Abstract
The burden of Clostridium difficile infection (CDI) is profound and growing. CDI now represents a common cause of health care–associated diarrhea, and is associated with significant morbidity, mortality, and health care costs. CDI disproportionally affects the elderly, possibly explained by the following risk factors: age-related impairment of the immune system, increasing antibiotic utilization, and frequent health care exposure. In the USA, recent epidemiological studies estimate that two out of every three health care–associated CDIs occur in patients 65 years or older. Additionally, the elderly are at higher risk for recurrent CDI. Existing therapeutic options include metronidazole, oral vancomycin, and fidaxomicin. Choice of agent depends on disease severity, history of recurrence, and, increasingly, the drug cost. Bezlotoxumab, a recently approved monoclonal antibody targeting C. difficile toxin B, offers an exciting advancement into immunologic therapies. Similarly, fecal microbiota transplantation is gaining popularity as an effective option mainly for recurrent CDI. The challenge of decreasing CDI burden in the elderly involves adopting preventative strategies, optimizing initial treatment, and decreasing the risk of recurrence. Expanded strategies are certainly needed to improve outcomes in this high-risk population. This review considers available data from prospective and retrospective studies as well as case reports to illustrate the merits and gaps in care related to the management of CDI in the elderly.
Introduction
Clostridium difficile (C. difficile) is increasingly being recognized as a major cause of gastrointestinal infections worldwide, with 70%–80% of C. difficile infections (CDIs) occurring in adults aged 65 and older.Citation1–Citation3 The inciting agent C. difficile is a ubiquitous anaerobic, spore-forming, Gram-positive bacterium. The elderly are especially vulnerable to CDI.Citation4 Indeed, reducing the incidence of CDI in this population is crucial because of the significant morbidity, mortality, and financial cost associated with this infection.Citation5 There are a number of therapeutic agents in development and currently being utilized for CDI, including antibiotics, probiotics, fecal transplantation therapy, antibody-based immunotherapy, and vaccines.Citation6–Citation9 In this article, we review the epidemiology of CDI, discuss risk factors, and outline current and emerging therapeutic options as it pertains to the geriatric population.
Pathogenesis and epidemiology
The pathogenesis of CDI lies in the dysregulation of the normal indigenous gastrointestinal microbiota typically secondary to systemic antimicrobial use.Citation10,Citation11 The histopathologic hallmark of CDI is damage to the mucosal epithelial cell lining with generation of an acute, neutrophil-predominant inflammatory response and the formation of pseudomembranes.Citation10,Citation12 Damage to the epithelium is caused by C. difficile virulence factors, the glucosyltransferase toxin A (TcdA) and toxin B (TcdB). The clinical manifestations of CDI range from mild diarrhea to life-threatening conditions such as pseudomembranous colitis and toxic megacolon. It should be noted, however, that C. difficile burden varies dramatically by geographic region, between institutions, and even between units of the same hospital.Citation12,Citation13
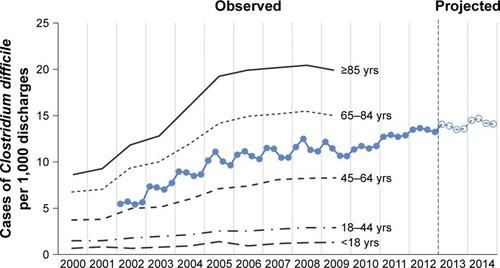
Over the last few decades there has been a dramatic rise in CDI incidence. Rates of CDI tripled in the USA and Canada.Citation1,Citation14 Of great concern is the fact that severe and fatal CDI predominantly affects elderly, nursing home patients, and those with poor functional status.Citation1,Citation15 A 2015 report from the Center for Disease Control and Prevention noted that one out of every three CDIs occurs in patients 65 years or older and two out of every three health care–associated CDIs occur in patients 65 years or older.Citation16 Indeed, CDI hospitalization rates were approximately fourfold for adults 65–84 years old and tenfold for adults ≥85 years old compared to adults 45–64 years old utilizing data from the Healthcare Cost and Utilization Project.Citation17 Another study found that US rates of hospital discharges with CDI increased from ~5 per 1,000 discharges in 2,000 to greater than 10 per 1,000 discharges in 2008; increases were especially prominent among those ≥65 years of age ().Citation3
Figure 1 Incidence of nosocomial Clostridium difficile infection.
According to national mortality data records, C. difficile-related deaths in the USA rose from 5.7 deaths per million in 1999 to 23.7 in 2004 with a median age of death reported as 82 years.Citation18 The substantial increase in CDI incidence has been primarily attributed to the emergence of a more virulent strain categorized as North American pulsed-field 1/PCR-ribotype 027 (NAP1/BI/027). NAP1/BI/027 virulence is characterized by increasing fluoroquinolone resistance, production of binary toxin, increased toxin production, and higher sporulation rates.Citation1,Citation12
The aging host: risk factors for CDI
Several prospective and retrospective trials have looked into risk factors, including advanced age, as being contributors to the development and severity of CDI. The three main factors are exposure to systemic antimicrobial therapy for other infections, exposure to C. difficile spores, and the host immune response ().Citation1,Citation2,Citation10,Citation19–Citation23
Table 1 Risk factors associated with CDI development and recurrence
The risk of CDI is the highest during systemic antimicrobial therapy and in the first month after cessation of antimicrobial therapy thereafter.Citation1 Antimicrobials that pose the greatest risk of CDI are clindamycin, cephalosporins, and fluoroquinolones, and to a lesser frequency macrolides and sulfonamides. A meta-analysis identified that fluoroquinolone use and age over 65 years were associated with a higher risk of CDI because of the NAP1/BI/027 strain.Citation23,Citation24 Studies also suggest probable association between proton-pump inhibitor (PPI) use and incident and recurrent CDI. In a 15-month prospective Canadian cohort study, Loo et al found that older age, use of antibiotics, and use of PPI were significantly associated with health care–associated CDI. Specifically, the authors found that for each additional year of age >18 years, the risk of health care–acquired CDI increased by 2% (odds ratio [OR] 1.02; 95% CI 1.00–1.04).Citation25 Among the many risk factors for CDI, the most readily modifiable is antimicrobial utilization. In the USA, 25%–75% of antibiotic prescriptions for long-term care residents have been found to be inappropriate.Citation26 Undeniably, reducing antimicrobial use also reduces CDI rates. For example, an effort to improve antimicrobial utilization and stewardship at a Veterans Affairs (VA) long-term care facility (LTCF) resulted in an infectious disease consult service achieving a 30% reduction in antimicrobial use, which correlated with a significant decrease in the rate of positive C. difficile tests.Citation27 Advanced age and receipt of non-CDI anti-microbials during or after CDI treatment were significantly associated with CDI recurrence.Citation28,Citation29 The validated results of a prediction tool by Hu et alCitation29 consistently predicted CDI recurrence in patients with three clinical factors: age >65 years, severe or fulminant underlying illness (assessed by Horn Index), and additional antimicrobial use after initial CDI treatment. Median age of patients in the cohort was 69 years.Citation29
Host factors are also important CDI risks, with advanced age, immunosuppression, prior hospitalization, and severity of underlying illness contributing to an increased risk. Aging alters important physiologic barriers to infection, ranging from changes in genitourinary physiology that impairs bladder function to decreased gastrointestinal microbial diversity.Citation30 In addition, the complex changes in the immune system related to advancing age, collectively called immunosenescence, play a key role in increased susceptibility in the elderly. Immunosenescence has been associated with a decrease in T-cell and B-cell counts as well as a decline in cell function.Citation31–Citation33 This age-related pathophysiology enhances morbidity and mortality risk as it limits the ability of older adults to respond to microbes. Indeed, older adults have been shown to exhibit an increase in incidence of infections compared to their younger counterparts.Citation30
In addition, decreased functional status is increasingly being recognized as an important and independent risk factor for poor outcomes among older adults, further enhancing the risk and severity of infections.Citation15,Citation34 Utilizing an assessment of activities of daily living prior to hospitalization and at onset of CDI, Rao et al identified impaired functional status as an independent risk factor for severe CDI in patients 50 years and older.Citation15
Therapeutic agents
The management of CDI involves three basic principles: 1) supportive care with fluid and electrolyte replacement, 2) discontinuation of the precipitating antimicrobials when appropriate, and 3) the initiation of effective anti-Clostridium difficile therapy. The drugs available in the USA for the treatment of CDI are listed in .
Table 2 Recommended medical therapy for Clostridium difficile infection
The goals of successful treatment are the elimination of symptoms and the prevention of recurrent CDI. Currently, CDI treatment regimens depend on severity of CDI and if the presentation is an index or recurrent episode ().Citation19–Citation21 While certainly a consideration for severe CDI, treatment recommendations are not currently stratified by patient age.
Table 3 Current treatment options available in the USA
Metronidazole and vancomycin
Early studies suggested that oral metronidazole and oral vancomycin had equivalent efficacy, with similar tolerability.Citation35 Newer data suggest higher treatment failure rates when metronidazole is used in severe or complicated CDI.Citation36–Citation38 In the first randomized controlled trial (RCT) comparing vancomycin to metronidazole for the treatment of CDI, vancomycin therapy was superior to metronidazole therapy overall, but this treatment benefit was limited to patients with severe disease. Approximately half of the study participants (N=150) were older than 60 years (47%). While age was not evaluated in subgroup analysis, patient characteristics that were statistically more common in the metronidazole treatment failure group were a low albumin level, admission to the intensive care unit, and the presence of pseudomembranous colitis on endoscopic examination.Citation37 In response to metronidazole’s lower drug cost, vancomycin efficacy data, and a theoretical risk of promoting vancomycin-resistant enterococci, major guidelines consider oral metronidazole as the primary agent for only mild-to-moderate CDI.Citation19–Citation21,Citation37 Of note, vancomycin is also inexpensive if the intravenous form of the drug is formulated for oral administration.
Tolevamer is a toxin-binding polymer that neutralizes the effects of C. difficile toxins A and B in vitro. Despite encouraging early-phase results, tolevamer failed to meet its primary endpoint of noninferiority to vancomycin in Phase III clinical trials.Citation39 In these Phase III trials comparing tolevamer with vancomycin and metronidazole, the investigators found that while tolevamer was inferior to both metronidazole and vancomycin, metronidazole was inferior to vancomycin (clinical success rates of 44.2%, 72.7%, and 81.1%, respectively). These differences were more pronounced in severe CDI (clinical success rates of 66.3% for metronidazole and 78.5% for vancomycin). Due to the randomization of patients to each tolevamer, metronidazole, and vancomycin treatment arm, this study actually represented the largest randomized study comparing metronidazole (n=278) to vancomycin (n=259) for the treatment of CDI. In post hoc analysis, age ≤65 years compared to age >65 years was not shown to influence clinical success.Citation39 Despite the tolevamer study providing no evidence for an impact of age on treatment success, advancing age has been shown in numerous studies to influence treatment outcomes. For example, a systematic review of 39 articles from 2001 to 2010 by Vardakas et al allowed an assessment of the impact of age on treatment failures.Citation36 The median age was greater than 65 years in 22 studies and 65 years and younger in 15 other studies. In age-specific analysis, more total treatment failures were reported in studies with older patients (median age >65 years) compared to younger patients (24.7% vs 19.6%; p=0.005). Total CDI recurrences were also higher in studies with older patients than in studies with younger patients (23.4% vs 19.4%; p=0.003). Treatment failure with metronidazole in studies with older patients was 27.4% and that of younger patients was 17.6% (p<0.001). The corresponding recurrence was 33.9% in older patients and 17.9% in younger patients (p<0.001). No age-related difference was observed in treatment failure and recurrence with vancomycin, suggesting that metronidazole may be associated with poorer outcomes in the elderly population.Citation36
Seventy patients were identified in a retrospective chart review (January–December 2006) to examine the clinical course of CDI in the patients 80 years and older (mean age: 84.0±4.1; range 80–94). The aim of this study was to characterize CDI in the “oldest” old population. Majority of patients received antibiotics (81.4%) and PPI (58.5%) during the 30 days prior to CDI presentation. Twelve patients (17.1%) died within 90 days of initial presentation, with one death directly attributable to CDI. Overall, treatment failure occurred in 18 (25.7%) patients and correlated with leukocytosis on presentation. While the small number of patients on vancomycin precluded a comparison of efficacy between metronidazole and vancomycin, the authors concluded that initial CDI therapy with vancomycin may be appropriate for elderly patients, especially those with elevated white blood cell counts.Citation40
Mounting evidence, therefore, suggests that in older adults with CDI, recurrence and treatment failure with metronidazole may be higher, so it may be reasonable to initiate therapy with vancomycin in all older adults with CDI.
Fidaxomicin
Fidaxomicin, US Food and Drug Administration (FDA) approved in May 2011 for CDI, is a bactericidal macrolide that inhibits nucleic acid synthesis by impairing bacterial RNA polymerase activity.Citation41 Fidaxomicin has a narrower spectrum of antimicrobial activity than metronidazole or vancomycin, thus limiting disruption to the normal gastrointestinal flora.Citation42 In addition, fidaxomicin has a prolonged post-antibiotic effect (~10 hours) allowing for twice-daily dosing.Citation43
The in vitro effect of fidaxomicin and its metabolite, OpT-1118, on C. difficile growth and sporulation dynamics was compared to vancomycin, metronidazole, and rifaximin. In comparison to the three comparator drugs, fidaxomicin and OpT-118 effectively inhibited C. difficile sporulation.Citation43 More recently, Housman et al sought to compare the number of C. difficile vegetative cells and spores in stool among patients receiving fidaxomicin or vancomycin as treatment for their first CDI episode. Thirty-four patients were enrolled, majority of them elderly: mean ages of the fidaxomicin (n=18) and vancomycin groups (n=16) were 69 years (±15 years) and 66 years (±15 years), respectively. Vancomycin and fidaxomicin therapy both resulted in rapid decreases in vegetative C. difficile counts throughout therapy; however, more patients receiving fidaxomicin achieved at least a 2 log10 colony-forming units/g reduction in spores at the 2-week follow-up visit (p=0.02).Citation44
Several clinical trials, with an adequate representation of elderly patients, have been conducted to compare the efficacy and safety of fidaxomicin in CDI treatment. In the two Phase III noninferiority RCTs, fidaxomicin was compared with vancomycin in the treatment of new-onset or first recurrence of CDI with a 28-day follow-up period.Citation45–Citation47 A total of 1,164 participants were evaluated in the pooled dataset. The mean reported age was 63 years and 61 years in the Louie study and Cornely study, respectively.Citation45,Citation46 Fidaxomicin was proven to be noninferior to vancomycin for CDI treatment and more effective than vancomycin in reducing the rate of recurrence. These findings were not influenced by age stratification (age <65 vs ≥65 years) in subgroup analysis. It should be noted that fidaxomicin was not associated with fewer recurrences among patients infected with the NAP1/BI/027 strain versus those infected with other C. difficile strains, possibly due to the small numbers of NAP1/BI/027 strain-infected patients. With regard to adverse events, fidaxomicin was well tolerated with a similar safety profile compared to oral vancomycin.Citation47
Utilizing combined data from the two RCTs conducted for fidaxomicin drug approval, Louie et al examined the effects of age (characterized in decades: ≤40, 41–50, 51–60, 61–70, 71–80, and >80 years) and study drug on CDI outcomes.Citation48 They reported a statistically significant linear effect of age on CDI outcomes, specifically a 17% lower clinical cure, 17% greater recurrence, and 13% lower sustained clinical response by advancing decade than in those younger than 40 years (p<0.01 each). Vancomycin and fidaxomicin were comparably effective in attaining clinical cure in all age strata; however, for participants who achieved clinical cure, fidaxomicin-treated participants were half as likely to have had a recurrence as participants treated with vancomycin (OR =0.46; 95% CI 0.32–0.67; p<0.001). Consequently, the authors suggest that fidaxomicin be considered an alternative to vancomycin for treatment of CDI, particularly in elderly adults, who have a higher likelihood of developing recurrent disease.Citation48
While fidaxomicin has a favorable safety and twice-a-day dosing profile, its current high drug acquisition cost poses a significant barrier to adoption in clinical practice. However, given its advantage in reducing the risk of recurrent CDI, targeting its use to populations at highest risk of relapse, including elderly patients, may prove to be cost-effective.Citation19–Citation23,Citation49,Citation50
Oral vancomycin and fidaxomicin are poorly absorbed; thus, systemic adverse effects are minimal. In addition, oral vancomycin and fidaxomicin do not require dose adjustment in the elderly or in patients with hepatic or renal dysfunction. On the other hand, the oral formulation of metronidazole is systemically absorbed but achieves effective concentrations in the colon after secretion back into the lumen.Citation19,Citation51 Intravenous and oral metronidazole have frequently been reported to cause diarrhea, nausea, gastrointestinal discomfort, and dysgeusia. Severe adverse effects of metronidazole include seizures, encephalopathy, and peripheral neuropathy. Metronidazole is also implicated in several drug interactions including an increased risk of bleeding with concomitant warfarin, a commonly utilized anticoagulant in the elderly. Fortunately, metronidazole dose adjustment is not required in the elderly.Citation52
Bezlotoxumab
Although metronidazole, vancomycin, and fidaxomicin are effective in the treatment of CDI, they each disrupt the indigenous gastrointestinal microbiota to varying degrees. This presents a considerable challenge in the risk reduction of recurrent CDI episodes. Because the pathogenesis of CDI is closely linked to the dysregulation of the gastrointestinal microbiota and host immune response, the development of immunotherapy is a rational therapeutic strategy and an area of increased interest. The severity and range of the symptoms of CDI are caused by the two C. difficile virulence factors, TcdA and TcdB. The magnitude of antibody response to these C. difficile virulence toxins is inversely correlated with the relative risk of developing recurrent disease. Indeed, studies have identified low endogenous anti-TcdA and -TcdB antibody levels as a risk factor for CDI recurrence.Citation53
Bezlotoxumab, approved in October 2016 by the FDA, is a human monoclonal antibody that binds to and neutralizes TcdB. This therapeutic strategy represents a recent advance in antibody-based immunotherapy for managing CDI. Bezlotoxumab binds to the combined repetitive oligopeptide domains of TcdB, and, through x-ray crystallography, has been shown to prevent binding of TcdB to mammalian cells.Citation54,Citation55 In addition to inciting a release of proinflammatory factors such as interleukin 8, TcdA and TcdB disrupt gastrointestinal epithelial cell tight junction resulting in acute diarrhea.Citation54–Citation57 The postulated mechanism of action of bezlotoxumab is direct toxin neutralization, thereby preventing the deleterious toxin effects and leading to restoration of a healthy microbiota.Citation58,Citation59
Bezlotoxumab is indicated in patients who are receiving standard-of-care anti-C. difficile treatment and are at a high risk for CDI recurrence.Citation60 The median age of participants was 66 years in the pivotal Phase III trials. CDI recurrence occurred in 16.5% of the bezlotoxumab group compared to 26.6% (p<0.0001) in the placebo group. Sustained cure (defined as initial clinical cure of the baseline episode of CDI and no recurrent infection through the 12-week follow-up period) was 64% with bezlotoxumab compared to 54% with placebo. Across prespecified groups who were at high risk for recurrent CDI, the rates of recurrent infection were lower with receipt of bezlotoxumab. In particular, among patients 65 years or older, bezlotoxumab was associated with a CDI recurrence rate that was 51% lower than that associated with placebo.Citation59 While bezlotoxumab was found to protect against CDI morbidity, like all medications, potential adverse events exist. Heart failure was more commonly reported in patients who received bezlotoxumab compared to placebo (12.7% vs 4.8%, respectively), prompting the FDA to require a warning label in the bezlotoxumab package insert.Citation59,Citation60
In addition, the impact of systemic concomitant antibiotics on the efficacy of bezlotoxumab is necessary to add valuation to this new therapy. Interestingly, actoxumab, developed in tandem with bezlotoxumab, is another human monoclonal antibody that neutralizes toxin A. However, actoxumab alone did not decrease C. difficile recurrence and had a worse adverse event profile.Citation59 Antibodies are poised to become an essential therapeutic strategy in the management of CDI and bezlotoxumab represents a significant advancement. However, like most first-in-class agents, concerns over real-world effectiveness and drug cost remain.
Fecal microbiota transplant
Relapse of CDI occurs in 10%–25% of patients treated with metronidazole or vancomycin. Furthermore, multiple relapses in the same individual are common.Citation28,Citation37 In recognition of the importance of restoring balance to the disrupted gastrointestinal flora, major guidelines have addressed the role of fecal microbiota transplant (FMT) but differ in their recommendations given the limited evidence at time of respective publications.Citation19–Citation21 For example, the 2010 Infectious Diseases Society of America/Society for Healthcare Epidemiology of America guidelines recognized FMT as a promising emerging therapy but due to a lack of randomized controlled trials were unable to evaluate its efficacy and safety.Citation19 On the other hand, for multiple recurrent CDIs unresponsive to repeated antibiotic treatment, European Society of Clinical Microbiology and Infection strongly recommends the use of FMT in combination with oral antibiotic treatment.Citation21 The American College of Gastroenterology offered a more reserved recommendation, “if there is a third recurrence after a pulsed vancomycin regimen, FMT should be considered (Conditional recommendation, moderate-quality evidence)”.Citation20
Since the major guidelines were published, interest in FMT has grown rapidly. A review of the literature reveals that FMT is gaining acceptance as an effective therapy for recurrent CDI.Citation61 Cumulative experience from case series and controlled trials shows that FMT is effective (80%–90%) when used to treat relapsing CDI.Citation62–Citation65 For example, a recent systemic review evaluated data from two RCTs, 28 case-series studies, and five case reports. The study subjects were predominantly elderly, and symptom resolution was seen in 85% of cases.Citation65
To better understand the impact of FMT on CDI in the elderly, Burke et al identified 115 patients from 10 pooled case studies, ranging in age from 60 to 101 years (mean age: 77 years). Durable remission of CDI was achieved in 103 (89.6%) patients over a follow-up period of 2 months to 5 years (mean 5.9 months). Cure rate in the older population (89.6%) was not significantly different from that of the 52 younger individuals (80.8%) in the included studies (p=0.26). Although most achieved bacteriological cure without complication, one patient died of peritonitis that may have resulted from nasogastric tube perforation during fecal transplantation.Citation66 In the subgroup analysis of a more recent meta-analysis, long-term outcomes of FMT for CDI were compared between older individuals (≥65 years old) and younger individuals (<65 years old). The primary cure rate (resolution of diarrhea without recurrence within 90 days of FMT) was higher in younger individuals compared to older individuals (99.4% vs 87.0%; p=0.0003). Among younger groups, the overall recurrence rate post-FMT was 4.6% compared to 9.3% for older individuals. The authors concluded that while FMT is likely a highly effective and robust therapy for recurrent CDI in adults, old age (≥65 years) should be considered as a risk factor for early CDI recurrence post-FMT therapy.Citation67
Identification of a healthy stool donor is an essential initial step to successful FMT. Because the indigenous gastrointestinal microbiota undergoes age-related changes, the selection of healthy FMT donors from among the elderly population may prove a challenge. In practice, younger donors tend to donate stool samples for their older relatives while older donors commonly donate specimens for their spouses. Guidelines do not suggest an upper limit of age to exclude donors for the purpose of FMT.Citation68 To address the lack of data regarding the effect of donor age on fecal microbiota and its clinical efficacy in patients with recurrent CDI, Anand et al utilized stool sample rRNA sequencing and demonstrated that while there was a decrease in the abundance of phylum Actinobacteria in donors above 60 years of age compared to the younger donor group (<60 years), there was no significant difference in the alpha diversity between the two donor groups.Citation69 Additional larger studies in both age and ethnic diverse populations are required to corroborate these findings.
Despite the growing support for FMT, clinicians and patients need to be cognizant of the inevitable risk of communicable disease transmission.Citation70 Another important consideration is the route of administration. A number of delivery modalities have been described for FMT: nasogastric or nasojejunal tube, colonoscopy, and enemas. Recently, the development of oral FMT capsules has garnered interest. The safety and rate of diarrhea resolution following administration of oral capsulized frozen FMT was evaluated in a feasibility study with 20 patients (median age 64.5 years; interquartile range 53.5–78.3). Resolution of diarrhea was achieved in 14 patients (70%; 95% CI 47%–85%) after a single-capsule-based regimen and in 90% of patients after non-responders were retreated. Age was not associated with CDI relapse.Citation71 Having a variety of delivery modalities, especially oral FMT capsules, may benefit the elderly population because of ease of administration and the avoidance of procedure-associated risk with invasive administration modalities such as colonoscopy.Citation70–Citation72
Probiotics
Probiotics, a nutritional supplement, contain either a single culture or a mixed culture of live microorganisms such as Lactobacillus and Bifidobacterium strains and the yeast Saccharomyces boulardii.Citation73,Citation74 They represent another therapeutic strategy targeting the restoration of microbiota flora.
Evidence around the probiotic effect has been mixed.Citation75,Citation76 For example, the largest placebo-controlled randomized trial conducted in 2,941 inpatients aged 65 years or older that received probiotics (multistrain preparation of Lactobacillus and Bifidobacterium) failed to demonstrate a reduction in antibiotic-associated diarrhea or C. difficile rates.Citation75 On the other hand, a recent meta-analysis, incorporating the aforementioned trial in addition to 25 other studies, did show a significantly lower risk of developing CDI in the probiotics group compared to the control group (relative risk [RR] =0.395; 95% CI 0.294–0.531; p<0.001).Citation77 Subgroup analysis identified that Lactobacillus, Saccharomyces, or a mixture of probiotics was beneficial in reducing the risk of developing CDI. Probiotics were beneficial for both adults (RR =0.405; 95% CI 0.294–0.556; p<0.001) and children (RR =0.341; 95% CI 0.153–0.759; p=0.008).Citation77
Though there are numerous studies and several systematic reviews evaluating the use of probiotics, the wide variety of probiotic strains, dosages, and durations of therapy makes it difficult to interpret. Overall, there is moderate-quality evidence supporting a protective effect of probiotics in preventing CDI in patients taking antibiotics.Citation10,Citation75,Citation77 In addition, the use of probiotics has been controversial because of the rare case reports of fungemia in both immunocompromised and immunocompetent patients.Citation75,Citation78 High-quality studies, utilizing standardized regimens, are certainly required in diverse populations including the elderly.
Combination antibiotics
There is a paucity of data on the efficacy of combination therapy in the management of CDI. Njoku et al sought to shed light on the impact of combination therapy versus monotherapy for CDI in a recent single-center study.Citation79 Median age was 59 years and 63 years in the monotherapy and combination therapy groups, respectively (p=0.08). Approximately 9% of patients were admitted from a nursing or LTCF. Overall, 177 of 248 patients (71.4%) achieved clinical cure. There were no differences in time to return of daily bowel movements to ≤2/day, clinical cure, length of stay, recurrence, or mortality, and while not clinically significant the combination therapy group had longer duration of therapy than the monotherapy group (15 vs 14 days; p=0.009).Citation79
In a systematic review comparing metronidazole mono-therapy with vancomycin monotherapy and combination therapy in CDI patients, no statistically significant difference was observed between monotherapy and combination therapy. The rate of adverse drug events was lower for monotherapy than that for combination therapy (OR =0.30; 95% CI 0.17–0.51; p<0.0001).Citation80
Miscellaneous agents
Besides the therapies discussed earlier, other therapeutic agents have been utilized for the treatment of CDI including rifaximin, nitazoxanide, and tigecycline. Most of the evidence for these agents comes from case reports and their utility in the elderly is largely unknown.
Nitazoxanide
Nitazoxanide, a nitrothiazolide, is FDA approved for the treatment of cryptosporidiosis and giardiasis, and is routinely employed in the management of parasitic intestinal infections through inhibition of anaerobic metabolism. However, for the treatment of CDI, there appears to be limited evidence.
In a noninferior, RCT, nitazoxanide was shown to be at least as effective as metronidazole in the treatment of C. difficile colitis. This study was conducted across seven VA medical centers with a predominance of elderly male patients.Citation81 Subsequently, a similarly designed study by the same investigators was designed to compare vancomycin and nitazoxanide therapy.Citation82 Among those who completed therapy, sustained response rates were 78% for the vancomycin group and 89% for the nitazoxanide group. Forty percent of patients were categorized as severe CDI and mean age was 59.6 years and 65.7 years in the nitazoxanide and vancomycin groups, respectively (p=0.19). The small sample (N=49) precluded any noninferiority analysis; nonetheless, the results suggest that nitazoxanide may be as effective as vancomycin.Citation82
Tigecycline
A derivative of minocycline, tigecycline has broad-spectrum activity against Gram-positive and Gram-negative organisms and anaerobic bacteria such as Bacteroides fragilis.Citation83 Several case reports have reported the use of intravenous tigecycline as salvage therapy for severe refractory cases of CDI with varying outcomes. A limited number of these reports involved elderly adults.Citation84,Citation85
In one such case series, Herpers et al present four patients with severe refractory CDI who were successfully treated with tigecycline. Three patients had previously failed standard CDI therapy while one patient was treated with tigecycline upon CDI onset. The pertinent demographics of the patients are as follows: 59-year-old male, 36-year-old female, 36-year-old male, and an 82-year-old female.Citation84
A single-center retrospective study by Thomas et al compared the outcomes of patients who received standard-of-care therapy with tigecycline (n=18) versus standard-of-care therapy without tigecycline (n=26) for severe CDI.Citation86 Median age of patients in the tigecycline group was 55 years and 63 years in the non-tigecycline group. No difference in treatment outcomes including overall survival, colectomy rates, and relapse rates were observed between the two groups.Citation86
Rifaximin
Rifaximin is a nonabsorbable derivative of rifamycin. It is primarily used in the management of irritable bowel syndrome, hepatic encephalopathy, and traveler’s diarrhea. Rifaximin shows potent activity against C. difficile, and clinical anecdotes have reported use as an adjunctive antibiotic for the treatment of recurrent and refractory CDI. For the treatment of mild-to-moderate CDI, clinical success with rifaximin (57%) was similar to vancomycin (64%) therapy but failed to achieve the goal of noninferiority in a RCT.Citation87
Johnson et al reported the clinical courses of the six CDI recurrent patients treated with rifaximin (post-vancomycin treatment).Citation88 The six patients were 88, 33, 78, 85, 81, and 66 years old, with a mean age of 72 years. Four of the six patients (67%) had no further diarrhea episodes, but two patients relapsed shortly after or during the rifaximin treatment. Of note, the two patients classified as treatment failure were elderly (88 and 85 years old), and one of these two patients had a C. difficile isolate minimum inhibitory concentration of >256 μg/mL to rifampin.Citation88
Cost-effectiveness
In addition to contributing to patient morbidity and mortality, CDI exerts a substantial financial toll on health systems, with a total US economic burden thought to exceed $1 billion per year.Citation89 As a result, hospitals and third-party payers are increasingly relying on the economic analysis of available and emerging therapeutic agents in their formulary decision-making. The varied purchasing, pricing, and insurance reimbursement structures utilized in different countries limit extrapolation of these analyses.
For example, analysis from a Scottish public health care provider perspective showed that compared to vancomycin, fidaxomicin is cost-effective in either patients with severe CDI or a first CDI recurrence.Citation50 In the USA, Konijeti et al compared four treatment strategies (metronidazole, vancomycin, fidaxomicin, and FMT via colonoscopy) for first-line treatment of recurrent CDI in a hypothetical cohort of patients with a median age of 65 years.Citation90 Initial treatment with FMT via colonoscopy was the most cost-effective strategy for recurrent CDI at cure rates greater than 88.4%. In clinical setting where FMT via colonoscopy is not available or cure rates are lower than threshold, oral vancomycin was more cost-effective.Citation90 Similarly, FMT by colonoscopy (or enema, if colonoscopy is unavailable) was concluded to be cost-effective for treating recurrent CDI in Canada. The modeled patient in this particular study was a 70-year-old community-dweller.Citation91
With regard to fidaxomicin in particular, cost-effectiveness analysis has been mixed given the varied methodological approaches. For example, utilizing a number-needed-to-treat of 7.1 for sustained clinical response from the two pivotal fidaxomicin trials, an epidemiologic study estimated that at $280 US dollars, fidaxomicin represents value for money in the treatment of CDAD.Citation92 On the other hand, Bartsch et al utilized a decision analytic simulation model to demonstrate that using fidaxomicin as a first-line treatment for CDI is not cost-effective when NAP1/BI/027 accounts for ~50% of infecting strains. In fact, a course of fidaxomicin would need to cost ≤$150 to be cost-effective in the treatment of all CDI cases. The authors suggest that treatment with fidaxomicin based on strain may be a reasonable approach.Citation93
Conclusion
Our understanding of CDI continues to evolve but it is apparent that advanced age is a major risk factor and one that results in substantial morbidity and mortality. Appropriate CDI prevention and management strategies involve antimicrobial and non-antimicrobial complimentary approaches. Metronidazole remains the initial treatment for mild-to-moderate CDI in majority of patients; however, evidence suggests that vancomycin or fidaxomicin may be considered as first-line options in the elderly. Certainly, there is no one-size-fits-all approach. For each elderly patient, therapeutic decisions should be guided by several factors, including the severity of the primary infection, underlying comorbidities, the severity of CDI, and the patient’s end-of-life wishes.
An updated C. difficile management guideline by Infectious Diseases Society of America/Society for Healthcare Epidemiology of America is anticipated in 2017 and will likely provide evidence-based recommendations on current and emerging treatment options, including FMT and bezlotoxumab, especially in populations at greatest risk of relapse. A concerted effort from national and state public health agencies, health care providers, and antimicrobial stewardship teams is required to decrease the burden of CDI in our aging population. Finally, the limited studies on CDI management among the elderly, especially LTCF residents, warrant further research to identify poor prognostic indicators and to validate interventions that may improve outcomes among this vulnerable population.
Disclosure
The authors report no conflicts of interest in this work.
References
- KellyCPLaMontJTClostridium difficile – more difficult than everN Engl J Med2008359181932194018971494
- SimorAEDiagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a reviewJ Am Geriatr Soc20105881556156420646106
- LefflerDALamontJTClostridium difficile infectionN Engl J Med2015372161539154825875259
- LessaFCMuYBambergWMBurden of Clostridium difficile infection in the United StatesN Engl J Med2015372982583425714160
- Centers for Disease Control and PreventionNearly half a million Americans suffered from Clostridium difficile infections in a single year2015 Available from: https://www.cdc.gov/media/releases/2015/p0225-clostridium-difficile.htmlAccessed August 12, 2017
- GerdingDNJohnsonSManagement of Clostridium difficile infection: thinking inside and outside the boxClin Infect Dis201051111306131320979491
- TsutsumiLSOwusuYBHurdleJGSunDProgress in the discovery of treatments for C. difficile infection: a clinical and medicinal chemistry reviewCurr Top Med Chem201414115217524236721
- GoldbergEJBhalodiaSJacobSClostridium difficile infection: a brief update on emerging therapiesAm J Health Syst Pharm201572121007101226025991
- OfosuAClostridium difficile infection: a review of current and emerging therapiesAnn Gastroenterol201629214715427065726
- GerdingDNYoungVBClostridium difficile infectionMandellGLBennettJCDolinRMandell, Douglas, and Bennett’s: Principles and Practice of Infectious Disease8th edPhiladelphia, PAElsevier201527442756.e3
- ChoIBlaserMJThe human microbiome: at the interface of health and diseaseNat Rev Gen2012134260270
- PruittRNLacyDBToward a structural understanding of Clostridium difficile toxins A and BFront Cell Infect Microbiol201222822919620
- McDonaldLCCoignardBDubberkeESongXHoranTKuttyPKAd Hoc Clostridium difficile Surveillance Working GroupRecommendations for surveillance of Clostridium difficile-associated diseaseInfect Control Hosp Epidemiol200728214014517265394
- PépinJValiquetteLAlaryMEClostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severityCMAJ2004171546647215337727
- RaoKMicicDChenowethEPoor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adultsJ Am Geriatr Soc201361101738174224083842
- Centers for Disease Control and PreventionAntibiotic resistance threats in the United States2013 Available from: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdfAccessed August 12, 2017
- LucadoJGouldCElixhauserAClostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #12420121Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]Rockville, MDAgency for Healthcare Research and Quality (US)2006 Available from: http://www.ncbi.nlm.nih.gov/books/NBK92613/Accessed August 12, 2017
- RedelingsMDSorvilloFMascolaLIncrease in Clostridium difficile-related mortality rates, United States, 1999–2004Emerg Infect Dis20071391417141918252127
- CohenSHGerdingDNJohnsonSSociety for Healthcare Epidemiology of AmericaInfectious Diseases Society of AmericaClinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA)Infect Control Hosp Epidemiol201031543145520307191
- SurawiczCMBrandtLJBinionDGGuidelines for diagnosis, treatment, and prevention of Clostridium difficile infectionsAm J Gastroenterol2013108447849823439232
- DebastSBBauerMPKuijperEJEuropean Society of Clinical Microbiology and Infectious DiseasesEuropean Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infectionClin Microbiol Infect201420Suppl 2126
- Abou ChakraCNPepinJSirardSValiquetteLRisk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. Paredes-Sabja D, edPLoS One201496e9840024897375
- VardakasKZKonstanteliasAALoizidisGRafailidisPIFalagasMERisk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysisInt J Infect Dis20121611e768e77322921930
- GoorhuisADebastSBDutilhJCType-specific risk factors and outcome in an outbreak with 2 different Clostridium difficile types simultaneously in 1 hospitalClin Infect Dis201153986086921914851
- LooVGBourgaultAMPoirierLHost and pathogen factors for Clostridium difficile infection and colonizationN Engl J Med2011365181693170322047560
- NicolleLEBentleyDWGaribaldiRNeuhausEGSmithPWAntimicrobial use in long-term–care facilities infection control and hospitalEpidemiology2000218537545
- JumpRLPOldsDMSeifiNEffective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic useInfect Control Hosp Epidemiol201233121185119223143354
- DrekonjaDMAmundsonWHDecarolisDDKuskowskiMALederleFAJohnsonJRAntimicrobial use and risk for recurrent Clostridium difficile infectionAm J Med2011124111081.e1e7
- HuMYKatcharKKyneLProspective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infectionGastroenterology200913641206121419162027
- YoshikawaTTNormanDCGeriatric infectious diseases: current concepts on diagnosis and managementJ Am Geriatr Soc201765363164128140454
- ZhengBHanSTakahashiYKelsoeGImmunosenescence and germinal center reactionImmunol Rev1997160163779476666
- RomeoJWärnbergJPozoTMarcosAPhysical activity, immunity and infectionProc Nutr Soc201069339039920569522
- NaylorKLiGVallejoANThe influence of age on T cell generation and TCR diversityJ Immunol2005174117446775215905594
- KyneLMerryCO’ConnellBKellyAKeaneCO’NeillDFactors associated with prolonged symptoms and severe disease due to Clostridium difficileAge Ageing199928210711310350405
- WenischCParschalkBHasenhündlMHirschlAMGraningerWComparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrheaClin Infect Dis19962258138188722937
- VardakasKZPolyzosKAPatouniKRafailidisPISamonisGFalagasMETreatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidenceInt J Antimicrob Agents20124011822398198
- ZarFABakkanagariSRMoorthiKMDavisMBA comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severityClin Infect Dis200745330230717599306
- LiRLuLLinYWangMLiuXEfficacy and safety of metronidazole monotherapy versus vancomycin monotherapy or combination therapy in patients with Clostridium difficile infection: a systematic review and meta-analysisPLoS One20151010e013725226444424
- JohnsonSLouieTJGerdingDNPolymer Alternative for CDI Treatment (PACT) investigatorsVancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trialsClin Infect Dis201459334535424799326
- CoberEDMalaniPNClostridium difficile infection in the “oldest” old: clinical outcomes in patients aged 80 and olderJ Am Geriatr Soc200957465966219392957
- ArtsimovitchISeddonJSearsPFidaxomicin is an inhibitor of the initiation of bacterial RNA synthesisClin Infect Dis201255Suppl 2S127S13122752861
- FinegoldSMMolitorisDVaisanenM-LSongYLiuCBolañosMIn vitro activities of OPT-80 and comparator drugs against intestinal bacteriaAntimicrob Agents Chemother200448124898490215561877
- BabakhaniFGomezARobertNSearsPPostantibiotic effect of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficileAntimicrob Agents Chemother20115594427442921709084
- HousmanSTThabitAKKutiJLQuintilianiRNicolauDPAssessment of Clostridium difficile burden in patients over time with first episode infection following fidaxomicin or vancomycinInfect Control Hosp Epidemiol201637221521826592763
- LouieTJMillerMAMullaneKMOPT-80-003 Clinical Study GroupFidaxomicin versus vancomycin for Clostridium difficile infectionN Engl J Med2011364542243121288078
- CornelyOACrookDWEspositoROPT-80-004 Clinical Study GroupFidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trialLancet Infect Dis201212428128922321770
- CrookDWWalkerASKeanYFidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trialsClin Infect Dis201255Suppl 2S93S10322752871
- LouieTJMillerMACrookDWEffect of age on treatment outcomes in Clostridium difficile infectionJ Am Geriatr Soc201361222223023379974
- MullaneKFidaxomicin in Clostridium difficile infection: latest evidence and clinical guidanceTher Adv Chronic Dis201452698424587892
- NathwaniDCornelyOAVan EngenAKOdufowora-SitaORetsaPOdeyemiIAOCost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infectionJ Antimicrob Chemother201469112901291225096079
- BoltonRPCulshawMAFaecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficileGut19862710116911723781329
- Flagyl (metronidazole) tablets [package insert]New YorkPfizer2013
- LeavBABlairBLeneyMSerum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI)Vaccine201028496596919941990
- BabcockGJBroeringTJHernandezHJHuman monoclonal antibodies directed against toxins A and B prevent Clostridium difficile- induced mortality in hamstersInfect Immun200674116339634716966409
- OrthPXiaoLHernandezLDMechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallographyJ Biol Chem201428926180081802124821719
- VothDEBallardJDClostridium difficile toxins: mechanism of action and role in diseaseClin Microbiol Rev200518224726315831824
- ShenAClostridium difficile toxins: mediators of inflammationJ Innate Immun20124214915822237401
- YangZRamseyJHamzaTMechanisms of protection against Clostridium difficile infection by the monoclonal antitoxin antibodies actoxumab and bezlotoxumabInfect Immun201583282283125486992
- WilcoxMHGerdingDNPoxtonIRMODIFY I and MODIFY II InvestigatorsBezlotoxumab for prevention of recurrent Clostridium difficile infectionN Engl J Med2017376430531728121498
- Zinplava (bezlotoxumab) intravenous injection [package insert]White-house Station, NJMerckl2016
- BakkenJSFecal bacteriotherapy for recurrent Clostridium difficile infectionAnaerobe200915628519778623
- BowdenTAJrMansbergerARJrLykinsLEPseudomembraneous enterocolitis: mechanism for restoring floral homeostasisAm Surg19814741787224366
- GoughEShaikhHMangesARSystematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infectionClin Infect Dis2011531099422002980
- KassamZHundalRMarshallJKLeeCHFecal transplant via retention enema for refractory or recurrent Clostridium difficile infectionArch Intern Med2012172219119322271132
- DrekonjaDReichJGezahegnSFecal microbiota transplantation for Clostridium difficile infection: a systematic reviewAnn Intern Med2015162963025938992
- BurkeKELamontJTFecal transplantation for recurrent Clostridium difficile infection in older adults: a reviewJ Am Geriatr Soc20136181394139823869970
- LiYTCaiHFWangZHXuJFangJYSystematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infectionAliment Pharmacol Ther201643444545726662643
- Current Consensus Guidance on Donor Screening and Stool Testing for FMTAmerican Gastroenterological Association20137 Available from: https://www.gastro.org/research/Joint_Society_FMT_Guidance.pdfAccessed August 13, 2017
- AnandRSongYGargSEffect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomesDig Dis Sci20176241002100828181098
- KassamZLeeCHYuanYHuntRHFecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysisAm J Gastroenterol2013108450050823511459
- YoungsterIRussellGHPindarCZiv-BaranTSaukJHohmannELOral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infectionJAMA2014312171772177825322359
- ZipurskyJSSidorskyTIFreedmanCASidorskyMNKirklandKBPatient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infectionClin Infect Dis201255121652165822990849
- IslamJCohenJRajkumarCLlewelynMJProbiotics for the prevention and treatment of Clostridium difficile in older patientsAge Ageing201241670671122718155
- HicksonMProbiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infectionTher Adv Gastroenterol201143185197
- AllenSJWarehamKWangDLactobacilli and bifido-bacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trialLancet201338299001249125723932219
- McFarlandLVProbiotics for the primary and secondary prevention of C. difficile infections: a meta-analysis and systematic review. Chang Y-F, edAntibiotics20154216017827025619
- LauCSChamberlainRSProbiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysisInt J Gen Med20169273726955289
- LiongMTSafety of probiotics: translocation and infectionNutr Rev200866419220218366533
- NjokuJCVan SchooneveldTCRuppMELack of benefit with combination therapy for Clostridium difficile infectionInfect Control Hosp Epidemiol201738560260528162100
- LiRLuLLinYWangMLiuXEfficacy and safety of metronidazole monotherapy versus vancomycin monotherapy or combination therapy in patients with Clostridium difficile infection: a systematic review and meta-analysisPLoS One20151010e013725226444424
- MusherDMLoganNHamillRJNitazoxanide for the treatment of Clostridium difficile colitisClin Infect Dis200643442142716838229
- MusherDMLoganNBresslerAMJohnsonDPRossignolJFNita-zoxanide versus vancomycin in Clostridium difficile infection: a randomized, double-blind studyClin Infect Dis2009484e41e4619133801
- SteinGECraigWATigecycline: a critical analysisClin Infect Dis200643451852416838243
- HerpersBLVlaminckxBBurkhardtOIntravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infectionClin Infect Dis200948121732173519435431
- LuCLLiuCYLiaoCHHuangYTWangHPHsuehPRSevere and refractory Clostridium difficile infection successfully treated with tigecycline and metronidazoleInt J Antimicrob Agents201035331131220045292
- ThomasAKhanFUddinNWallaceMRTigecycline for severe Clostridium difficile infectionInt J Infect Dis20142617117225064460
- PardiDSBrennanRSpinnellMThe efficacy and safety of rifaximin vs. vancomycin in the treatment of mild to moderate C. difficile infection: a randomized double-blind active comparator trialGastroenterology20121425S-599
- JohnsonSSchrieverCPatelUPatelTHechtDWGerdingDNRifax-imin Redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatmentAnaerobe200915629029119698797
- McGloneSMBaileyRRZimmerSMThe economic burden of Clostridium difficileClin Microbiol2012183282289
- KonijetiGGSaukJShrimeMGGuptaMAnanthakrishnanANCost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysisClin Infect Dis201458111507151424692533
- Lapointe-ShawLTranKLCoytePCCost-effectiveness analysis of six strategies to treat recurrent Clostridium difficile infectionPLoS One2016112e014952126901316
- SclarDARobisonLMOganovAMSchmidtJMBowenKACastilloLVFidaxomicin for Clostridium difficile-associated diarrhoea: epidemiological method for estimation of warranted priceClin Drug Investig2012328e17e24
- BartschSMUmscheidCAFishmanNLeeBYIs fidaxomicin worth the cost? An economic analysisClin Infect Dis201357455556123704121
