Abstract
Purpose
To present optical coherence tomography (OCT) angiography features in patients with idiopathic full-thickness macular hole before and after vitrectomy.
Study design
Prospective case series study.
Materials and methods
Patients presenting with an idiopathic full-thickness macular hole (IMH) who underwent posterior vitrectomy with internal limiting membrane peeling and gas tamponade were included in the study. En face OCT and OCT angiography (OCTA) was performed pre- and postoperatively using 3×3 mm scans (Optovue, XR Avanti). Foveal avascular zone (FAZ) area, macular hole size (MHS), central retinal thickness (CRT), macular parafoveal choriocapillary flow area (MCFA), and fovea vessel density (FVDS) were measured and assessed using OCTA. Best-corrected visual acuity (BCVA) was examined before and 3 months after surgery.
Results
Twenty-eight eyes of 28 patients were included in the study. The mean age of patient group was 68.28 years. The hole was closed in all eyes after the initial surgery. OCTA showed enlargement of FAZ and increased CRT in foveal area. Mean preoperative FAZ area was 0.39±0.07 mm2. En face images of the middle retina showed a range of preoperative cystic patterns surrounding the hole. BCVA was improved from 0.1±0.11 preoperatively to 0.42±0.17 postoperatively. Mean FAZ area was reduced to 0.24±0.07 mm2 postoperatively with resolution of macular hole and adjacent cystic areas. Mean CRT was reduced from 396±62.6 µm pre-operatively to 272±30.7 µm postoperatively. After vitrectomy, the parafoveal choriocapillary flow area and FVDS of IMH eyes increased compared with the preoperative measurements.
Conclusion
Quantitative evaluation of vascular and morphological changes following IMH surgery using OCTA shows the potential for recovery due to vascular and neuronal plasticity. OCTA showing vascular changes and their quantitative characteristics might be a useful tool for the assessment of macular holes before and after surgical treatment.
Introduction
A macular hole (MH) is a tissue defect in the foveal retina involving its full thickness from internal limiting membrane (ILM) to the outer segment of photoreceptor layer. Although full-thickness macular hole (FTMH) was first described by Knapp in 1869,Citation1 in relation to trauma,Citation1–Citation3 inflammation, and myopia,Citation2,Citation3 more recent clinical studies have shown that the vast majority are idiopathicCitation4–Citation16 and can occur with a prevalence of 1/3,300 usually in the sixth and seventh decades of life.Citation17 There have been many studies investigating the cause of idiopathic MH that have implicated traction at the level of the vitreofoveal interface the underlying mechanism of FTMH formation.Citation17,Citation18
Pars plana vitrectomy (PPV) with gas tamponade first described by Kelly and WendelCitation19 in 1991 is the method of choice in the treatment of MH.Citation19 The introduction of vitrectomy has shown that surgical intervention is beneficial in >90% of cases with FTMH in promoting the anatomical closure of MH,Citation19–Citation22 with subsequent improvement of visual acuity in the majority of cases. After the introduction of optical coherence tomography (OCT), fine morphologic changes in MHs have been extensively investigated,Citation23–Citation28 which has increased the understanding of development and healing of MH.
Recovery of MH after vitrectomy begins with connection of the inner retina and formation of a bridge-like glial proliferation, followed by gradual restoration of the outer retina.Citation29 Various changes in the fine structure of the macula, during the healing process, have been described.Citation30–Citation42 These changes suggest that retinal morphologic remodeling occurs during the recovery process after MH surgery. Retinal vessels are another important component of the retinal structure and are known to be involved in the development and healing of retinal diseases.Citation43
OCTA is a novel imaging platform that utilizes motion contrast to visualize retinal microvasculature in a rapid, noninvasive way (without the use of a dye) and depth-resolved fashion. This technology has enabled to investigate vascular changes in eyes with retinal disease.Citation44 Previous studies have reported characteristics of OCTA images in eyes with MH.Citation45,Citation46 In this study, we investigated the characteristics of the foveal structure and retinal microvasculature in eyes with MH before and after surgery using OCTA.
Materials and methods
This prospective case series study comprises 28 consecutive patients who underwent vitrectomy for an idiopathic MH and a follow-up examination 3 months after surgery using OCTA. All procedures took place at Professor K. Gibinski University Clinical Center, Medical University of Silesia, Katowice, Poland, between April 2016 and January 2017. The research was approved by Bioethics Committee of Silesian Medical Chamber in Katowice (Nr 8/2017). All patients provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. We included cases of idiopathic MH. The patient group had a mean age of 68.28 years and included 9 males and 19 females. Fifteen patients were phakic and 13 pseudophakic. We excluded patients with history of trauma, high myopia (spherical equivalent ≥6.0 diopters or axial length 26 mm), MH combined with retinal detachment, reoperation for unclosed MH after vitrectomy, other vitreoretinal disease, glaucoma, uveitis or retinitis in history, patients with retinal dystrophy and central retinal degeneration, anti-vascular endothelial growth factor treatment in history, patients with cataract, or patients who underwent cataract surgery 3 months prior to PPV. We excluded patients with poor OCTA image quality (signal strength index <50). In cases of bilateral MH, the first affected eye was included in the analysis. Two surgeons performed PPV with ILM peeling and SF6 gas tamponade in all cases. ILM peeling was performed symmetrically around the fovea after staining with trypan blue. After the operation, the patient maintained a facedown position for 7 days. Each patient underwent a comprehensive ophthalmic examination pre- and postoperatively, including measurement of best-corrected visual acuity (BCVA) using Snellen charts, slit-lamp biomicroscopy with dilated Volk 90D fundoscopy, Goldmann applanation tonometry, and cross-sectional OCT as well as OCTA images using a commercial SD-OCT system (RTVue-XR Avanti, Optovue, Inc, Fremont, CA, USA) for imaging. The OCT images were used to measure the preoperative basal and minimum diameters of the MH. The pre- and postoperative foveal thickness was determined from the OCTA images.
The AngioVue OCTA device (Optovue, Inc) was used to obtain amplitude-decorrelation angiography images. All the OCTA measurements were performed preoperatively and 3 months after vitrectomy.
The scanning area was captured in 3×3 mm sections centered on the fovea. Only images with a signal strength index >50 were used, and images with motion artifacts and other artifacts were excluded. The en face images of the superficial capillary plexus (SCP) were segmented with an inner boundary at 3 µm beneath the inner limiting membrane and an outer boundary at 15 µm beneath the inner plexiform layer. The en face images of the deep capillary plexus (DCP) were segmented with inner and outer boundaries at 15 and 70 µm, respectively, beneath the inner plexiform layer. The segmentation errors in SCP and DCP slices in preoperative MH B-scans are due to central retinal defect and thickening associated with MH.
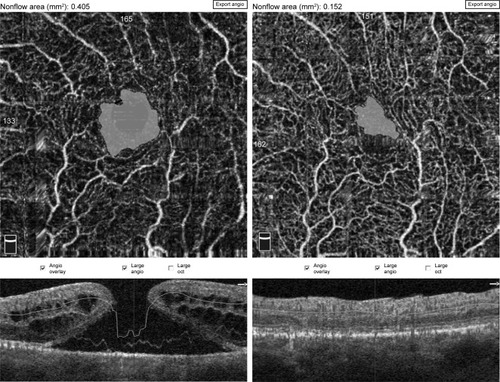
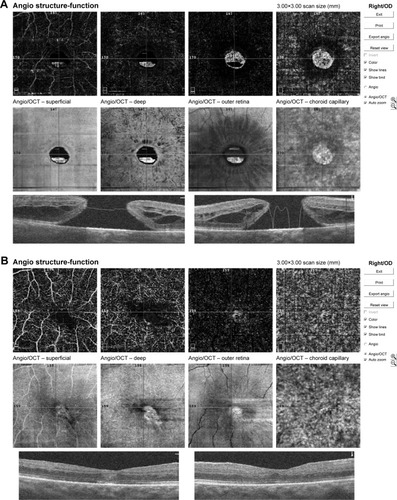
Calculation of foveal avascular zone (FAZ) area was performed on the superficial retinal OCTA slab (SCP) using the nonflow function of the AngioAnalytics imaging software embedded in the AngioVue OCTA device ().
Figure 1 Pre- and postoperative FAZ measurement using a nonflow software in an 82-year-old male patient.
Abbreviation: FAZ, foveal avascular zone.
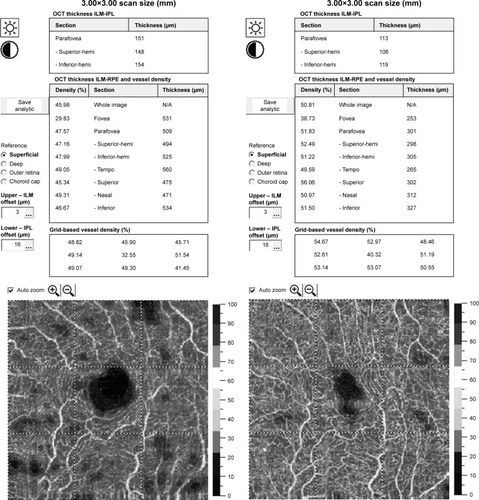
The fovea vessel density (FVDS) was measured on SCP images using a density function on the AngioAnalytics software. Qualitative data consisted of retinal vascular density color perfusion maps generated for each microvascular layer. In the color maps, bright red represents a density of greater than 50% perfused vessels, dark blue represents no perfused vessels, and intermediate perfusion densities are color coded accordingly. From OCT thickness inner limiting membrane:retinal pigment epithelium (ILM-RPE) and Vessel Density Report, we obtained the data about FVDS (in % perfused vessels) as well as a central retinal thickness (CRT) values (in µm) ().
Figure 2 The fovea vessel density (FVDS) measured in superficial capillary plexus (SCP) using a density function on the imaging software.
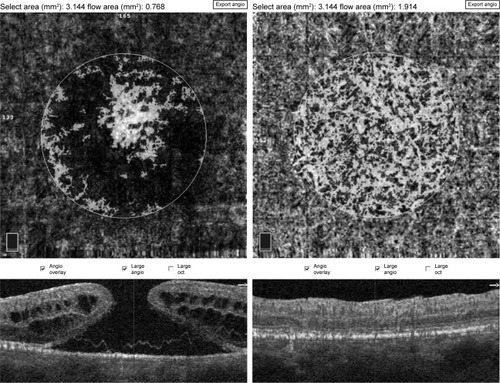
The en face images of the choriocapillary plexus were segmented with inner and outer boundaries at 31 and 59 µm, respectively, beneath the RPE-Reference Offset. Parafoveal choriocapillary flow area (in mm2) was measured on the choriocapillary slab using the flow function of the Angio-Analytics software with a circle of 1.0 mm radius centered in the middle of image. The flow area in choriocapillaries within the circle in chosen area is measured automatically by imaging software. The selected measurement area pre- and postoperatively was always 3.14 mm2 (based on a circle of 1 mm radius) ().
Figure 3 Pre- and postoperative choriocapillary flow area measurement using a flow tool of imaging software.
Statistical analysis was performed using IBM SPSS Statistics software. Statistical results were considered significant at P<0.05. Normality of parameters was assessed with the Shapiro–Wilk test. Student’s t-test was used for dependent samples. We used Pearson correlation coefficient (r) to measure the strength of linear relationship between two variables.
Results
Mean preoperative BCVA of MH eyes was 0.1±0.11 and improved to 0.42±0.17 at the final visit (P<0.001). Mean basal hole size in MH eyes was 937.39 µm, mean minimum hole diameter was 446.14 µm. The hole was closed in all cases after initial surgery, which was confirmed in OCT examination. The mean area of superficial FAZ preoperatively was 0.39±0.07 mm2 and was reduced to 0.24±0.07 mm2 postoperatively (P<0.001). The mean CRT in MH eyes was 396.75±62.6 µm preoperatively and was reduced to 272.17±30.7 µm postoperatively (P<0.001). En face images of the middle retina showed the preoperative cystic areas surrounding the hole in all MH eyes with resolution of the MH and adjacent cystic areas in all cases. A representative case is shown in and .
Figure 4 Pre- and post- operative OCTA of 82-year-old patient’s left eye.
Notes: (A) Preoperative OCTA report of 82-year-old male patient’s left eye showing numerous cystic changes in middle retina in en face scans. Macular basal hole size is 2,146 µm, minimum hole diameter is 427 µm. BCVA preoperatively was 0.08. (B) Postoperative OCTA scans of the same case as presented above. We observe in OCTA and en face scans the resolution of macular hole and adjacent cystic areas with normalization of foveal morphology and reduction of central retinal thickness. BCVA improved to 0.3 after surgery.
Abbreviations: BCVA, best-corrected visual acuity; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography.
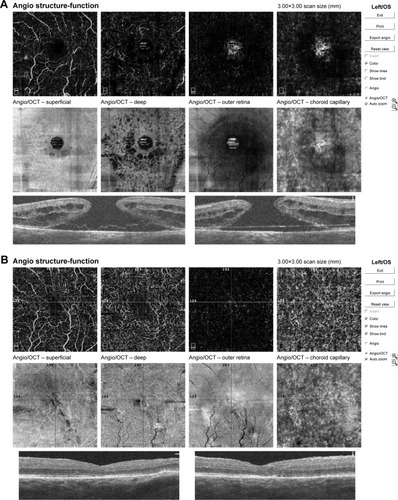
Figure 5 Pre- and post- operative OCTA of 75-year-old patient’s right eye.
Abbreviations: BCVA, best-corrected visual acuity; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography.
The mean preoperative parafoveal choriocapillary flow area in MH eyes was 1.59±0.28 mm2. Postoperative mean choriocapillary flow area in parafovea increased significantly to 1.93±0.03 mm2. Mean FVDS in SCP was 29.84%±4.17% preoperatively and increased to 35.02%±3.47% postoperatively (P<0.001).
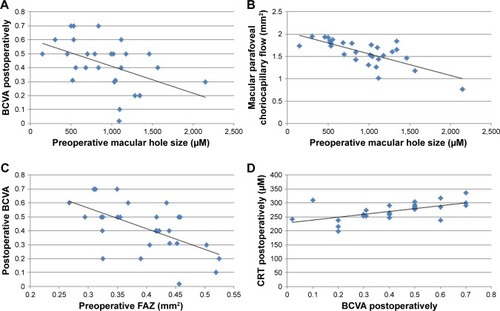
The r-Pearson correlation coefficient between the initial MHS and the BCVA after the treatment was measured: r=−0.47; P<0.05, the correlation was statistically significant. There is a moderate and negative correlation between these two variables. High values of one variable are accompanied by low values of the other variable (). There is a very strong correlation between the initial MHS and the MCFA before the treatment r=−0.729; P<0.01. The bigger MHS was corresponding with lower parafoveal choriocapillary flow area (). There was no statistical correlation between the initial MHS and FVDS in SCP before (r=0.018; P>0.05) and after the surgical treatment (r=0.222; P>0.05). There was also no statistically significant correlation between preoperative FAZ and BCVA before the treatment (r=0.369; P>0.05), but we found a relatively strong negative correlation between preoperative FAZ and postoperative BCVA (r=−0.59, P<0.01) (). There was also a strong correlation between BCVA and CRT after the treatment (r=0.6; P<0.01); higher postoperative CRT values were corresponding with lower BCVA outcomes postoperatively (). Moreover, there was neither a correlation between preoperative superficial fovea vessel density (FVDS) and BCVA postoperatively nor between preoperative MCFA and BCVA after the surgical treatment.
Figure 6 The r-Pearson correlation coefficient between the initial MHS and the BCVA.
Abbreviations: CRT, central retinal thickness; FAZ, foveal avascular zone.
Discussion
In our study, we investigated the morphological and vascular OCTA features of idiopathic MHs before and after surgical treatment. Regarding the superficial and deep vascular network as seen on OCTA, pre- and postoperative comparisons confirmed structural recovery following surgery. As previously described by Shahlaee et al,Citation47 in our study we observed resolution of the cystic changes in the middle retina on en face scans, which likely resulted in contraction of the FAZ with blood vessels and retinal tissue replacing the fluid-filled cystic areas with subsequent reduction of CRT. Other previous OCT studies have similarly demonstrated the dynamic healing process that occurs after surgical repair of MHs.Citation48 In our study, we observed postoperatively a statistically important increase of FVDS in SCP as well as an increase of flow area in choriocapillary network in OCTA. Moreover, we observe a statistically important reduction of FAZ postoperatively. This shows the potential for recovery due to neuronal and vascular plasticity and suggests that the healing process after MH surgery may be involved in both anatomic and hemodynamic changes of the inner retina.
We evaluated the relationship between vascular changes in OCTA and visual outcomes (BCVA postoperatively). Neither FVDS in superficial capillary network nor choriocapillary flow area (MCFA) was associated with final visual acuity, but we found a relatively strong correlation between preoperative FAZ and postoperative BCVA. The influence of FAZ size and its reduction following surgery on visual acuity outcomes may be an area worth exploring. We observed a significant increase of choriocapillary flow postoperatively. The mechanisms of impairment or restoration of choriocapillaris in FTMH remain unclear. A possible explanation would be that the restoration of choriocapillaris is associated with restoration of retinal structure.Citation49 The major blood supply to the retina is the choroid because the photoreceptors are extremely metabolically active. There is a continuous flow of ions and water across the retina and RPE into the choroid.Citation52 It has been suggested that the modulation of this flow may modulate the choroid.Citation53 The choriocapillaris in the foveomacular zone has a homogeneous structure. It has been suggested that foveomacular blood flow is determined by pressure gradients and differences in metabolic requirements within the macular retina.Citation54 The choroidal lobuli comprising the choriocapillaris layer of the choroid is also thought to determine the flow of blood based on both structure (lobuli) and function (neuroretinal activity).Citation55 Restoration of ions and water flow from sealed MH could help explain the restoration of choriocapillaris after the closure of FTMH.
Previous studies confirmed that postoperative photoreceptor layer status was associated with visual acuity.Citation50 This could mean that retinal vascular change might not be related to visual prognosis, which is consistent with other previous studies.Citation41,Citation42,Citation51 Our report has several limitations, such as small number of eyes and short postoperative observation period. We also did not evaluate in this study other than BCVA functional outcomes such as microperimetry or visual field. The relatively large MHS might be a bias, so the observation may not apply to all MHs, and further investigations are needed.
Although we showed that retinal vascular change was not related to visual outcome, further studies on a larger sample size and longer observation period with diverse tools for measurement of visual function are needed.
Conclusion
In summary, surgically closed MH eyes showed reduction of FAZ and increase of FVDS and choriocapillary flow area compared with these parameters preoperatively. We observed reduction of CRT and resolution of cystic changes in en face images after surgery. These results suggest that both anatomic and hemodynamic changes may be involved in the healing process of MH after surgery.
Quantitative evaluation of vascular and morphological changes following idiopathic full-thickness MH surgery using OCTA shows the potential for recovery due to vascular and neuronal plasticity.Citation47 OCTA showing vascular changes and their quantitative characteristics might be a useful tool for the assessment of MHs before and after surgical treatment.
Institution where the work was completed
University Clinical Center, Department of Ophthalmology, School of Medicine in Katowice, Medical University of Silesia, Katowice, Poland.
Disclosure
The authors report no conflicts of interest in this work.
References
- KnappHÜber isolierte zerreissungen der aderhaut in folge von traumen auf dem augapfelArch Augenheilkd18691612
- CollinsETUnusual changes to the macular regionTrans Ophthalmol Soc UK190020196197
- NoyesHDDetachment of the macula with laceration of the macula luteaTrans Am Ophthalmol Soc187118128134
- YaoedaHClinical observation on macular holeActa Soc Ophthalmol Jpn196771917231736 Japanese
- AabergTMBlairCJGassJDMacular holesAm J Ophthalmol19706945555625437820
- AabergTMMacular holes: a reviewSurv Ophthalmol197015139162
- GassJDStereoscopic Atlas of Macular Diseases: Diagnosis and TreatmentSt LouisCV Mosby1970195199
- MargheriaRRSchepensCLMacular breaks. 1. Diagnosis, etiology, and observationsAm J Ophthalmol19727422192324559896
- JamesMFemanSSMacular holesAlbrecht von Graefes Arch Klin Exp Ophthalmol1980215159636906170
- McDonnellPJFineSLHillisAIClinical features of idiopathic macular cysts and holesAm J Ophthalmol19829367777867091263
- MorganCMSchatzHIdiopathic macular holesAm J Ophthalmol19859944374443985081
- MorganCMSchatzHInvolutional macular thinning. A pre-macular hole conditionOphthalmology19869321531613951821
- TrempeCLWeiterJJFurukawaHFellow eyes in cases of macular hole. Biomicroscopic study of the vitreousArch Ophthalmol1986104193953942551
- GassJDStereoscopic Atlas of Macular Diseases: Diagnosis and TreatmentSt LouisCV Mosby1987684693
- GassJDIdiopathic senile macular hole: its early stages and developmentArch Ophthalmol198810656296393358729
- JohnsonRNGassJDIdiopathic macular holes. Observations, stages of formation, and implications for surgical interventionOphthalmology19889579179243174041
- EzraEIdiopathic full thickness macular hole: natural history and pathogenesisBr J Ophthalmol200185110210911133724
- SmiddyWEFlynnHWJrPathogenesis of macular holes and therapeutic implicationsAm J Ophthalmol2004137352553715013877
- KellyNEWendelRTVitreous surgery for idiopathic macular holes. Results of a pilot studyArch Ophthalmol199110956546592025167
- WendelRTPatelACKellyNESalzanoTCWellsJWNovackGDVitreous surgery for macular holesOphthalmology199310011167116768233393
- RyanEHJrGilbertHDResults of surgical treatment of recent-onset full-thickness idiopathic macular holesArch Ophthalmol199411212154515537993209
- BrooksHLJrMacular hole surgery with and without internal limiting membrane peelingOphthalmology20001071019391948 discussion 1948–194911013203
- DukerJSKaiserPKBinderSThe International Vitreomacular Traction Study group classification of vitreomacular adhesion, traction, and macular holeOphthalmology2013120122611261924053995
- KusuharaSTeraoka EscañoMFFujiiSPrediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holesAm J Ophthalmol2004138570971615531303
- MatetASavastanoMCRispoliMEn face optical coherence tomography of foveal microstructure in full-thickness macular hole: a model to study perifoveal Müller cellsAm J Ophthalmol201515961142.e31151.e325728860
- TannerVChauhanDSJacksonTLWilliamsonTHOptical coherence tomography of the vitreoretinal interface in macular hole formationBr J Ophthalmol20018591092109711520763
- WoonWHGreigDSavageMDMovement of the inner retina complex during the development of primary full-thickness macular holes: implications for hypotheses of pathogenesisGraefes Arch Clin Exp Ophthalmol2015253122103210925673252
- YunCOhJHwangSYTogloomAKimSWHuhKMorphologic characteristics of chronic macular hole on optical coherence tomographyRetina201232102077208422617832
- MichalewskaZMichalewskiJNawrockiJContinuous changes in macular morphology after macular hole closure visualized with spectral optical coherence tomographyGraefes Arch Clin Exp Ophthalmol201024891249125520379735
- BalducciNMoraraMVeroneseCTorrazzaCPichiFCiardellaAPRetinal nerve fiber layer thickness modification after internal limiting membrane peelingRetina201434465566324670998
- IshidaMIchikawaYHigashidaRTsutsumiYIshikawaAImamuraYRetinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular holeAm J Ophthalmol2014157597197724503409
- ItohYInoueMRiiTHiraokaTHirakataACorrelation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closureOphthalmology201211971438144622424577
- KawanoKItoYKondoMDisplacement of foveal area toward optic disc after macular hole surgery with internal limiting membrane peelingEye (Lond)201327787187723703632
- KimJHKangSWParkDYKimSJHaHSAsymmetric elongation of foveal tissue after macular hole surgery and its impact on metamorphopsiaOphthalmology2012119102133214022867977
- KumagaiKHangaiMLarsonEOginoNProgressive changes of regional macular thickness after macular hole surgery with internal limiting membrane peelingInvest Ophthalmol Vis Sci20135474491449723696603
- NukadaKHangaiMOotoSYoshikawaMYoshimuraNTomographic features of macula after successful macular hole surgeryInvest Ophthalmol Vis Sci20135442417242823471893
- OhIKOhJYangSMAhnSEKimSWHuhKHyperreflective external limiting membranes after successful macular hole surgeryRetina201232476076622105500
- OhJSmiddyWEFlynnHWJrGregoriGLujanBPhotoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgeryInvest Ophthalmol Vis Sci20105131651165819850825
- OhJYangSMChoiYMKimSWHuhKGlial proliferation after vitrectomy for a macular hole: a spectral domain optical coherence tomography studyGraefes Arch Clin Exp Ophthalmol2013251247748422623115
- OhtaKSatoAFukuiERetinal thickness in eyes with idiopathic macular hole after vitrectomy with internal limiting membrane peelingGraefes Arch Clin Exp Ophthalmol201325151273127923052721
- OokaEMitamuraYBabaTKitahashiMOshitariTYamamotoSFoveal microstructure on spectral-domain optical coherence tomographic images and visual function after macular hole surgeryAm J Ophthalmol20111522283.e1290.e121669402
- WakabayashiTFujiwaraMSakaguchiHKusakaSOshimaYFoveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysisOphthalmology201011791815182420472291
- YunCAhnJKimMCharacteristics of retinal vessels in surgically closed macular hole: an optical coherence tomography angiography studyGraefes Arch Clin Exp Ophthalmol2017255101923193428744658
- SpaideRFKlancnikJMJrCooneyMJRetinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiographyJAMA Ophthalmol20151331455025317632
- RizzoSSavastanoABacheriniDSavastanoMCVascular features of full-thickness macular hole by OCT angiographyOphthalmic Surg Lasers Imaging Retina2017481626828060396
- TengYYuMWangYLiuXYouQLiuWOCT angiography quantifying choriocapillary circulation in idiopathic macular hole before and after surgeryGraefes Arch Clin Exp Ophthalmol2017255589390228236003
- ShahlaeeARahimyEHsuJGuptaOPHoACPreoperative and postoperative features of macular holes on en face imaging and optical coherence tomography angiographyAm J Ophthalmol Case Rep20175202529503940
- GrewalDSReddyVMahmoudTHAssessment of foveal microstructure and foveal lucencies using optical coherence tomography radial scans following macular hole surgeryAm J Ophthalmol20151605990.e1999.e126297503
- AhnJYooGKimJTKimSWOhJChoriocapillaris layer imaging with swept-source optical coherence tomography angiography in lamellar and full-thickness macular holeGraefes Arch Clin Exp Ophthalmol20182561112129032413
- SanoMShimodaYHashimotoHKishiSRestored photoreceptor outer segment and visual recovery after macular hole closureAm J Ophthalmol20091472313.e1318.e118835472
- HashimotoYSaitoWFujiyaAChanges in inner and outer retinal layer thicknesses after vitrectomy for idiopathic macular hole: implications for visual prognosisPLoS One2015108e013592526291526
- RymerJWildsoetCFThe role of the retinal pigment epithelium in eye growth regulation and myopia: a reviewVis Neurosci200522325126116079001
- NicklaDLWallmanJThe multifunctional choroidProg Retin Eye Res201029214416820044062
- FryczkowskiAWAnatomical and functional choroidal lobuliInt Ophthalmol19941831311417852018
- LovasikJVKergoatHSystemic determinantsSchmettererLKielJWOcular Blood FlowSpringerNew York2012173210
