Abstract
Docetaxel remains a cornerstone of therapy for the patient with metastatic castration-resistant prostate cancer (CRPC). However, the landscape of CRPC therapy is changing rapidly – recently, data from the phase III TROPIC study revealed a survival advantage with the novel taxane cabazitaxel/prednisone (compared with mitoxantrone/prednisone) in a cohort of 755 men with docetaxel-refractory metastatic CRPC. Interestingly, cabazitaxel bears substantial structural similiarity to docetaxel but appears to be mechanistically distinct. In preclinical studies, the agent has antitumor activity in a variety of docetaxel-refractory in vitro and in vivo models. Subsequent to phase I testing in advanced solid tumors (where neutropenia was identified as a dose-limiting toxicity), the agent was assessed in a phase II trial in advanced, taxane-refractory breast cancer and in the aforementioned phase III TROPIC study. This review describes in detail the preclinical and clinical development of cabazitaxel.
Introduction
In 2010, it is estimated that prostate cancer will account for 28% of newly diagnosed cancers among males in the United States.Citation1 A large majority of these cases (approximately 92%) will be diagnosed at a local or regional stage, with 5-year survival rates approaching 100%. However, for individuals who are diagnosed with (or subsequently develop) metastatic prostate cancer the prognosis remains limited.Citation2 Until recently, the treatment algorithm for metastatic disease remained relatively simple. Observations by Huggins et al in 1941suggested that castration could induce regression of prostatic tumors.Citation3,Citation4 Thereafter, permutations of synthetic luteinizing hormone releasing hormone (LHRH) agonists and antiandrogen therapy supplanted surgical intervention.Citation5–Citation8 Upon failure of these therapies, further options were limited until recently. Two large, randomized phase III trials demonstrated an overall survival (OS) advantage with docetaxel compared to mitoxantrone-based regimens.Citation9,Citation10 Beyond docetaxel, strategies such as crossing over to mitoxantrone-based regimens appear to be of limited efficacy.Citation11,Citation12
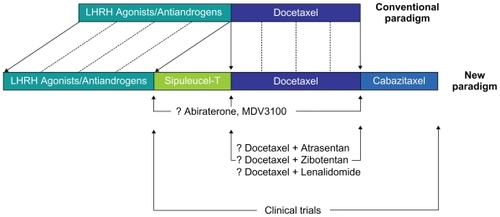
Clinical data have amassed over the past several years that now position several agents in either the pre-or postdocetaxel space (and potentially both) in the prostate cancer treatment paradigm.Citation13 The phase III IMPACT trial assessed sipuleucel-T, an autologous cellular vaccine, in a largely chemotherapy-naïve cohort of patients with castration-resistant prostate cancer (CRPC).Citation14 Relative to placebo, sipuleucel-T significantly prolonged OS (25.8 vs 21.7 months, P = 0.04), leading to Food and Drug Administration (FDA) approval of this agent. As an alternative, several novel endocrine therapies have shown substantial efficacy in the setting of CRPC. Promising phase II data for abiraterone, MDV3100, and TAK700 have led to the design of large, randomized trials.Citation15–Citation21 Notably, a placebo-controlled, phase III study enrolling patients with docetaxel-refractory CRPC demonstrated a survival advantage with abiraterone therapy.Citation22 Just as these novel therapies challenge the paradigm of ‘castration resistance’ in the setting of CRPC (), clinical data for the novel taxane cabazitaxel suggest that a chemotherapeutic strategy may be effective even after failure of docetaxel. Herein, the development and clinical implementation of cabazitaxel are reviewed in detail.
Mechanism of action/preclinical data
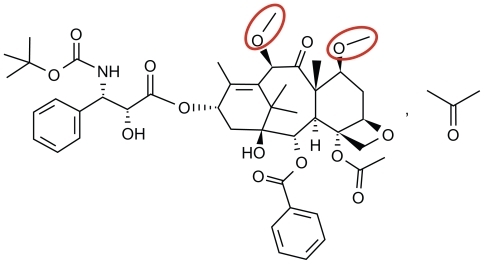
Whereas vinca alkaloids inhibit incorporation of tubulin into microtubules, the taxanes appear to inhibit microtubule disassembly.Citation23–Citation25 Although the microtubular binding mechanism of cabazitaxel does not appear to be distinct from docetaxel or paclitaxel, the agent is structurally distinct. As noted in , hydroxyl groups present in docetaxel are replaced with methoxy groups in cabazitaxel.
Figure 2 The chemical structure of cabazitaxel (C45H57 NO14…C3H6O, MW = 894.01). Highlighted in red are methoxy side chains that substitute hydroxyl groups found in docetaxel.
Bissery et al reported preclinical data suggesting the in vitro activity of cabazitaxel.Citation26 Four cell lines were assessed, including P388 (lymphoblastic leukemia), HL60 (promyelocytic leukemia), KB (cervical adenocarcinoma), and Calc18 (breast carcinoma). With a 4-day exposure to the drug, cytotoxicity was noted with relatively low cabazitaxel concentrations (IC50 = 3–29 ng/mL). In accompanying in vivo models, the agent was noted to have significant antitumor activity. In murine tumor xenografts (colon C38 and pancreas P03), cabazitaxel elicited complete tumor regressions. Two schedules of the drug were assessed: 1) a day 1 and 5 schedule with a dose of 58 mg/kg and 2) thrice daily dosing on a day 1 to 5 schedule at 12 mg/kg. The maximally tolerated dose (MTD) was 4.8-fold higher using the former schedule. Notably, in cell lines resistant to a variety of other cytotoxic agents (ie, anthracyclines, vinca alkaloids, and the older taxanes), cabazitaxel was noted to still induce tumor regression.
The activity of cabazitaxel was subsequently documented in human tumor xenografts using a variety of intravenous schedules.Citation27 In 3 human colorectal cell lines (HCT-116, HCT-8, and HT-29), high antitumor activity was observed. For instance, on a thrice daily schedule given every 3 days, cabazitaxel induced a 3.34 log cell kill (LCK) at the total highest nontoxic dose (THNTD), 36 mg/kg. In lung models, dosing at the THNTD yielded 2.7 LCK in the NCI-H460 cell line, and 2.2 LCK in the A549 cell line. As observed in murine tumor xenograft studies, multiple cases of complete regression were observed using human tumor xenografts. Notably, long-term tumor-free survival (exceeding 133 days) and complete tumor regression were observed in pancreatic xenografts (MIA PaCa-2), head and neck xenografts (SR475), and prostate xenografts (DU145, a cell line that represents a hormone-resistant entity established from a prostate cancer brain metastasis).Citation28
Pharmacokinetics/pharmacodynamics
Pharmacokinetic parameters associated with cabazitaxel were first documented in animal studies.Citation29 Using 14C-labeled cabazitaxel, doses of 15, 30, and 90 mg/m2 were delivered to mice as either 1-minute or 1-hour infusions. Radioactivity was measured in the blood, plasma, and brain. There was a correlation between dose and plasma exposure within the aforementioned dosing range, whereas brain exposure increased more than proportionally over the same range. The peak of brain concentrations occurred between 2 minutes and 1 hour post-infusion. Parallel assessments performed in dogs using a dose of 15 mg/m2 over 80 minutes suggested lesser brain exposure as compared to mice. Of note, brain concentrations of 14C-labeled cabazitaxel were detectable up to 168 hours after infusion in mice, and for up to 24 hours in dogs. This ability to concentrate in the brain is not typical for other taxanes.
The role of P-glycoprotein (P-gp) in the accumulation of cabazitaxel in the brain was assessed more extensively in a report by Cisternino et al.Citation30 Again using 14C-labeled cabazitaxel, doses ranging between 15 and 90 mg/m2 were delivered to mice, and doses of either 15 or 60 mg/m2 were delivered to rats. It was noted that brain uptake of cabazitaxel was enhanced when concentrations exceeded 11 μM. These saturable kinetics suggested the role of a critical transporter (ie, P-gp) in transporting cabazitaxel across the blood–brain barrier (BBB) upon a certain threshold (saturation was found to be at 13 μM). To further test this hypothesis, animals were concomitantly dosed with the P-gp inhibitor verapamil. Verapamil co-administration led to a 2.9-fold and 4.7-fold increase in brain uptake in mice and rats, respectively. Harnessing these pharmacokinetic properties, the activity of cabazitaxel has been documented in brain tumor models. Citation31 Using SF-295 and U251 human glioblastoma cell lines, both orthotopic and subcutaneous murine xenografts were generated. Cabazitaxel treatment led to complete regression in the majority of subcutaneously implanted tumors. Furthermore, in orthotopic models, cabazitaxel led to complete tumor regression in 4 out of 10 U251 tumors.
A phase I clinical trial of 3-weekly cabazitaxel enrolled patients with advanced solid malignancies refractory to conventional treatments.Citation32 With respect to prior therapy, patients were limited to less than 2 prior chemotherapy regimens for metastatic disease and radiation affecting less than 25% of the available hematopoietic reserve. A starting dose of 10 mg/m2 was selected, representing one-tenth the severe toxic dose in mice (STD10). Given that the STD10 in mice corresponded to a plasma level of 10.8 μg/mL, pharmacokinetic monitoring was performed during the first course of therapy and dose-escalation was to be terminated for plasma levels beyond this value.
In total, 25 patients were treated with 102 courses of 3-weekly cabazitaxel at 4 dose levels, ranging from 10 mg/m2 to 25 mg/m2.Citation32 A total of 22 patients had received prior chemotherapy (88%), and 8 patients had received prior taxane-based therapy (32%). Although a diverse array of tumor types was enrolled, the largest subgroup comprised patients with prostate cancer (8 patients, 32%). A median of 4 cycles (range 1–9) was administered. Pharmacokinetic analyses suggested that cabazitaxel absorption best fit a triphasic model. A rapid initial phase was followed by a longer intermediate phase (t1/2 = 2.5 minutes and 1.3 hours, respectively). Finally, a prolonged terminal phase (t1/2 = 77.3 hours) was observed.
The dose-limiting toxicity (DLT) of cabazitaxel was neutropenia, with 1 case of febrile neutropenia and 2 cases of grade 4 neutropenia occurring at a dose of 25 mg/m2. Accordingly, the recommended phase II dose emerging from this study was 20 mg/m2.Citation32 Notably, support with granulocyte colony-stimulating factor (G-CSF) or granulocyte macrophage colony-stimulating factor was not utilized in these studies, although it was ultimately administered in patients incurring grade 4 neutropenia. Nonhematologic toxicities were generally mild in nature; the most commonly encountered adverse events were diarrhea (52%), nausea (40%), and vomiting (15%). Only 1 grade 3 nonhematologic event was recorded – diarrhea in a patient dosed at 15 mg/m2 (resolving shortly after therapy with loperamide). In this initial clinical experience, 2 confirmed partial responses were observed, both in patients with prostate cancer. One patient had previously received mitoxantrone, while the other had progressed on docetaxel. An unconfirmed partial response was observed in a patient with bladder cancer, and minor responses were seen in 2 patients with osteosarcoma and prostate cancer, respectively. Stable disease (SD) was recorded as a best response in 12 patients (48%).
Phase II data in breast cancer
A phase II study in breast cancer was originally designed as a randomized 3-arm study to explore 2 distinct dosing regimens of cabazitaxel and to further assess the activity of the novel taxane larotaxel. (the activity of larotaxel has been documented in phase I and II studies in breast and lung cancer).Citation33–Citation36 Due to poor accrual, it was ultimately modified to be a single-arm study evaluating cabazitaxel alone in patients with taxane-resistant metastatic breast cancer. In the setting of patients who had received adjuvant or neoadjuvant taxane therapy, resistance was defined as metastatic progression within 12 months of systemic therapy. For patients with metastatic disease, the definition was more complex; resistance was characterized as: 1) progressive disease (PD) representing the best response to treatment, 2) PD occurring within 4 months after first- or second-line therapy (after an initial clinical benefit), or 3) SD representing the best response if a taxane had been administered for 3 or more months. Patients were treated initially with a dose of 20 mg/m2, which was escalated to 25 mg/m2 in those patients who did not incur a significant adverse event during the first cycle of therapy. Patients who were HER2-positive were allowed to enroll if they had progressed on a trastuzumab-based regimen; otherwise, the study was limited to HER2-negative patients.
The study was powered to assess objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, with secondary endpoints including duration of response, time to progression, and OS.Citation36 The study was stratified by the number of lines of previous taxane-based therapy. Stratum 1 consisted of 47 patients who had progressed after either first-line systemic therapy for advanced disease or adjuvant/neoadjuvant taxanes; stratum 2 consisted of 20 patients who had progressed on second-line therapy for advanced disease. The median age of enrolled patients was 53 years, with an expected distribution of hormone receptor-positive and HER2-positive tumors (52% and 27%, respectively). The majority of patients had received prior chemotherapy for advanced disease, with only 1 patient having received adjuvant therapy. Seven patients (10%) had received multiple forms of taxane therapy.
Among treated patients, the ORR was 14% (95% confidence interval [CI], 7%–24%), with no differences between the two pre-defined strata (14% for stratum 1 and 12% for stratum 2).Citation36 The median duration of response was 7.6 months (range 2.6–18.7 months). A significant proportion of patients also exhibited SD as a best response (38%). Two patients were noted to have a complete response to cabazitaxel therapy. Mirroring the phase I experience, the most common grade 3/4 toxicity incurred was neutropenia, present in 73% of the patients. Two patients developed febrile neutropenia, while 3 patients developed neutropenic infections. Two deaths were recorded within 30 days of on-study therapy; both were secondary to nonhematologic toxicities. In the first patient, death occurred due to respiratory failure that was possibly related to study therapy, and in the second patient, the cause of death was unknown. The results for cabazitaxel in breast cancer have drawn multiple comparisons to the novel epothilone ixabepilone, which also impacts microtubule function and has been assessed in phase III trials in this disease.Citation37
Phase III data
The information garnered from phase I and II studies, encompassing multiple malignancies, were used to inform the design of the phase III TROPIC trial comparing cabazitaxel/prednisone with mitoxantrone/prednisone in patients with docetaxel-refractory prostate cancer.Citation38 The study itself represented somewhat of a paradigm shift, given the absence of prior phase II studies assessing cabazitaxel specifically in the setting of prostate cancer. However, no viable therapeutic options were available to the docetaxel-refractory patient at the time the study was initiated, generating a substantial area of need. Furthermore, abundant preclinical data in docetaxel-refractory cell lines and an initial clinical demonstration of safety and efficacy in solid tumors supported this larger undertaking.
In TROPIC, progression on docetaxel was defined by RECIST in patients with measurable disease, or by 2 consecutive prostate-specific antigen (PSA) rises (at least 1 week apart) in patients with nonmeasurable disease.Citation38 Orchiectomy or prior pharmacologic androgen deprivation was mandated, and patients who were receiving LHRH agonists were instructed to continue taking them during protocol therapy.
Ultimately, 755 men were randomized (378 to cabazitaxel and 377 to mitoxantrone) in a total of 26 countries. The median age of the study population was 68 years, and the majority of patients were Caucasian (84%).Citation38 Although enrollment was originally conducted irrespective of the amount of prior docetaxel therapy, the study was ultimately modified to exclude patients who had received a cumulative dose of less than 225 mg/m2. This amendment was made in light of guidelines suggesting that castrate-resistant prostate cancer therapy be maintained for a period of at least 3 cycles prior to instituting any change. The mean docetaxel dose in the experimental arm was 576.6 mg/m2, compared with 529.2 mg/m2 in the control arm. A substantial proportion of patients progressed on docetaxel therapy either during treatment (29%) or within 3 months of its completion (45%); the mean time from the last docetaxel dose to disease progression was 0.8 months in the experimental arm and 0.9 months in the control arm. Although most patients had bony metastases (84%), a considerable proportion did have visceral metastases (25%).
Whereas the phase II experience in breast cancer initiated 3-weekly dosing of cabazitaxel at 20 mg/m2, in TROPIC, patients were initiated at 25 mg/m2. Patients randomized to receive mitoxantrone were started on a conventional dose of 12 mg/m2 every 3 weeks. Both arms received prednisone 10 mg oral daily. In order to limit the risk of mitoxantrone-induced cardiac dysfunction, therapy on both arms was limited to a total of 10 cycles. While growth factor support was not allowed at the initiation of therapy, it was permitted to treat extended neutropenia (>7 days), neutropenic infection, or neutropenic fever.
The primary endpoint of the study was OS, with a secondary endpoint of progression-free survival (PFS). PFS was defined by the occurrence of one of several clinical events, including PSA progression, radiographic progression, progression of pain (measured by the McGill-Melzack present pain intensity scale, PPI) or death. The study met its primary endpoint, with an improvement in OS of 2.4 months favoring cabazitaxel therapy (15.1 vs 12.7 months; hazard ratio [HR] 0.70, 95% CI 0.59–0.83, P < 0.001). The benefit of cabazitaxel for survival appeared to extend across the majority of subgroups assessed, including subgroups divided by performance status (ECOG 0-1 or ECOG 2), measureable disease (absent or present), number of previous chemotherapeutic agents (1 or ≥2), age (<65 or ≥65), and pain (at baseline, absent or present). Furthermore, subset analyses favored cabazitaxel across groups divided by cumulative docetaxel dose. Cumulative PFS (using the composite endpoint) was also improved with cabazitaxel therapy (2.8 vs 1.4 months, HR 0.74, 95% CI 0.64–0.86, P < 0.0001), although time to pain progression (as defined by the PPI inventory) did not significantly improve. PSA response rate was 39.2% vs 17.8% (P = 0.002) and median time to PSA progression was 6.1 vs 3.1 months (P = 0.001), both favoring cabazitaxel.
Mirroring the phase I and II experiences, the most common toxicity associated with cabazitaxel therapy was neutropenia. Grade ≥ 3 neutropenia occurred in 82% of cabazitaxel patients, with 8% of patients developing febrile neutropenia. Common nonhematologic toxicities in patients receiving cabazitaxel included diarrhea, fatigue, and asthenias (all grades: 47%, 37%, and 20%, respectively). A total of 18 patients (5%) died within 30 days of the last cabazitaxel infusion, compared with 9 patients (2%) receiving mitoxantrone therapy within the same time frame. In the cabazitaxel arm, 7 patients (2%) died of complications related to neutropenia, while 5 patients (1%) died of cardiac causes.
Safety considerations
Several factors may influence the toxicities associated with cabazitaxel therapy. In the TROPIC trial, diarrhea appeared to be more prevalent in older patients (55.7% vs 44.5% in patients aged ≥75 or <75, respectively; P < 0.1) and in patients who had previously received radiotherapy (50.0% vs 41.4% in patients with and without prior exposure, respectively).Citation39 The most prevalent toxicity, neutropenia, occurred at a frequency 6.6% higher in patients aged ≥65 compared with those <65. Furthermore, the incidence of neutropenia varied significantly by region, with rates of neutropenia in North America exceeding those in the Europe. Analyses are currently underway to determine the extent of growth factor use both in the study population at large and within these subgroups (notably, cycle 1 prophylaxis with growth factors was not allowed in the TROPIC protocol). Until these data are available, the currently available FDA label suggests the use of primary prophylaxis with G-CSF in those patients who are considered high risk, as delineated in .
Table 1 Special precautions for use of cabazitaxel
As yet, there are no head-to-head trials comparing docetaxel and cabazitaxel, making it challenging to juxtapose both the efficacy and toxicity of these agents. Nonetheless, the rates of neuropathy with cabazitaxel were relatively low, only 1% of patients reporting a grade 3/4 event (14% for all grades).Citation38 It should be noted that patients with grade 2 or higher peripheral neuropathy in association with docetaxel were excluded from TROPIC, confounding any comparisons with this agent. Another important distinction between cabazitaxel and docetaxel is the premedication regimen proposed for each. In SWOG 9916 and TAX 327, patients receiving 3-weekly docetaxel received 60 mg and 24 mg of oral dexamethasone divided over 3 doses, respectively.Citation9,Citation10 In contrast, patients receiving cabazitaxel in the TROPIC study received 8 mg of intravenous dexamethasone in conjunction with an antihistamine and H2-antagonist.Citation38 In the setting of certain co-morbidities (ie, diabetes), the latter regimen may be preferable.
Conclusions
Therapy with cabazitaxel in docetaxel-refractory CRPC has already been adopted as a category 1 recommendation in National Comprehensive Cancer Network Criteria.Citation40 However, the challenge that lies ahead is multifold. Given the efficacy of cabazitaxel in the heavily pretreated population in the TROPIC study, could cabazitaxel potentially be moved forward in the current therapeutic algorithm for prostate cancer ()? Furthermore, underway are numerous clinical studies assessing synergy of docetaxel with a range of agents. Some of the reports thus far have been sobering. For instance, the phase III Cancer and Leukemia Group B (CALGB) 90401 trial showed no OS benefit with the addition of bevacizumab to docetaxel.Citation41 Nonetheless, several other phase III efforts are underway, including studies pairing docetaxel with the endothelin antagonists zibotentan and atrasentan, and the antiangiogenic/immunomodulatory agent lenalidomide.Citation42–Citation44 With its efficacy now demonstrated, the investigator may be inclined to assess cabazitaxel in the same combinations currently being investigated with docetaxel. The research community is cautioned to perform appropriate preclinical and clinical safety testing prior to embarking on larger efforts assessing such combinations. Several ongoing clinical trials of cabazitaxel both alone and in combination with other cytotoxic agents are denoted in . Furthermore, cabazitaxel/prednisone (dosed at both 20 and 25 mg/m2) will be compared to docetaxel/prednisone (at a standard dose of 75 mg/m2) as first-line chemotherapy in metastatic CRPC. The primary endpoint in this study is OS. Problematic in the trial design is the fact patients progressing on docetaxel (but not cabazitaxel) will have a known effective salvage therapy.
Table 2 Listed studies evaluating the clinical efficacy and safety of cabazitaxelCitation47–Citation50
The role of docetaxel in distinct settings of prostate cancer may similarly guide clinical implementation of cabazitaxel. For instance, CALGB 90203 is a randomized, phase III effort comparing 6 cycles of neoadjuvant docetaxel therapy preceding prostatectomy with prostatectomy alone in the setting of high-risk, localized disease.Citation45 If the trial yields promising results, the application of cabazitaxel as neoadjuvant therapy could be explored. Further, it remains to be seen whether cabazitaxel has specific activity in the context of aggressive prostatic cancer histologies, such as tumors bearing neuroendocrine features. Available clinical data suggest limited efficacy of docetaxel and other standard cytotoxic agents in this setting.Citation46 Questions remain about the dosing regimen chosen in the TROPIC study; ie, could toxicity have been mitigated by starting with a dose of 20 mg/m2? As previously noted, this represented the initial dose utilized in a phase II study of cabazitaxel in breast cancer. In that study, allowance of dose escalation to 25 mg/m2 was contingent upon completion of the first cycle of therapy with no toxicity. The aforementioned phase III first line trial in metastatic CRPC will help to address this issue.
Finally, it is not known yet whether the activity of cabazitaxel in docetaxel-refractory CRPC will translate to other tumor types. The previously noted phase II study assessing the agent in taxane-refractory advanced breast cancer may stimulate further trials in this disease.Citation36 Furthermore, urothelial carcinoma, lung cancer, ovarian cancer, and countless other malignancies where taxanes have a described clinical benefit may represent new domains where cabazitaxel therapy could be examined.
Acknowledgments
Dr Pal’s efforts are supported by the NIH Loan Repayment Plan (LRP), the CBCRP 15IB-0140 (California Breast Cancer Research Program Junior IDEA Award) and NIH K12 2K12CA001727-16A1. The authors would also like to acknowledge the generous support of Nancy and Ira Norris.
Disclosure
Sumanta Kumar Pal, MD: Honoraria: Glaxo-Smith-Kline, Pfizer, Sanofi-Aventis, Novartis; Research Support: Amgen; Consulting: Novartis, Genentech; Przemyslaw Twardowski, MD: Honoraria: Sanofi-Aventis; Oliver Sartor, MD: Research Support: Sanofi-Aventis, Johnson & Johnson; Consulting: Sanofi-Aventis, Johnson & Johnson, Medivation.
References
- JemalASiegelRXuJWardECancer StatisticsWiley Subscription Services, Inc., A Wiley Company2010
- WaylandHKelvinAMMichaelGAsheshBJPeterJRVirajAMStage IV prostate cancer: survival differences in clinical T4, nodal and metastatic diseaseJ Urol2010184251251820620410
- HugginsCHodgesCVStudies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostateCancer Res194114293297
- HugginsCStevensREJrHodgesCVStudies on prostate cancer: II. The effects of castration on advanced carcinoma of the prostate glandArch Surg1941432209223
- Aragon-ChingJBWilliamsKMGulleyJLImpact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancerFront Biosci2007124957497117569623
- EisenbergerMO’DwyerPFriedmanMGonadotropin hormone-releasing hormone analogues: a new therapeutic approach for prostatic carcinomaJ Clin Oncol1986434144242936872
- ScherHKellyWFlutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancerJ Clin Oncol1993118156615727687666
- ScherHLiebertzCKellyWBicalutamide for advanced prostate cancer: the natural versus treated history of diseaseJ Clin Oncol1997158292829389256137
- PetrylakDPTangenCMHussainMHDocetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancerN Engl J Med2004351151513152015470214
- TannockIFde WitRBerryWRDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancerN Engl J Med2004351151502151215470213
- BertholdDRPondGRde WitREisenbergerMTannockIFSurvival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versaAnn Oncol200819101749175318487550
- ArmstrongAJGarrett-MayerEde WitRTannockIEisenbergerMPrediction of survival following first-line chemotherapy in men with castration- resistant metastatic prostate cancerClinical Cancer Research201016120321120008841
- LongoDLNew therapies for castration-resistant prostate cancerN Engl J Med.363547948120818868
- KantoffPWHiganoCSShoreNDSipuleucel-T immuno-therapy for castration-resistant prostate cancerN Engl J Med363541142220818862
- NCT0887198: Abiraterone acetate in asymptomatic or mildly symptomatic patients with metastatic castration-resistant prostate cancer http://www.clinicaltrials.govAccessed 2009 Jul 29
- NCT0638690: Abiraterone acetate in castration-resistant prostate cancer previously treated with docetaxel-based chemotherapy http://www.clinicaltrials.govAccessed 2009 Jul 29
- NCT1193257: A phase 3, randomized, double-blind, multicenter trial comparing orteronel plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or following docetaxel-based therapy http://www.clinicaltrials.govAccessed 2010 Oct 2
- AttardGReidAHMA’HernRSelective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancerJ Clin Oncol200927233742374819470933
- AttardGReidAHMYapTAPhase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone drivenJ Clin Oncol200826284563457118645193
- ScherHIBeerTMHiganoCSAntitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 studyLancet2010 Apr 437597241437144620398925
- NCT0974311: AFFIRM: a multinational phase 3, randomized, double-blind, placebo-controlled efficacy and safety study of oral MDV3100 in patients with progressive castration-resistant prostate cancer previously treated with docetaxel-based chemotherapy http://www.clinicaltrials.govAccessed 2010 Oct 2
- de BonoJSLogothetisCFizaziKAbiraterone acetate plus low dose prednisone improves overall survival in patients with metastatic castration-resistant prostate cancer (CRPC) who have progressed after docetaxel-based chemotherapy: results of COU-AA-301, a randomized double-blind placebo-controlled phase 3 study2010 European Society for Medical Oncology (ESMO) Annual Meeting2010 Oct 11
- KumarNTaxol-induced polymerization of purified tubulin. Mechanism of actionJ Biol Chem19812562010435104416116707
- CaplowMZeebergBDynamic properties of microtubules at steady state in the presence of taxolEur J Biochem198212723193246128228
- WhiteJRaoGEffects of a microtubule stabilizing agent on the response of platelets to vincristineBlood19826024744836124288
- BisseryM-CBouchardHRiouJVrignaudPCombeauCBourzatJDPreclinical evaluation of TXD258, a new taxoidProceedings of the American Association for Cancer Research200041Abstract1364
- VrignaudPLejeunePChaplinDLavelleFBisseryMCIn vivo efficacy of TXD258, a new taxoid, against human tumor xenograftsProceedings of the American Association for Cancer Research200041Abstract1365
- StoneKRMickeyDDWunderliHMickeyGHPaulsonDFIsolation of a human prostate carcinoma cell line (DU 145)Int J Cancer1978213274281631930
- ArchimbaudYGiresPPellerinRNicheleGPouletDPharmacokinetics of a new taxoid, 14C-TXD258, in blood, plasma and brain of the mouse, rat and dogProceedings of the American Association for Cancer Research200041Abstract1375
- CisterninoSBourassetFArchimbaudYSemiondDSanderinkGScherrmannJMNonlinear accumulation in the brain of the new taxoid TXD258 following saturation of P-glycoprotein at the blood-brain barrier in mice and ratsBr J Pharmacol200313871367137512711638
- DykesDSarsatJBisseryMCEfficacy evaluation of TXD258, a taxoid compound against orthotopic and subcutaneous glioblastomasProceedings of the American Association for Cancer Research200041Abstract1916
- MitaACDenisLJRowinskyEKPhase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumorsClin Cancer Res200915272373019147780
- DierasVLimentaniSRomieuGPhase II multicenter study of larotaxel (XRP9881), a novel taxoid, in patients with metastatic breast cancer who previously received taxane-based therapyAnn Oncol20081971255126018381372
- Metzger-FilhoOMoulinCde AzambujaEAhmadALarotaxel: broadening the road with new taxanesExpert Opin Investig Drugs200918811831189
- YamamotoNBokuNMinamiHPhase I study of larotaxel administered as a 1-h intravenous infusion every 3 weeks to Japanese patients with advanced solid tumoursCancer Chemother Pharmacol200965112913619437020
- PivotXKoralewskiPHidalgoJLA multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patientsAnn Oncol20081991547155218436520
- HortobagyiGNGomezHLLiRKAnalysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanesBreast Cancer Res Treat2010122240941820454927
- De BonoJSOudardSOzgurogluMPrednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trialLancet201037697471147115420888992
- De BonoJSOudardSOzgurogluMPrednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial3769747 Web Appendix 1
- NCCN Clinical Practice Guidelines in OncologyProstate Cancer22009 http://www.nccn.orgAccessed. 2009 Jul 29
- KellyWKHalabiSCarducciMAA randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): survival results of CALGB 90401J Clin Oncol (Meeting Abstracts)201028Suppl 18LBA4511
- NCT0617669: A phase III, randomised, double-blind, placebo-controlled study to assess the efficacy and safety of 10 mg ZD4054 (zibotentan) in combination with docetaxel in comparison with docetaxel in patients with metastatic hormone-resistant prostate cancer http://www.clinicaltrials.govAccessed 2010 Oct 7
- NCT0134056: Phase III study of docetaxel and atrasentan versus docetaxel and placebo for patients with advanced hormone refractory prostate cancer http://www.clinicaltrials.govAccessed 2010 Oct 7
- NCT0988208: A phase 3 study to evaluate the efficacy and safety of docetaxel and prednisone with or without lenalidomide in subjects with castrate-resistant prostate cancer http://www.clinicaltrials.govAccessed 2010 Oct 7
- NCT0430183: Randomized phase III study of neo-adjuvant docetaxel and androgen deprivation prior to radical prostatectomy versus immediate radical prostatectomy in patients with high-risk, clinically localized prostate cancer http://www.clinicaltrials.govAccessed 2010 Nov 7
- CulineSEl DemeryMLamyP-JIborraFAvancèsCPinguetFDocetaxel and cisplatin in patients with metastatic androgen independent prostate cancer and circulating neuroendocrine markersJ Urol2007178384484817631339
- NCT1140607: Phase I safety and pharmacokinetic study of XRP6258 (cabazitaxel) in advanced solid tumor patients with varying degrees of hepatic impairment http://www.clinicaltrials.govAccessed 2010 Oct 5
- NCT0925743: A dose-escalation study of the safety, tolerability, and pharmacokinetics of cabazitaxel in combination with cisplatin administered every 3 weeks in subjects with advanced solid malignancies http://www.clinicaltrials.govAccessed 2010 Oct 5
- NCT1001221: A dose-escalation, single arm, combination study of cabazitaxel with gemcitabine to determine the safety, and pharmacokinetics in subjects with advanced solid malignancies http://www.clinicaltrials.govAccessed 2010 Oct 5
- NCT1087021: QT-Cab: an open-label study to investigate the effect of cabazitaxel on the QTc interval in cancer patients http://www.clinicaltrials.govAccessed 2010 Oct 5