subjects were all French-speaking community-dwelling individuals. The study was approved by the Research Ethics Committee of CIUSSS de l’Estrie-CHUS, and written informed consent was obtained from all participants. All procedures performed were in accordance with the ethical standards of the local institutional research committee and with the Declaration of Helsinki of 1964 and its later amendments or comparable ethical standards.
Experimental design
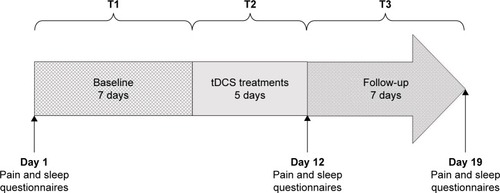
A randomized, parallel, double-blind, sham-controlled design was used. The study lasted 19 days and was divided into 3 phases: 1) a 7-day baseline evaluation; 2) a 1-week double-blind treatment period, which consisted of 5 consecutive daily treatment sessions of sham or active tDCS and 3) a 7-day follow-up period (). Throughout the 19 days of the study, daily measures of sleep and pain were recorded. Randomization to sham or active tDCS was performed using a random numbers table with a ratio of 1:1, based on order of entry of the participants in the study. The randomized table was designed to assign a total of 16 subjects to the study (8 in each group).
Figure 1 The study lasted 19 days and was divided into 3 phases: T1 (baseline), T2 (tDCS treatments) and T3 (follow-up). Pain and sleep questionnaires were completed at the beginning of the study (day 1), after the 5 tDCS sessions (day 12) and after the 7 days of follow-up (day 19). Actigraphic measures were taken from day 1 to day 19. Pain and sleep logbooks were completed each day, at home, by the participants, from day 1 to day 19.
Pain measurements
Pain intensity was evaluated with a visual analog scale (VAS) and a pain logbook. The VAS is a self-assessment scale of 10 cm that ranges from “no pain” (0 cm) to “the worst imaginable pain” (10 cm). This scale is widely used to evaluate pain outcome in studies, and its validity is well established.Citation32,Citation33 Participants had to rate pain intensity in the laboratory with a VAS once on day 1 and day 19, and before and after each tDCS session (day 8 to day 12). In addition to the VAS pain score, participants were asked to rate their daily pain in a pain assessment logbook containing 3 numerical rating scales (NRSs) ranging from 0 to 10 (0= no pain; 10= maximal pain). These 3 NRSs were used to evaluate the pain felt by the participant 1) at its least during the last 24 hours (minimal pain), 2) at its worst during the last 24 hours (maximal pain) and 3) on average in the last 24 hours (average pain). The pain assessment logbook was filled out by the participant at the end of each day throughout the duration of the study. The NRSs have been shown to be reliable and valid to measure pain intensity in elderly patients with persistent pain.Citation34 Pain assessments with the VAS were used in the laboratory to measure current pain, while the pain logbook was used to reflect the pain for each day. Although the pain intensity measurement is essential, it captures only part of the pain experience in older patients and should be supplemented by other pain measures.Citation33,Citation34 Consequently, pain questionnaires such as the McGill Pain Questionnaire (MPQ) and the Short Form of the Brief Pain Inventory (SF-BPI) were used to assess qualitative aspects of pain and physical functioning, respectively. These questionnaires were completed 3 times during the study (ie, on day 1, 12 and 19; ). The validity and reliability of both questionnaires have been previously documented.Citation35–Citation38
Sleep measurements
Sleep efficiency, which is the ratio of total time spent in bed to total time actually spent sleeping, was evaluated with actigraphy (Model Actiwatch; Philips – Respironics, Murrysville, PA, USA). Actigraphic records were also used to quantify nocturnal awakenings and estimate sleep-onset latency. Participants were asked to wear the actigraph device on their nondominant wrist throughout the duration of the study. Actigraphy has been shown to be a reliable method of recording activity during sleep.Citation39,Citation40 Actigraphic recordings were supplemented with the data collected in the sleep diary; during their participation in the study, from day 1 to day 19, participants were asked to fill out a sleep diary collecting information on the quality and quantity of sleep that they perceived. In addition to the actigraph and the sleep diary, the Pittsburgh Sleep Quality Index (PSQI) was used to assess the participant’s sleep quality. The PSQI showed a strong validity and reliability in both clinical and nonclinical samples.Citation41 Finally, 1 question of the SF-BPI, based on a 0–10 NRS, and labeled “Circle the one number that describes how, during the past 24 hours, pain has interfered with your sleep”, was used to assess pain interference with sleep (0= does not interfere; 10= completely interferes).
tDCS protocol
Participants were seated comfortably in an armchair during the 5 tDCS treatment sessions. The stimulations for a given subject were always done by the same investigator who was different from the evaluator. The investigator was responsible for the assignment of participants to the active or sham tDCS group, while the evaluator and the participants were blinded. The stimulations were always given in the afternoon or in the evening to get as close as possible to the actual time period of the night of sleep. Direct current was transferred to the subject by a saline-soaked pair of surface sponge electrodes (5×7 cm) and delivered by a constant current stimulator, battery-driven, 1×1 tDCS device (Model 1300-A; Soterix Medical Inc, New York, NY, USA). Participants received either anodal stimulation of the primary motor cortex (M1) or sham stimulation of M1. The anodal electrode was placed over M1, contralateral to the most painful site (C3 or C4 per the electroencephalogram 10/20 system), and the cathodal electrode was placed on the supraorbital area contralateral to the anode. During active tDCS, a constant anodal current of 2 mA was delivered for 20 minutes. This anodal tDCS procedure is known to increase cortical excitability and to reduce pain.Citation20,Citation21,Citation23,Citation42,Citation43 This procedure has also been shown to improve sleep in patients suffering from fibromyalgia.Citation20 For the sham stimulation, the electrodes were placed in the same montage as the active tDCS; however, current was applied only for the initial and final 30 seconds. Therefore, the patients felt the ramp-up and ramp-down itching sensation of the current, but received no current for the rest of the stimulation period. This placebo procedure has been validated and is now successfully used in most studies using tDCS.Citation44–Citation46 The tDCS device was set by the manufacturer to automatically provide this type of sham stimulation.
Data analysis
The feasibility of the study was evaluated with regard to the challenges incurred by the data collection and the number of missing data for every pain and sleep measurement tool. Also documented were the number of participants who completed the study, the ability to maintain blinding for the evaluator and participants, as well as randomization issues.
Preliminary analysis on the effectiveness of the tDCS intervention was also conducted. To facilitate interpretation and to reduce the number of statistical comparisons, pain intensity ratings (pain logbook) and actigraphy measurements (sleep efficiency, sleep-onset latency and number of nocturnal awakenings) were averaged into 3 scores, reflecting the 3 phases of the study (ie, before, during and after tDCS treatment). As shown in , T1 represents the 7 days of baseline, T2 corresponds to the 5 days of tDCS treatments and T3 represents the 7 days of follow-up. The mean values were used for all analyses. Percentages of change were also calculated to directly compare the efficacy of active tDCS and sham tDCS on pain and sleep, based on the following formula: percentage of change = [(score during or after treatment (T2 or T3) − score before treatment (T1))/score before treatment (T1)] ×100.
The study was designed to detect a clinically important difference of 2 points on the 0–10 pain intensity scale.Citation47 To detect this difference with 80% power and a 5% significance level, we determined that 16 individuals had to be enrolled in the study (estimated standard deviation of 1.3, based on previous tDCS studiesCitation23,Citation24). Because of the low number of subjects, and since visual inspection of the histograms did not allow us to assume that the data were normally distributed, nonparametric tests were used for all the statistical analyses. Specifically, Mann–Whitney tests (continuous variables) and Fisher’s exact tests (categorical variables) were used to compare the 2 groups (between-subject analyses). This allowed us to evaluate if the outcome measures were different between the active and sham tDCS groups. Friedman tests and Wilcoxon signed-rank tests were also used to compare if the intervention affected the outcome measures in each group (intragroup analyses). All tests were performed using SPSS (version 17.0 for Windows; SPSS Inc., Chicago, IL, USA), and differences were considered to be significant if P>0.05 was obtained. Bonferroni corrections were applied to prevent type I errors.
Results
Participants’ characteristics
Sixteen elderly individuals aged between 62 and 84 years (mean age 71±7 years; 3 men) were included in the study. Two participants from the active tDCS group dropped out of the study, 1 because of a family event (death of a loved one) and 1 because of a personal matter (withdrawal of driver’s license). The demographic and general clinical characteristics of the remaining 14 participants are summarized in . Participants had no change in medication during the last 3 months. Exploratory analyses and visual inspection of the data revealed that the medication had no effect on the response to tDCS, for pain as well as for sleep.
Table 1 Clinical and demographic characteristics of the participants
Pain outcomes
There were no missing data for all pain outcomes. The participants correctly filled all pain measures and questionnaires. However, the VAS often needed explanations before the participants could fully grasp the concept evaluated. After the first laboratory session, the concept of pain measures was well understood by the participants, and all the daily pain logbooks were completed correctly.
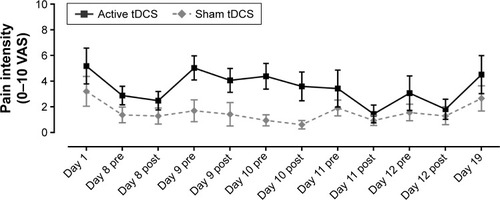
The VAS pain scores obtained on day 1 and day 19, and before and after each tDCS session (day 8 to day 12) in the laboratory are presented in . As it can be seen from this figure, no significant change was observed immediately after each tDCS session, for both the active and sham tDCS group. The absence of short-term effect was confirmed by the Friedman and Wilcoxon signed-rank tests, which revealed no significant intragroup differences for both treatment groups (all P≥0.17). Mann–Whitney tests also showed that there were no between-group differences for all time measures (all P≥0.11) suggesting, again, that tDCS has no short-term effect on pain.
Figure 2 Pain intensity scores measured with VAS for active and sham tDCS groups. Pain scores were obtained once on day 1 and day 19, and before and after each tDCS session (day 8 to day 12). Each point represents group mean ± standard error of mean.
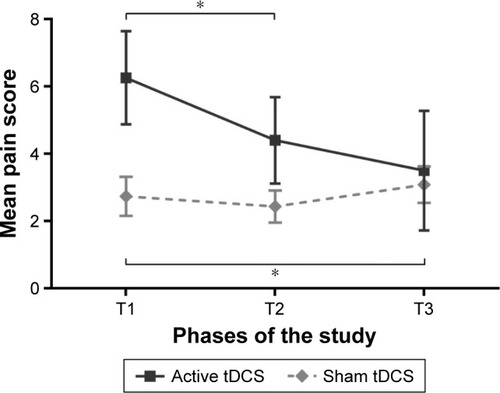
Daily average pain ratings, obtained via the pain logbook for the 3 phases of the study, are presented in and in . As can be seen from this figure, daily average pain ratings decreased among the active tDCS group and slightly increased among the sham tDCS group. This pattern of results was confirmed by the Friedman tests, which revealed a change in pain for both tDCS conditions (both P≤0.03). For the active tDCS group, post hoc Wilcoxon signed-rank tests revealed that there was a significant reduction in daily average pain during (T2; P=0.04) tDCS treatments, when compared to baseline (T1). A significant trend was also observed for T3 (P=0.06). In contrast, post hoc Wilcoxon signed-rank tests showed that sham tDCS slightly increased pain at T3 when compared to T1 (+0.34±0.29; P=0.04).
Table 2 Etiology of pain and daily average pain ratings of the 3 phases of the study
Figure 3 The average daily pain for sham and active treatment groups gathered using the pain logbook. T1 represents the 7 days of baseline, T2 corresponds to the 5 days of tDCS treatments and T3 represents the 7 days of follow-up. Each point represents a group mean ± standard error of mean. There was a significant difference between T1 and T2 in the active tDCS group and between T1 and T3 in sham tDCS group. *Statistically significant (P<0.05).
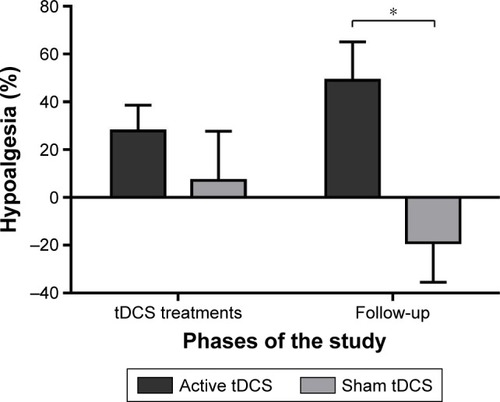
To better evaluate the effect of active and sham tDCS, percentages of hypoalgesia were calculated (). On one hand, active tDCS produced a reduction in pain of 28% at T2 and of 49% at T3. Both these percentages of pain reduction are clinically significant.Citation48 On the other hand, sham tDCS reduced pain by 7% at T2 and increased pain by 19% at T3. These percentages of change observed among the sham tDCS group are not clinically significant. Mann–Whitney tests comparing the active tDCS group and the sham tDCS group revealed a significant difference between the groups at T3 (P=0.008), but not at T2 (P=0.31).
Figure 4 Percentages of hypoalgesia calculated with the average pain on the day measured using the pain logbook. The first 2 columns represent hypoalgesia during the week of tDCS treatments (comparing T2 to T1), and the next 2 columns represent hypoalgesia during the 7 days of follow-up (comparing T3 to T1). Each column represents mean ± standard error of mean. *Statistically significant (P<0.05).
shows the results of pain questionnaires for each time point. Active tDCS generated a significant change in MPQ scores. Post hoc Wilcoxon signed-rank tests revealed that tDCS reduced MPQ scores at T2 and T3, when compared to baseline (T1) (all P≤0.04), suggesting that active tDCS can affect the qualitative aspects of pain. No changes over time were noted for physical functioning, as measured by the SF-BPI for both active and sham tDCS groups (P≥0.07). There were no between-group differences, according to both questionnaires, for all time measures (all P≥0.07).
Table 3 Pain and sleep questionnaires
Sleep outcomes
The actigraphic measures generated several missing data, and the analyses of sleep efficiency and sleep-onset latency were done only for 4 participants (2 participants by group), which dangerously reduced the power of the statistical analysis. Still, visual inspection of the diagrams showed no trend toward a difference between the 2 participants who received active tDCS and the 2 participants who received sham tDCS. Intragroup inspection revealed that active tDCS seems to decrease sleep efficiency at T2 and T3 (approximately −7% and −1%, respectively) (all P≥0.20), to increase sleep-onset latency at T2 (+12 minutes) and to decrease sleep latency at T3 (−34 minutes) (all P≥0.22).
There were no missing data for the number of nocturnal awakenings evaluated with actigraphy and for the sleep diary measures and sleep questionnaires (PSQI and SF-BPI). There was no change in nocturnal awakenings at T2 and T3 when compared to T1 in the active tDCS group (approximately +4 and +5 awakenings, respectively), nor in the sham tDCS group (approximately −8 awakenings for both time measures) (all P≥0.57). Mann–Whitney tests showed that there were no between-group differences in nocturnal awakenings for all time measures (all P≥0.69). The data collected with the sleep diaries revealed that patients observed no difference in their sleep during or after the active and sham tDCS treatments. shows that there was no change in sleep questionnaires scores during and after tDCS when compared to baseline, for both the active and sham tDCS group (all P≥0.12). Mann–Whitney tests also showed that there were no between-group differences for all time measures (all P≥0.22).
Discussion
The first objective of the present study was to provide information regarding the feasibility of conducting a randomized, double-blind, sham-controlled trial considering the efficacy of tDCS to reduce pain and improve sleep in older adults suffering from chronic musculoskeletal pain. Although no major obstacles prevented the completion of the study, some important recommendations can be made. First, using a stratified randomization strategy for key factors (eg, baseline pain levels) appears to be of primary importance.Citation49,Citation50 This is particularly true for small-sample-size studies, as conventional simple randomization methods can generate imbalances in baseline characteristics among groups.Citation49,Citation50 In the present study, despite randomization, the group that received active tDCS treatments had higher levels of baseline pain, compared to the group that received sham tDCS. Even if this difference was not statistically significant, baseline differences in core outcome measures are always problematic. In the present case, it remains possible that the pattern of results observed (ie, greater effect of active tDCS than sham tDCS) is partly attributable to the fact that it is easier to provide pain relief when initial pain is high.Citation48 Despite the fact that increasing the number of participants could help attenuate baseline group differences, we suggest that future studies should randomize patients according to their initial pain. Second, although pain measures and questionnaires were correctly understood by the participants, these measures often required several explanations before participants could fully grasp the concept evaluated. Even though the pain scales and questionnaires used in this study (eg, VAS, MPQ, Brief Pain Inventory) are designed to be self-administered, these observations indicate that the presence of a member of the research team is essential to ensure valid pain measures in studies conducted in elderly individuals. Of importance, the results show that the use of NRS (included in the pain logbooks) is appropriate to evaluate changes in the daily pain of elderly chronic pain patients. NRS appears to be a simpler tool to understand than VAS, an observation that is coherent with that of Dworkin et alCitation33 and Herr.Citation51
Blinding of the participants and of the evaluators is always a major concern of randomized controlled studies. In a commendable study published recently by O’Connell et al, the authors suggest that the use of a sham tDCS treatment, applied at an intensity of 2 mA, is hardly attainable.Citation52 Indeed, based on the sensation of the stimulation, 65% of the participants correctly judged the stimulation condition (active or sham), while evaluators noticed skin changes (redness) under the reference electrode more often following active tDCS than sham tDCS.Citation52 In our case, none of the participants, nor the evaluator, were able to distinguish between the 2 types of stimulation (sham or active tDCS).Citation52 Contrary to O’Connell et al, the participants included in this study were elderly individuals. Perhaps the age-related changes observed in tegumentary and sensory functions (eg, decreased sensation and skin thickness) could explain these discrepancies.Citation53–Citation55 Indeed, all the participants (including those who received sham tDCS, and therefore, only 60 seconds in total of real stimulation) presented some redness under the reference electrode.
Participants wore the actigraph without problems during the 19 days of the study. Although some studies consider that actigraphy is a valid method to assess sleep, it is important to mention that in our study, the use of actigraphy led to many missing data.Citation56,Citation57 The analyses revealed that many participants tended to be immobile in bed, even when awake, thus leading to several incorrect or missing data concerning sleep-onset latency (a measure that is also used to calculate sleep efficiency). Consequently, measuring sleep efficiency with actigraphy is probably not the best strategy in older populations. Although costlier and more time consuming, future studies should consider using polysomnography instead of actigraphy.Citation58 However, the absence of changes noted in the subjective measures of sleep (sleep diaries and questionnaires) sheds some doubt on the potential utility of tDCS to decrease sleep problems in elderly individuals and somewhat diminishes the interest for the implementation of a polysomnography study in this population. As for pain questionnaires, many participants needed help to complete the sleep questionnaires, highlighting once again the key role of the research team.
Effect of tDCS on pain intensity
Among the active tDCS group, there was a significant reduction in daily average pain during tDCS treatments (T2), when compared to baseline (T1). Of importance, the reduction in pain observed at T2 was clinically significant for patients suffering from chronic musculoskeletal pain.Citation48 Moreover, despite the fact that the reduction of the average daily pain in the follow-up (T3) was not statistically significant when compared with T1, it is important to note that the pain reduction provided by the active tDCS between T1 and T3 is almost 3 points on the NRS. Thus, this reduction in the average daily pain is clinically significant and associated with the concept of a “much better” improvement in pain.Citation48,Citation59 The large variability in tDCS-induced pain reductions observed at T3 in the active tDCS group can probably explain why this important difference did not reach statistical significance. Concerning the sham tDCS group, there was an increase in the average daily pain at T3 when compared to T1. Although these differences are statistically significant, they are not clinically significant.
Many of the previous studies considering the analgesic efficacy of tDCS were conducted with young or age-heterogeneous populations, and on patients suffering from neuropathic pain. In one of the first studies published on the effect of tDCS on pain, Fregni et alCitation21 evaluated the analgesic efficacy of tDCS in a group of 17 subjects with spinal cord injuries (mean age: 35.7±13.3 years). The authors reported that tDCS, applied for 20 minutes at 2 mA for 5 consecutive days, reduced pain in this patient population, suggesting that this stimulation protocol is effective in adults suffering from neuropathic pain, a conclusion that was substantiated by many other studies.Citation23,Citation42 These results contrast with those of Wrigley et al who observed no effect of tDCS on the pain in patients suffering from neuropathic pain conditions.Citation60 Very few studies looked into the analgesic efficacy of tDCS on chronic musculoskeletal pain. Antal et al have assessed the analgesic quality of tDCS in patients suffering from chronic musculoskeletal or neuropathic pain (age range: 41–70 years).Citation22 They reported that anodal tDCS produced a 30% reduction in pain after the fifth and last session of stimulation. Interestingly, this decrease in pain after 5 anodal sessions of tDCS was like that of the present study (average reduction of 28% when comparing T2 to T1). For their part, Schabrun et al observed a decrease in pain for almost 60% of patients with chronic low back pain (CLBP) (mean age: 30±2 years).Citation61 However, the age difference between the population in Schabrun et al’s study and the current study makes any comparison somewhat hazardous. A recent study published by Concerto et al assessed the analgesic efficiency of tDCS on 10 elderly patients with plantar fasciitis (mean age: 68.8±3.3 years).Citation62 This study showed a decrease in pain that was relatively identical to that of the current study (36.9% vs 28% during the 5 days of treatment and 42.4% vs 49% on the week after the treatment). To our knowledge, Concerto et al’s pilot study is the only other study that looked at the analgesic effect of tDCS in elderly individuals. Although interesting, it should be noted that the results of Concerto et al were obtained using an open-label design, with no placebo condition. This can be problematic given the important placebo effect noted in studies looking into the analgesic effect of brain stimulation techniques.Citation63
The long-term effect of tDCS has been measured in several studies. In one of their studies, Fregni et al reported that the pain improvement induced by 5 sessions of tDCS in women with fibromyalgia was still apparent 3 weeks after the stimulation sessions.Citation64 Similarly, Valle et al demonstrated that 10 sessions of tDCS could reduce pain in women suffering from fibromyalgia (mean age: 54.8±9.6 years) for a period of 60 days, whereas Auvichayapat et al reported that 20 sessions of tDCS produced a decrease in pain for a period of 12 weeks in patients suffering from migraines (mean age: 28.6±6.8 years).Citation65,Citation66 Increasing the number of tDCS sessions could produce cumulative and more long-lasting effects.Citation67 In contrast, O’Connell et al showed that tDCS did not produce a significant effect on the pain in patients with CLBP (mean age: 45±10 years), regardless of the number of sessions.Citation68
Effect of tDCS on sleep
To our knowledge, only 1 study specifically focused on the effect of tDCS on sleep structure in patients suffering from chronic pain. In this study, Roizenblatt et al aimed to determine if the application of tDCS in women suffering from fibromyalgia could decrease pain, as well as improve sleep quality.Citation20 Using polysomnography, the authors observed that 5 sessions of anodal tDCS stimulation applied over the primary motor cortex improved sleep parameters and architecture in patients suffering from fibromyalgia. In the present study, no benefits were observed on sleep variables following tDCS applications. It is important to note that, although the populations examined in this study and in the study of Roizenblatt et al both suffered from chronic pain, the type of pain, as well as the sex and the age of participants, was quite different.Citation20 Past research has shown that sleep mechanisms are different in elderly individuals when compared to young adults (eg, sleep–wake cycle, more frequent and longer periods of awakening, wake-up time in the morning, sleep satisfaction, percentage of slow-wave sleep and rapid eye movement sleep).Citation11,Citation14,Citation69–Citation72 These differences may explain why tDCS did not have an impact on the elderly, whereas it was effective in younger adults. Furthermore, contrary to fibromyalgia (in which sleep disorder is generally recognized as a primary symptomCitation73), the sleep disorders experienced by the participants of this study were secondary to pain problems. Perhaps, a longer follow-up would have allowed us to detect sleep changes in our participants.
Although they did not assess the effect of tDCS on sleep structure, other authors have focused on the effect that tDCS has on sleep. In one of these studies, Borckardt et al measured the analgesic effect of tDCS in patients who had undergone endoscopic retrograde cholangiopancreatography (ERCP).Citation26 They found that the patients who received real tDCS (10 women, mean age: 37.8±10.8 years) reported less pain interference with sleep (according to 1 question of the BPI) than those who received sham tDCS. In the present study, no change was noted following tDCS for the same question from the BPI. It is however important to note that the results of Borckardt et al’s study are the result of the analyses of only 24 hours post-ERCP.
Conclusion
The present study provides preliminary evidence on the efficacy of tDCS to reduce pain and improve sleep in older adults, as well as guidelines for the implementation of future studies. tDCS appears to be a promising therapeutic avenue for older individuals suffering from chronic musculoskeletal pain. Future studies evaluating the effect of tDCS on sleep should consider the shortcomings of actigraphic measures and should strongly consider using polysomnography.
Acknowledgments
The authors would like to thank Ms Marie-Claude Girard, Mr Antoine Guillerand and Ms Isabelle Viens for their help with data collection, as well as Mr Yannick Tousignant-Laflamme and Ms Anaïs Lacasse for their thoughtful comments on the manuscript. They also thank all the subjects who participated in this project. Part of this work served as an M.Sc. degree fulfillment by Marie-Philippe Harvey. Preliminary results of this research were presented as a poster at the International Neuromodulation Society 12th World Congress Neuromodulation: Medicine Evolving Through Technology in June 2015, and the abstract was published online at www.onlinelibrary.wiley.com/doi/10.1111/ner.12333/abstract. G Léonard is supported by the Fonds de Recherche en Santé (FRQ-S, Montréal, QC, Canada). This project was partially supported by the Neuroscience Centre of Excellence of the Université de Sherbrooke (CeNUS, Sherbrooke, QC, Canada) and an internal start-up fund from the Research Centre on Aging (Initiatives stratégiques du Centre de recherche sur le vieillissement, Sherbrooke, QC, Canada).
Disclosure
The authors report no conflicts of interest in this work.
References
- ManocchiaMKellerSWareJESleep problems, health-related quality of life, work functioning and health care utilization among the chronically illQual Life Res200110433134511763246
- MarchandSLe phénomène de la douleur [The Phenomenon of Pain]MontrealLes Éditions de la Chenelière Inc2009 French
- ChoCHJungSWParkJYSongKSYuKIIs shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance?J Shoulder Elbow Surg201322222222822738644
- FishbainDAColeBLewisJEGaoJWhat is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based reviewPain Med201011215817919788712
- OnenSHOnenFCourpronPDubrayCHow pain and analgesics disturb sleepClin J Pain200521542243116093748
- PurushothamanBSinghALingutlaKBhatiaCPollockRKrishnaMPrevalence of insomnia in patients with chronic back painJ Orthop Surg (Hong Kong)2013211687023629992
- Schuh-HoferSWodarskiRPfauDBOne night of total sleep deprivation promotes a state of generalized hyperalgesia – a surrogate pain model to study the relationship of insomnia and painPain201315491613162123707287
- StiefelFStagnoDManagement of insomnia in patients with chronic pain conditionsCNS Drugs200418528529615089114
- MorinCMLeBlancMDaleyMGregoireJPMeretteCEpidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviorsSleep Med20067212313016459140
- SmithMTHaythornthwaiteJAHow do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literatureSleep Med200482119132
- CrowleyKSleep and sleep disorders in older adultsNeuropsychology Rev20112114153
- GibsonSJLussierDPrevalence and relevance of pain in older personsPain Med201213Suppl 2S23S2622497744
- HelmeRDGibsonSJThe epidemiology of pain in elderly peopleClin Geriatr Med200117341743111459713
- Rissling MaA-ISUoCaSDSleep in agingHandbook of the Neuroscience of Aging2009373379
- BeaulieuPLussierDPorrecaFDickensonAHPharmacology of PainSeattleIASP Press2010
- MarchandSPharmacologie de la douleur [Pharmacology of Pain]1st edBeaulieuPMontrealPresses de l’Université de Montréal2005 French
- RoehrsTRothTInsomnia pharmacotherapyNeurotherapeutics20129472873822976558
- BenningerDKuntzerTTreatment of chronic pain: transcranial stimulation of the motor cortex?Rev Med Suisse2012833993593622675823
- BallentineNHPolypharmacy in the elderly: maximizing benefit, minimizing harmCrit Care Nurs Q2008311404518316935
- RoizenblattSFregniFGimenezRSite-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled studyPain Pract20077429730617986164
- FregniFBoggioPSLimaMCA sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injuryPain20061221–219720916564618
- AntalATerneyDKühnlSPaulusWAnodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibitionJ Pain Symptom Manage201039589090320471549
- KimYJKuJKimHJRandomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathyAnn Rehabil Med201337676677624466511
- YoonEJKimYKKimHRKimSELeeYShinHITranscranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic pet studyNeurorehabil Neural Repair201428325025924213958
- BorckardtJJReevesSTRobinsonSMTranscranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA)Clin J Pain2013291192592823370085
- BorckardtJJRomagnuoloJReevesSTFeasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot studyGastrointest Endosc20117361158116421470608
- De VisJBHendrikseJBhogalAAdamsAKappelleLJPetersenETAge-related changes in brain hemodynamics; a calibrated MRI studyHum Brain Mapp201536103973398726177724
- SowellERPetersonBSThompsonPMWelcomeSEHenkeniusALTogaAWMapping cortical change across the human life spanNat Neurosci20036330931512548289
- CrivelloFTzourio-MazoyerNTzourioCMazoyerBLongitudinal assessment of global and regional rate of grey matter atrophy in 1,172 healthy older adults: modulation by sex and agePLoS One2014912e11447825469789
- ClarkBCTaylorJLAge-related changes in motor cortical properties and voluntary activation of skeletal muscleCurr Aging Sci20114319219921529329
- MorinCMBellevilleGBelangerLIversHThe insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment responseSleep201134560160821532953
- PriceDDMcGrathPARafiiABuckinghamBThe validation of visual analogue scales as ratio scale measures for chronic and experimental painPain198317145566226917
- DworkinRHTurkDCFarrarJTIMMPACTCore outcome measures for chronic pain clinical trials: IMMPACT recommendationsPain20051131–291915621359
- WoodBMNicholasMKBlythFAsghariAGibsonSAssessing pain in older people with persistent pain: the NRS is valid but only provides part of the pictureJ Pain20101121259126620579940
- MelzackRThe McGill pain questionnaire: major properties and scoring methodsPain1975132772991235985
- MendozaTMayneTRubleeDCleelandCReliability and validity of a modified brief pain inventory short form in patients with osteoarthritisEur J Pain200610435336116051509
- PoundjaJFikretogluDGuaySBrunetAValidation of the French version of the brief pain inventory in Canadian veterans suffering from traumatic stressJ Pain Symptom Manage200733672072617531912
- VeilleuxSSDBohuonALe défi de la douleur [The Challenge of Pain]3rd edMelzackRWallPDEdisemSaint-Hyacinthe, QC, Canada1989 French
- MarinoMLiYRueschmanMNMeasuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnographySleep201336111747175524179309
- SadehAHauriPJKripkeDFLaviePThe role of actigraphy in the evaluation of sleep disordersSleep19951842883027618029
- MollayevaTThurairajahPBurtonKMollayevaSShapiroCMColantonioAThe Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysisSleep Med Rev201625527326163057
- MoriFCodecaCKusayanagiHEffects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosisJ Pain201011543644220018567
- NitscheMAPaulusWExcitability changes induced in the human motor cortex by weak transcranial direct current stimulationJ Physiol2000527Pt 363363910990547
- GandigaPCHummelFCCohenLGTranscranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulationClin Neurophysiol2006117484585016427357
- KesslerSKTurkeltaubPEBensonJGHamiltonRHDifferences in the experience of active and sham transcranial direct current stimulationBrain Stimul20125215516222037128
- PalmUReisingerEKeeserDEvaluation of sham transcranial direct current stimulation for randomized, placebo-controlled clinical trialsBrain Stimul20136469069523415938
- FarrarJTPortenoyRKBerlinJAKinmanJLStromBLDefining the clinically important difference in pain outcome measuresPain200088328729411068116
- SalaffiFStancatiASilvestriCACiapettiAGrassiWMinimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scaleEur J Pain20048428329115207508
- AltmanDGBlandJMHow to randomiseBMJ1999319721170370410480833
- KangMRaganBGParkJHIssues in outcomes research: an overview of randomization techniques for clinical trialsJ Athl Train200843221522118345348
- HerrKPain assessment strategies in older patientsJ Pain2011123 Suppl 1S3S1321396599
- O’ConnellNECossarJMarstonLRethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2 mAPLoS One2012710e4751423082174
- O’SullivanSBSchmitzTJBehrmanALPhysical RehabilitationPhiladelphiaF.A. Davis2007
- BowdenJLMcNultyPAAge-related changes in cutaneous sensation in the healthy human handAge (Dordr)20133541077108922661298
- FiroozARajabi-EstarabadiAZartabHPazhohiNFanianFJananiLThe influence of gender and age on the thickness and echo-density of skinSkin Res Technol2017231132027273751
- BlackwellTRedlineSAncoli-IsraelSComparison of sleep parameters from actigraphy and polysomnography in older women: the SOF studySleep200831228329118274276
- MartinJLHakimADWrist actigraphyChest201113961514152721652563
- KnauertMPYaggiHKRedekerNSMurphyTEAraujoKLPisaniMAFeasibility study of unattended polysomnography in medical intensive care unit patientsHeart Lung201443544545225023504
- HaefeliMElferingAPain assessmentEur Spine J200615Suppl 1S17S2416320034
- WrigleyPJGustinSMMcIndoeLNChakiathRJHendersonLASiddallPJLongstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trialPain2013154102178218423831866
- SchabrunSMJonesEElgueta CancinoELHodgesPWTargeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation interventionBrain Stimul20147345145924582372
- ConcertoCAl SawahMChusidEAnodal transcranial direct current stimulation for chronic pain in the elderly: a pilot studyAging Clin Exp Res201628223123726174129
- de CamposGCPlacebo effect in osteoarthritis: why not use it to our advantage?World J Orthop20156541642026085983
- FregniFGimenesRValleACA randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgiaArthritis Rheum200654123988399817133529
- ValleARoizenblattSBotteSEfficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trialJ Pain Manag20092335336121170277
- AuvichayapatPJanyacharoenTRotenbergAMigraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trialJ Med Assoc Thai20129581003101223061303
- CharvetLEKasschauMDattaARemotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocolsFront Syst Neurosci201592625852494
- O’ConnellNECossarJMarstonLTranscranial direct current stimulation of the motor cortex in the treatment of chronic nonspecific low back pain: a randomized, double-blind exploratory studyClin J Pain2013291263423221623
- LorrainDBoivinDPrécis pratique de gériatrie [Precis Practice of Geriastrics]3rd edArcandMHébertRActon ValeEdisem2007 French
- LandoltHPDijkDJAchermannPBorbélyAAEffect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged menBrain Res199673822052128955514
- LandoltHPBorbélyAAAge-dependent changes in sleep EEG topographyClin Neurophysiol2001112236937711165543
- MillmanRPSleep and agingMed Health R I2012953889022533226
- ChakrabartySZoorobRFibromyalgiaAm Fam Physician200776224725417695569
