Abstract
Objective
Vascular abnormality includes two forms, arteriosclerosis (ARS) and atherosclerosis (ATS), which coexist in patients with cardiovascular (CV) diseases. However, whether their combination may lead to a worsening status in those patients remains unclear. We therefore aimed to investigate the association of ARS and/or ATS with hypertensive target organ damage (TOD).
Methods
From June 2014 to August 2015, a total of 1,599 community-dwelling elderly subjects (aged >65 years) from northern Shanghai were recruited. Vascular measurements, such as carotid–femoral pulse wave velocity (cf-PWV), ankle–brachial index (ABI) and carotid plaque, were conducted on each participant, and ARS was defined as cf-PWV >12 m/s, while ATS was defined as participants who have carotid plaque or ABI <0.9. Within the framework of comprehensive CV examinations, CV risk factors were assessed, and asymptomatic TOD was evaluated by measuring participants’ left ventricular mass index (LVMI), peak transmitral pulsed Doppler velocity/early diastolic tissue Doppler velocity (E/Ea), urinary albumin–creatinine rate (UACR) and estimated glomerular filtration rate (eGFR).
Results
Although LVMI, E/Ea and eGFR were significantly different among subjects with or without ARS and/or ATS (P<0.02), in full adjustment model, only E/Ea showed the independent and significant difference (P=0.023). Moreover, E/Ea was significantly different between participants with ARS or ATS and those without ARS or ATS (P=0.045), while there was no significant difference between participants with ARS and ATS and those without ARS or ATS (P=0.28). Similar results were obtained in the multivariate logistic regression of left ventricular diastolic dysfunction (LVDD). With similar adjustment, LVDD was significantly associated with ATS (P=0.01) but not with ARS (P=0.99).
Conclusion
In the community-dwelling elderly Chinese, among hypertensive TOD, LVDD was significantly associated with ATS but not with ARS. The proportion of patients with LVDD was not significantly different despite the presence of both ATS and ARS, when compared to patients with ATS alone.
Introduction
Many cardiovascular (CV) events and deaths are tightly associated with vascular structural and functional changes. From a pathophysiological point of view, vascular abnormality includes two forms; one is arteriosclerosis (ARS) and the other is atherosclerosis (ATS). Both are critical, fatal or event-related.Citation1
Carotid–femoral pulse wave velocity (cf-PWV) is recognized as a gold standard for the assessment of patients’ arterial stiffness, while carotid plaque detection by ultrasound and measurement of ankle–brachial index (ABI) by the four-limb blood pressure (BP) monitoring are the common methods to detect patients’ ATS in routine clinical practice.Citation2 Several reviews and articles have reported that patients may simultaneously suffer from ARS and ATS, and these two vascular disorders were tightly linked.Citation3–Citation6 For instance, a large population study with 3,000 elderly subjects aged 60–101 years reported that arterial stiffness was intensively associated with ATS (P for trend <0.01).Citation7 Moreover, Huynh et alCitation8 proposed that arterial stiffening may affect endothelial cells in the vessel wall with aging and promote atherogenesis. In addition, Demer suggested a relationship between functional stiffening and ATS progression.Citation9 Therefore, it seems that ARS and ATS may commonly coexist in patients with cardiovascular diseases (CVD). Several previous studies have investigated the associations of ARS or ATS with some target organ damage (TOD).Citation10–Citation13 However, whether the combination of ARS and ATS would lead to a worsening prognosis or severe TOD remains unclear. Herein, we aimed to investigate the association of ARS and/or ATS with hypertensive TOD, within a framework of CV risk assessment in a community-dwelling elderly cohort.
Methods
Study population
The Northern Shanghai Study (NSS) is a community- dwelling prospective study. From June 2014 to August 2015, 1,721 residents were invited, of whom 1,599 (92.9%) participants were enrolled. All residents were included if they were, 1) aged 65 years or more; 2) local residents from urban communities in the north of Shanghai, and 3) available for long-term follow-up. Exclusion criteria were as follows: 1) severe cardiac disease (New York Heart Association IV) or end-stage renal disease (chronic kidney disease >4); 2) malignant tumor with life expectancy <5 years, and 3) stroke history within 3 months. The NSS was conducted and financially supported by the Shanghai municipal government (Grant ID, 2013ZYJB0902) and had the approval of Shanghai Tenth People’s Hospital Institutional Review Board. Written informed consent was obtained from all participants.
Measurement of social, clinical and biological parameters
Every participant was required to answer a questionnaire containing information such as gender, age, weight and height, smoking habits, family history of premature CVD, history of diabetes mellitus/hypertension/cardio-cerebrovascular diseases/renal diseases and usage of medications.
Venous blood samples and urine samples were obtained from fasting participants in the morning. Biological markers, including plasma low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, plasma creatinine (PCr) and urinary microalbumin and creatinine, were assayed by standard methods in the Department of Laboratory Medicine of Shanghai Tenth People’s Hospital. Fasting plasma glucose was measured by the glucose oxidase method. Estimated glomerular filtration rate (eGFR) was calculated by the modified MDRD (modification of diet in renal disease) formula for Chinese as follows: eGFR (mL/min/1.73 m2) =175× PCr−1.234 × age−0.179 (women ×0.79).Citation14 Urinary microalbumin divided by urinary creatinine was defined as urinary albumin–creatinine ratio (UACR).
Measurement of BP, ABI and PWV
BP of each participant was measured three times in the morning using an electronic device, by specialized physicians, with at least 10 minutes’ rest each time in a sitting position. The recorded BP value was calculated by the average of the three BP readings.
Bilateral brachial and ankle BPs were simultaneously and automatically measured to calculate ABI (the ratio of brachial systolic BP and ankle systolic BP) using the VP1000 system (Omron, Tokyo, Japan).
Cf-PWV was evaluated by one physician (who did not perform the ultrasound examination) using applanation tonometry (SphygmoCor; AtCor Medical, Sydney, Australia), according to the European Expert Consensus on Arterial Stiffness.Citation15 Two pressure waves were recorded transcutaneously in the right carotid and right femoral arteries. PWV was identified as the foot-to-foot velocity. Pulse transit time for each artery was automatically calculated via electrocardiogram (ECG) data. The traveling distance covered by the pulse wave between the two recording sites was measured over the body surface. cf-PWV was calculated by traveling distance divided by traveling time, and an operator index >80% indicated a high-quality measurement.
Ultrasonography
Ultrasound examinations were performed by a single experienced sonographer using the MyLab 30 Gold CV system (ESAOTE SpA, Genoa, Italy), with a 7.5 MHz probe. The presence or absence of plaque in the right carotid artery was assessed by evaluating the ultrasonographic images. No attempt was made to record the quantity and size of the lesions. “Plaque =0” referred to the absence of plaque, while “plaque =1” referred to the presence of plaque.
M-mode or two-dimensional echocardiography was used to assess the measurement of left ventricular end-diastolic diameter (LVEDd), interventricular septal diameter (IVSd), posterior wall thickness at end-diastole diameter (PWTd) and left ventricular end-systolic diameter (LVESd) from the parasternal view,Citation16,Citation17 and then left ventricular mass (LVM) was calculated through those parameters using related formulas.Citation18 Left ventricular mass index (LVMI) was assessed by LVM divided by body surface area (BSA).Citation19 LVM (g) =0.8×{1.04× [(LVEDd + PWTd + IVSd)Citation3 − (LVEDd)Citation3 +0.6 and LVMI (g/m2) = LVM/BSA. Left ventricular ejection fraction (LVEF) was calculated through left ventricular end-diastolic volume (EDV) and end-systolic volume (ESV) estimates, using the following formula: LVEF = (EDV − ESV)/EDV.Citation20 Peak transmitral pulsed Doppler velocity/early diastolic tissue Doppler velocity (E/Ea) was performed by continual wave Doppler in the four-chamber view and was calculated for the evaluation of LV diastolic function. The abovementioned measurements were made according to the guidelines laid out by the American Society of Echocardiography (ASE).Citation16
Definition of ARS and ATS
ARS was defined as cf-PWV >12 m/s, while ABI <0.9 or the presence of carotid plaque represented ATS.Citation2,Citation21,Citation22
Definition of hypertensive TOD
Asymptomatic hypertensive TOD investigated in this study included cardiac and renal TOD. Left ventricular hypertrophy (LVH) was defined as LVMI ≥115 g/m2 (male) or LVMI ≥95 g/m2 (female). Left ventricular diastolic dysfunction (LVDD) was assessed by E/Ea and other evidence of abnormal left ventricular relaxing and filling, such as enlarged left atrial volume and increased LVM.Citation23 Specifically, diastolic dysfunction was defined as E/Ea ≥15 or 15> E/Ea >8 with the following (LVMI >149 g/m2 [male] or LVMI ≥122 g/m2 [female]).Citation23,Citation24 Renal TOD was defined as microalbuminuria (MAU) (UACR >30) and renal dysfunction (RD) (creatinine clearance rate <60 mL/min/1.73 m2).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were presented as numbers with the percentage in parentheses. Participants were divided into three groups according to the participants’ presence of ARS and/or ATS, such as normal (without ARS and ATS), ARS/ATS (with either ARS or ATS) and ARS+ATS (with both ARS and ATS). The association of conventional CV risk factors with participants with or without ARS and/or ATS (normal, ARS/ATS, ARS+ATS) was assessed by general linear model for variance analysis, such as age, gender, body mass index (BMI), smoking status, systolic BP, fasting plasma glucose and LDL cholesterol. Three models were set with different adjustments: Model 1 (without adjustment), Model 2 (with adjustment for age and gender) and Model 3 (with adjustment for age, gender, BMI, smoking status, systolic BP and blood glucose). The comparisons of all parameters of asymptomatic TOD among the three groups were carried out using variance analysis, including LVMI, E/Ea, UACR and eGFR. Furthermore, multivariate logistic regressions were conducted to investigate the association of hypertensive TOD with ARS/ATS and ARS+ATS. Statistical analysis was performed using standard procedures from the Statistical Analysis System (SAS), version 9.3 (SAS Institute, Cary, NC, USA). P<0.05 was considered statistically significant.
Results
Participants
Characteristics of participants by gender are presented in , including conventional CV risk factors, asymptomatic hypertensive TOD and diseases and treatment. The 1,599 participants included 711 (44.5%) men, 312 (19.5%) diabetics and 843 (52.7%) hypertensives, of whom 799 (93.9%) were taking antihypertensive agents. Men, compared with women, had lower total cholesterol (4.92±0.99 vs 5.46±0.96 mmol/L, P<0.001), triglycerides (1.54±0.85 vs 1.66±1.00 mmol/L, P=0.008), HDL cholesterol (1.28±0.33 vs 1.46±0.36 mmol/L, P<0.001), LDL cholesterol (3.04±0.85 vs 3.33±0.83 mmol/L, P<0.001), pulse pressure (54.4±14.0 vs 56.2±16.1 mmHg, P=0.02), eGFR (88.3±20.0 vs 95.8±22.5 mL/min/1.73 m2, P<0.001), E/Ea (9.29±3.51 vs 10.11±3.71, P<0.001) but they had significantly more smokers (49.4% vs 1.7%, P<0.001) and greater incidence of carotid plaque (54.3% vs 48.9%, P=0.03).
Table 1 Characteristics of participants by gender
Association of ARS and/or ATS with CV risk factors
Conventional CV risk factors, including age, gender, BMI, smoking status, systolic BP, fasting plasma glucose and LDL cholesterol, were put into a variance analysis model to investigate their association with ARS and ATS. As shown in , age, BMI, smoking status, systolic BP and fasting plasma glucose were significantly different between participants with or without ARS and/or ATS (P≤0.02).
Table 2 Association of CV risk factors with ARS and/or ATS
Association of asymptomatic hypertensive TOD with ARSand/or ATS
In the variance analysis of ARS and ATS with hypertensive TOD, all parameters of asymptomatic TOD (P<0.02) were significantly different among the three groups, except LVEF (P=0.11; , Model 1) and UACR (P=0.16; , Model 1). Of note, in Model 3 with full adjustment, only E/Ea significantly differed among the three groups (P=0.02; , Model 3). Moreover, E/Ea was significantly different between ARS/ATS and normal (P=0.045), while there was no significant difference between ARS+ATS and normal (P=0.28). As LVEF was not significantly different among these groups (P=0.10; , Model 3), we did not adjust for LVEF in the subsequent multivariate logistic regressions.
Table 3 Association of ARS and/or ATS with parameters of asymptomatic hypertensive TOD analyzed by variance analyses with different adjustments
As shown in , with similar adjustment strategy analyzed by the multiple logistic regression model, most asymptomatic TOD was significantly associated with ARS/ATS and ARS+ATS, but only ARS/ATS was significantly associated with LVDD in the full adjustment model, with an odds ratio (OR) =1.50 (95% confidence interval [CI] 1.07–2.09; ).
Table 4 Association of ARS and/or ATS with parameters of asymptomatic hypertensive TOD analyzed by multivariate logistic regressions with different adjustments
Figure 1 Association of ARS and/or ATS with asymptomatic hypertensive TOD in the full adjustment model.
Abbreviations: ARS, arteriosclerosis; ATS, atherosclerosis; BMI, body mass index; CI, confidence interval; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; MAU, microalbuminuria; OR, odds ratio; RD, renal dysfunction; TOD, target organ damage.
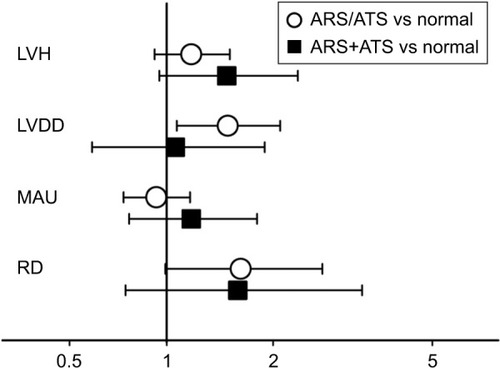
Finally, when ARS and ATS were separately put in the logistic regression model, in the full adjustment model, LVDD was significantly associated with ATS (OR 1.55; 95% CI 1.11–2.17, P=0.01) but not with ARS (OR 0.99; 95% CI 0.45–2.18, P=0.99) ().
Table 5 Association of ARS and ATS with LVDD analyzed by multivariate logistic regressions with different adjustments
Discussion
There are two major findings in this study. First, age, BMI, smoking status, systolic BP and fasting plasma glucose were significantly different between participants with or without ARS and/or ATS. Second, among hypertensive TOD, LVDD as measured by E/Ea was significantly associated with ATS but not with ARS, and the proportion of patients with LVDD was not significantly different despite the presence of both ATS and ARS, when compared to patients with ATS alone in the community-dwelling elderly Chinese.
ARS and ATS are pathologically different and possess different clinical manifestations.Citation25,Citation26 ARS is a degenerative process of the media of elastic arteries and the calcification in arterial wall under the effect of aging and risk factors.Citation27 The mechanism of ARS is the chronic vascular inflammation derived from mitochondrial oxidative stress in smooth muscle cells.Citation28 It would only cause stiffness and thickness of the arterial wall but without luminal stenosis and syndromes of tissue/organ ischemia.Citation4,Citation29 However, ATS is the underlying cause of most ischemic diseases, such as myocardial infarction and ischemic stroke. Pathologically, ATS is a chronic inflammatory response to the accumulation of lipid in the artery wall and causes intimal plaques and stenosis in arteries. Disruption of atherosclerotic plaques will trigger the formation of a thrombus and subsequently cause acute ischemia of the end organ. Although its prevalence is also age-related, the major risk factor that promotes the development of ATS is high level of cholesterol.Citation30,Citation31 As ARS and ATS are the two most common types of modifications in vascular structure and they are prevalent in the elderly, it is meaningful to investigate their respective and combined association with TOD. In this study, we found that, among cardiac and renal TOD, only LVDD was significantly associated with ATS but not with ARS. The association of LVDD with different indicators of arterial stiffness and ATS has been previously reported. Some previous studies indicated that arterial stiffness, assessed by the cardio-ankle vascular index (CAVI), was significantly correlated with LVDD.Citation32–Citation34 Some other reports discussed a strong association between LVDD and arterial stiffness assessed by PWV.Citation35,Citation36 Albu et alCitation37 conducted an investigation into 96 postmenopausal women and proposed that aortic PWV was an independent predictor of LVDD (OR 2.15, 95% CI 1.39–3.31, P=0.0006), while carotid plaque score did not show correlation with LVDD after adjustment. However, some investigators reported that the presence of carotid ATS was significantly related to LVDD,Citation38–Citation40 which was in accordance with our finding. Other studies concerning arterial stiffness were not consistent with our results. Several possible reasons may probably contribute to the discrepancies. First, arterial stiffness in those studies was assessed mainly by the CAVI but not by PWV. Although the CAVI, as a new index of arterial stiffness, was suggested to be superior to PWV due to its BP independence, different indicators in arterial stiffness may lead to miscellaneous results. Second, those studies were mainly conducted on patients with high CV risk or with coronary artery disease (CAD) or heart failure (HF) or other established cardiac diseases, while we focused on the community-dwelling elderly population. The last point may be the most important. Since participants in those studies may suffer both ATS and ARS, the observed significant association between arterial stiffness of those participants and LVDD would be due to ATS, and the association was therefore overestimated. However, in our analysis, ARS and ATS were separately investigated.
Nevertheless, there are limited data concerning the mutual association of ARS and ATS with LVDD. In our study, we found that their combination did not actually worsen patients’ LV diastolic function. This finding seems to conflict with the finding that LVDD was significantly associated with ATS. Of note, since our results were double checked by the variance analysis and multivariate logistic regression models and with sufficient adjustments, we believe that our findings are reliable. The possible explanation may lie in the fact that arterial stiffening is very prevalent in the elderly. Therefore, PWV, as influenced by biological aging, become less significant in predicting events and deaths in the geriatric population.Citation41–Citation43 Further investigations focusing on the younger population or middle-age population are warranted to confirm the present finding.
Since hypertensive TOD is of great importance and is recognized as the intermediate outcome connecting CV risk factors and CV events and mortality, it is meaningful to investigate some potential markers to screen TOD. LVDD is considered as one of the major causes of HF with preserved ejection fraction. Our finding indicated that, in accordance with other studies, LVDD was tightly associated with ATS. Therefore, the presence of ATS, even in the elderly, may indicate the high risk for the development of LVDD and early and intensified intervention may be needed in preventing future heart failure with preserved ejection fraction.
Limitations
Findings of this study need to be interpreted within the context of its limitations. First, we could not fully adjust for the potential influence from the various medications on cf-PWVs, ABI and carotid plaque, etc., such as antihypertensive, antidiabetic and anti-hyperlipidemic drugs. Second, as a cross-sectional analysis, this study cannot tell the cause–effect relationship between ATS and LVDD.
Conclusion
In the community-dwelling elderly Chinese, among hypertensive TOD, LVDD was significantly associated with ATS but not with ARS. The proportion of patients with LVDD was not significantly different despite the presence of both ATS and ARS, when compared to patients with ATS alone.
Acknowledgments
We thank all the investigators, staff and patients involved in this study for their assistance and cooperation. Jiadela Teliewubai and Yiwu Zhou provided purely technical help, while Jun Zhang and Hongwei Ji provided writing assistance. This study was authorized and financially supported by the Shanghai municipal government (Grant IDs, 2013ZYJB0902; 15GWZK1002). Dr Yi Zhang was supported by the National Nature Science Foundation of China (Grant IDs, 81300239; 81670377).
Disclosure
The authors report no conflicts of interest in this work.
References
- BenetosASafarMRudnichiAPulse pressure: a predictor of long-term cardiovascular mortality in a French male populationHypertension1997306141014159403561
- ZureikMBureauJMTemmarMEchogenic carotid plaques are associated with aortic arterial stiffness in subjects with subclinical carotid atherosclerosisHypertension200341351952712623953
- OberoiSSchoepfUJMeyerMProgression of arterial stiffness and coronary atherosclerosis: longitudinal evaluation by cardiac CTAJR Am J Roentgenol2013200479880423521451
- PalomboCKozakovaMArterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indicationsVascul Pharmacol2016771726643779
- VakhovskaiaTVBalakhonovaTVLuk’ianovMMTitovVNMasenkoVPBoitsovSAArterial stiffness and blood levels of glycation end-products in patients with arterial hypertension and carotid atherosclerosisKlin Med (Mosk)201391549
- SudanoIRoasSNollGVascular abnormalities in essential hypertensionCurr Pharm Des201117283039304421861833
- van PopeleNMGrobbeeDEBotsMLAssociation between arterial stiffness and atherosclerosis: the Rotterdam StudyStroke200132245446011157182
- HuynhJNishimuraNRanaKAge-related intimal stiffening enhances endothelial permeability and leukocyte transmigrationSci Transl Med20113112112ra122
- DemerLLEffect of calcification on in vivo mechanical response of rabbit arteries to balloon dilationCirculation1991836208320932040058
- BrietMBoutouyriePLaurentSLondonGMArterial stiffness and pulse pressure in CKD and ESRDKidney Int201282438840022534962
- KolliasAStergiouGSDolanEO’BrienEAmbulatory arterial stiffness index: a systematic review and meta-analysisAtherosclerosis2012224229130122632918
- TriantafyllidiHTzortzisSLekakisJAssociation of target organ damage with three arterial stiffness indexes according to blood pressure dipping status in untreated hypertensive patientsAm J Hypertens201023121265127220671719
- RomanMJPickeringTGSchwartzJEPiniRDevereuxRBAssociation of carotid atherosclerosis and left ventricular hypertrophyJ Am Coll Cardiol199525183907798531
- MaYCZuoLChenJHChinese eGFR Investigation CollaborationImproved GFR estimation by combined creatinine and cystatin C measurementsKidney Int200772121535154217898698
- Van BortelLMLaurentSBoutouyriePArtery SocietyEuropean Society of Hypertension Working Group on Vascular Structure and FunctionEuropean Network for Noninvasive Investigation of Large ArteriesExpert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocityJ Hypertens201230344544822278144
- LangRMBierigMDevereuxRBAmerican Society of Echocardiography’s Nomenclature and Standards CommitteeTask Force on Chamber QuantificationAmerican College of Cardiology Echocardiography CommitteeAmerican Heart AssociationEuropean Association of Echocardiography, European Society of CardiologyRecommendations for chamber quantificationEur J Echocardiogr2006727910816458610
- ZhangYProtogerouADIariaPSafarMEXuYBlacherJPrognosis in the hospitalized very elderly: the PROTEGER studyInt J Cardiol201316832714271923578896
- DevereuxRBAlonsoDRLutasEMEchocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findingsAm J Cardiol19865764504582936235
- BrillaCGWhat is the value of the left ventricular muscle mass index (LVMI) in echocardiographic diagnosis of left heart hypertrophy?Internist (Berl)199334109868225850
- LangRMBadanoLPMor-AviVRecommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular ImagingJ Am Soc Echocardiogr20152811.e39.e25559473
- AsmarRBenetosATopouchianJAssessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studiesHypertension19952634854907649586
- CarterSAIndirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremitiesCirculation19683746246375649086
- ZhangYKolliasGArgyrisAAAssociation of left ventricular diastolic dysfunction with 24-h aortic ambulatory blood pressure: the SAFAR studyJ Hum Hypertens201529744244825391758
- KaneGCKaronBLMahoneyDWProgression of left ventricular diastolic dysfunction and risk of heart failureJAMA2011306885686321862747
- WilkinsonIBMcEnieryCMCockcroftJRArteriosclerosis and atherosclerosis: guilty by associationHypertension20095461213121519884560
- PickeringGArteriosclerosis and atherosclerosis. The need for clear thinkingAm J Med19633471813943324
- SpronckBHeusinkveldMHVanmolkotFHPressure-dependence of arterial stiffness: potential clinical implicationsJ Hypertens201533233033825380150
- UngvariZKaleyGde CaboRSonntagWECsiszarAMechanisms of vascular aging: new perspectivesJ Gerontol A Biol Sci Med Sci201065101028104120576649
- ZhangJLiYWangYArterial stiffness and asymptomatic intracranial large arterial stenosis and calcification in hypertensive ChineseAm J Hypertens201124330430921164493
- OtsukaFKramerMCWoudstraPNatural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: a pathology studyAtherosclerosis2015241277278226058741
- GoldmanLSchaferAIGoldman’s Cecil Medicine24th edPhiladelphia, PAElsevier2011409411978-1-4377-1604-7
- NambaTMasakiNMatsuoYArterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular diseaseInt Heart J201657672973527829641
- MasugataHSendaSGodaFTissue Doppler echocardiography for predicting arterial stiffness assessed by cardio-ankle vascular indexTohoku J Exp Med2009217213914619212107
- SakaneKMiyoshiTDoiMAssociation of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic functionJ Atheroscler Thromb200815526126818981651
- MizuguchiYOishiYTanakaHArterial stiffness is associated with left ventricular diastolic function in patients with cardiovascular risk factors: early detection with the use of cardio-ankle vascular index and ultrasonic strain imagingJ Card Fail200713974475117996823
- ErenMGorguluSUsluNCelikSDagdevirenBTezelTRelation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or bothHeart2004901374314676238
- AlbuAFodorDBondorCPoantaLArterial stiffness, carotid atherosclerosis and left ventricular diastolic dysfunction in postmenopausal womenEur J Intern Med201324325025423276453
- HaradaMTabakoSCarotid atherosclerosis is associated with left ventricular diastolic functionJ Echocardiogr201614312012927364492
- ChahalNSLimTKJainPChambersJCKoonerJSSeniorRThe distinct relationships of carotid plaque disease and carotid intima-media thickness with left ventricular functionJ Am Soc Echocardiogr201023121303130920864314
- MizuguchiYTanakaHOishiYPredictive value of associations between carotid arterial sclerosis and left ventricular diastolic dysfunction in patients with cardiovascular risk factorsJ Am Soc Echocardiogr200720780681217617306
- Ben-ShlomoYSpearsMBoustredCAortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjectsJ Am Coll Cardiol201463763664624239664
- MiljkovicDPerret-GuillaumeCAllaFSalviPErpeldingMLBenetosACorrelation between peripheral blood pressure and pulse-wave velocity values in the institutionalized elderly persons 80 years of age and older: the PARTAGE studyAm J Hypertens201326216317323382400
- BenetosAGautierSLabatCMortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: the PARTAGE (predictive values of blood pressure and arterial stiffness in institutionalized very aged population) studyJ Am Coll Cardiol201260161503151122999729
