Abstract
Purpose: Enlarged perivascular spaces (EPVS) have been widely considered as a feature of cerebral small vessel disease (cSVD) but the pathogenesis of EPVS remains unclear. Compromised blood–brain barrier (BBB) integrity may play a role since previous studies have shown that BBB breakdown is a critical contributor to the pathogenesis of other cSVD markers. This study aimed to investigate the association of EPVS in the centrum semiovale (CSO) and basal ganglia (BG) with BBB permeability.
Patients and methods: Consecutive participants free of symptomatic stroke history presented for physical examination were enrolled in this cross-sectional study. CSO- and BG-EPVS on T2-weighted (T2-W) magnetic resonance imaging (MRI) were rated using a five-point validated scale. Dynamic contrast-enhanced (DCE)-MRI and Patlak pharmacokinetic model were applied to quantify BBB permeability in the CSO and BG.
Results: A total of 109 participants aged 49–90 years (mean age of 69.85 years) were enrolled. The proportions of participants presenting high-grade (>10) EPVS in the CSO and BG were 50.5% and 44.0%, respectively. After adjustments for potential confounders by logistic regression, leakage rate and fractional blood plasma volume were correlated with the severity of BG-EPVS (OR: 5.33; 95%CI: 1.95–14.60 and OR: 0.93; 95%CI: 0.87–0.99).
Conclusion: Our study demonstrates that BG-EPVS are associated with compromised BBB integrity, supporting the hypothesis that the BBB dysfunction may be involved in the pathogenesis of BG-EPVS. EPVS in the CSO and BG may have distinct pathophysiology.
Introduction
Enlarged perivascular spaces (EPVS), or Virchow–Robin spaces, are visible in axial T2-weighted (T2-W) magnetic resonance imaging (MRI) as round or tubular hyperintensities in the centrum semiovale (CSO) and basal ganglia (BG).Citation1 These spaces are cerebrospinal fluid (CSF)-filled cavities surrounding small penetrating cerebral arterioles and important drainage conduits for metabolic waste and cerebral interstitial fluid.Citation2 EPVS have been widely considered as a feature of cerebral small vessel disease (cSVD)Citation3 and are associated with the pathogenesis of cognitive impairment in the elderly.Citation4
The pathogenesis of EPVS is as yet unclear. Previous studies have reported compromised blood–brain barrier (BBB) integrity in patients with lacunar stroke, white matter hyperintensities (WMH), cerebral microbleeds (CMBs), and mild vascular cognitive impairment (mVCI).Citation5–Citation8 Thus, BBB breakdown has been widely considered as a critical contributor to the pathogenesis of cSVD.Citation9 However, the association between BBB permeability and EPVS has not been clarified. Only two papersCitation10,Citation11 revealed the association of compromised BBB integrity with EPVS through prolonged MRI signal enhancement of contrast agent in patients with lacunar stroke, but quantitative data is absent and the effect of the symptomatic lacunar stroke on BBB permeability cannot be ruled out.
In order to explore the pathogenesis of EPVS, we applied dynamic contrast-enhanced (DCE)-MRI and Patlak pharmacokinetics to quantitatively evaluate BBB permeability,Citation12 which is the best method so far to distinguish between cSVD-related and age-related BBB permeability changes.Citation13 Therefore, considering that stroke may change BBB permeability,Citation14 participants with symptomatic stroke history were excluded from our study.
The present study aimed to investigate the association of BBB permeability with the severity of EPVS. In addition, we tentatively examined whether EPVS in the CSO and BG share the same pathogenesis.
Material and methods
Study design and settings
This cross-sectional study was conducted from April 2016 to March 2017. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting of observational studies were followed.
Study population
We recruited consecutive participants presented for physical examination at the department of Neurology of Beijing Chao-Yang Hospital, Capital Medical University, from April 2016 to March 2017. Exclusion criteria included: (1) history of symptomatic stroke or carotid stenosis of ≥50%, neurodegenerative disease, epilepsy, Alzheimer's disease or other neurological disorders; (2) brain trauma, tumor, or systemic inflammatory disease; (3) contraindication for MRI (eg pacemaker, metal implants, and claustrophobia) or the use of the contrast agent (eg allergy to gadolinium or renal failure); or (4) psychiatric disorders, alcohol or drug abuse.
Ethics statement
All participants consented to participate in our study and signed an informed consent to the use of data for research. The design of this study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University and was performed in accordance with the Declaration of Helsinki.
MRI protocol and assessment
Structural MRI
All participants underwent structural brain MRI on a 3T MRI scanner (Prisma; Siemens AG, Erlangen, Germany). Sequences included T1-weighted (T1-W), T2-W, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery (FLAIR), respectively.
DCE-MRI
MRI examinations were performed on a 3T MRI scanner (Prisma; Siemens AG). The T1 dynamic protocol comprised precontrast T1 measurements with 2 different flip angles (3°, 15°) for T1 mapping, as well as continuous serial acquisitions of 60 volumes of T1-weighted images, the sequence was applied (repetition time, TR/echo time, TE: 5.08/1.8 ms, field of view, FOV: 230×230 mm, voxel size 1.2×1.2×3 mm). The contrast agent (Gadolinium, 1.0 mmol/mL; 0.1 mmol/kg body weight, range 5–10 mmol per person) was injected after four volumes of T1-W images after start of acquisition in the antecubital vein at a rate of 2.5 mL/second using a power injector, followed by a 20 mL saline flush.
MR imaging analysis
DCE data were processed offline using Nordic ICE (Nordic Neuro Lab, Bergen, Norway). The concentration of contrast agent in tissue was calculated using relative signal change and T1 mapping. Individual vascular input functions were derived from the superior sagittal sinusCitation15 using a semi-automated method in Nordic ICE. The Patlak graphical approach was applied per voxel since it was considered as the most appropriate model for low-leakage regimen.Citation13 It could provide BBB leakage rate (Ktrans), area under the leakage curve (AUC) and fractional blood plasma volume (Vp). The following tissue regions of interest (ROIs) were selected: EPVS in the CSO and BG (size=5 mm2), respectively. Each ROI was measured for four times and averaged to obtain the average BBB leakage parameters. An experienced radiologist performed this procedure manually.
Assessment of EPVS
EPVS were defined as sharply delineated ovoid, round or linear structures depending on the imaging plane, size <3 mm, following the path of perforating arterioles, located in the CSO and BG, and with CSF intensity signal in T2-W images.Citation1 EPVS were counted in the slice and the side with the highest number. A 5-point visual rating ordinal scale (0, no EPVS; 1, 1–10 EPVS; 2, 11–20 EPVS; 3, 21–40 EPVS; 4, >40 EPVS) was used to evaluate the severity of EPVS in the CSO and BG.Citation3 We dichotomized the severity of EPVS into low-grade (EPVS scores 0–1) and high-grade (EPVS scores 2–4).Citation16
All images were analyzed by two experienced radiologists blinded to the clinical data. An interobserver reliability test was performed in 30 subjects and the κ-coefficient for CSO-EPVS and BG-EPVS were 0.812 and 0.845, respectively. The disagreement was resolved by discussing with other coauthors.
Statistical analysis
Continuous variables with normal distribution were presented as mean with standard deviation and compared using independent t-tests, those with non-normal distribution were presented as median with interquartile ranges and compared using Mann–Whitney U tests. Categorical variables were compared using chi-squaredtests. Logistic regression was used to assess the relationship between BBB permeability and the severity of EPVS in the CSO and BG as a binary variable. All multivariable analyses were first adjusted for age and sex (model 1) and additionally adjusted for all variables (including age, sex, hypertension, diabetes mellitus, hyperlipidemia, current smoker, BMI, and Fazekas score; model 2). Statistical significance was established at P<0.05. Analysis was performed with Statistical Package for Social Sciences (SPSS version 24, IBM Corporation, Armonk, NY, USA).
Results
Participants characteristics
A total of 142 participants were recruited and 33 participants were excluded (9 participants with incomplete injection of contrast or contraindication for MRI, 16 participants with history of symptomatic stroke or carotid stenosis, and 8 participants with history of tumor). Finally, 109 participants (mean age: 69.85±9.18 years; 49.5% male) were enrolled. Baseline characteristics of the study population are presented in .
Table 1 Baseline characteristics of the study population
Among these participants, 50.5% had high-grade EPVS in the CSO and 44.0% had high-grade EPVS in the BG. Clinical characteristics of participants stratified by the severity of EPVS are presented in . High-grade EPVS in the CSO were related to sex as well as the presence of diabetes mellitus, while high-grade EPVS in the BG were related to increasing age, sex and Fazekas score. There was no significant difference in laboratory tests between different groups of EPVS in the CSO and BG ().
Table 2 Demographic and clinical characteristics of participants with different severity of EPVS
Association between BBB permeability and the severity of EPVS
An example of leakage rate, AUC and fractional blood plasma volume map in the CSO is displayed in ; that in the BG is displayed in . Higher leakage rate as well as the AUC and lower fractional blood plasma volume were significantly associated with high-grade BG-EPVS. There was no significant difference in these BBB permeability parameters between different groups of CSO-EPVS.
Figure 1 An example map in the CSO (×1). (A) Axial FLAIR image of a 67-year-old man and ROIs; (B) Ktrans map; (C) AUC map; (D) Vp map.
Abbreviations: CSO, centrum semiovale; FLAIR, fluid-attenuated inversion recovery; ROIs, regions of interest; Ktrans, BBB leakage rate; BBB, blood–brain barrier; AUC, area under the leakage curve; Vp, fractional blood plasma volume.
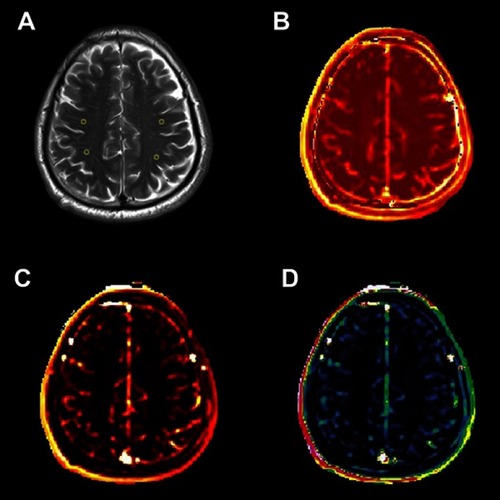
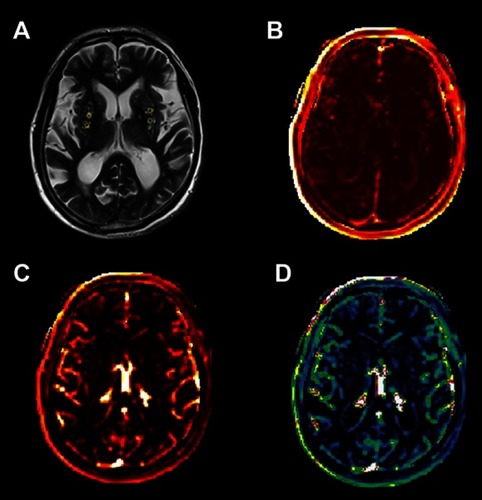
Figure 2 An example map in the BG (×1). (A) Axial FLAIR image of a 71-year-old man and ROIs; (B) Ktrans map; (C) AUC map; (D) Vp map.
Abbreviations: BG, basal ganglia; FLAIR, fluid-attenuated inversion recovery; ROIs, regions of interest; Ktrans, BBB leakage rate; BBB, blood-brain barrier; AUC, area under the leakage curve; Vp, fractional blood plasma volume.
The results of the binary logistic regression of the EPVS are displayed in . After adjusting for all confounders, both increased leakage rate and reduced fractional blood plasma volume were related with the high-grade BG-EPVS (OR: 5.33; 95%CI: 1.95–14.60 and OR: 0.93; 95%CI: 0.87–0.99).
Table 3 Leakage rate and fractional blood plasma volume in relation to severity of BG-EPVS
Discussion
In this study, we demonstrated that high-grade BG-EPVS were associated with higher BBB leakage rate and lower fractional blood plasma volume, but there was no significant difference in BBB permeability parameters between high-grade and low-grade EPVS in the CSO.
Using DCE-MRI and Patlak pharmacokinetics model in combination, previous studies revealed that increased BBB permeability is associated with the presence and extent of lacunar stroke, WMH as well as CMBs.Citation5–Citation7 Besides, compromised BBB integrity is also associated with total MRI cSVD burden including lacunes, WMH, CMBs and EPVS, which could more comprehensively reflect the severity of cSVD.Citation17 BBB dysfunction is now widely considered as a critical contributor to the pathogenesis of cSVD. An experiment using spontaneously hypertensive stroke-prone rats revealed that BBB breakdown may be the starting point of cSVD.Citation18 BBB breakdown might cause a series of pathophysiological changes which eventually lead to cSVD.
Although more and more evidence suggests that EPVS are related to lacunar stroke and WMH,Citation3,Citation19 only two studiesCitation10,Citation11 revealed that signal enhancement after gadolinium was associated with enlarged perivascular spaces in patients with lacunar stroke. However, no quantitative studies exploring the relationship between BBB permeability and EPVS, which is considered as a novel marker of cSVD, are available to date.Citation20 Nonquantitative methods could not distinguish WMH-related BBB permeability change from age-related BBB permeability change.Citation7
Our study revealed the association of BBB breakdown with EPVS, yielding more evidence that compromised BBB integrity is part of the pathological processes of EPVS.Citation21 Furthermore, we also demonstrated that CSO-EPVS and BG-EPVS may have a different pathogenesis, which is new for the understanding the BBB permeability of EPVS.
EPVS are important conduits for drainage of cerebral interstitial fluid into the ventricles and hence to the venous system,Citation22 but the exact pathogenesis of EPVS is uncertain. Early-stage experimental studies showed that EPVS could be affected by compromised BBB integrity and endothelial inflammation.Citation23 BBB is a selective barrier structure composed of basement membrane, capillary endothelial cells, tight junctions (TJs), pericytes and astrocytes.Citation24 The damage of TJs will cause leakage of plasma content, alteration of cell polarity and change of transport mechanisms.Citation25 Leakage of interstitial fluid and changes of microvascular wall will lead to obstruction of drainage space, accumulation of toxic substances and consequent occurrence of EPVS.Citation26 Apart from affecting the integrity of TJs between vascular endothelial cells, increased BBB permeability also affects endothelial function,Citation27 which is closely related to cSVD.Citation28 Vessels in the vicinity of EPVS in pathological conditions show arterial wall thickening and tortuosity, venular widening, endothelial inflammation and BBB breakdown.Citation29 Thus, EPVS are considered as a manifestation of cerebral small vessel pathology and a possible marker for BBB dysfunction.Citation3
We found that participants with high-grade BG-EPVS had a lower fractional blood plasma volume. As blood plasma volume is associated with the cerebral blood flow (CBF), this observation was in line with the result of a previous studyCitation30 suggesting that patients with high-grade EPVS were significantly associated with Moyamoya disease which features lower CBF and reduced cerebral perfusion pressure. This may suggest that hypoperfusion is involved in the pathogenesis of EPVS.
We showed that BG-EPVS and CSO-EPVS may have different pathogenesis, in line with the result of a previous study suggesting that BG-EPVS were linked to cognitive impairment,Citation31 but CSO-EPVS were not.Citation32 Most studies reckoned that EPVS in the BG, and not in the CSO, are specifically related to WMH and cSVD.Citation3,Citation33 A possible mechanism is that CSO-EPVS and BG-EPVS have different morphological features and related perforating arteries, thus, they may have unsynchronized pathological processes and different susceptibility to BBB dysfunction.Citation34 In addition, there is a widely accepted hypothesis that cerebrovascular amyloid angiopathy and microbleed is associated with CSO-EPVSCitation35,Citation36 while hypertension is a risk factor of BG-EPVS.Citation37
There are some limitations in our study. First, the study population are those who came for physical examination in a single center. They may have higher EPVS grade than those of the same age from the community since they may have a higher incidence of underlying diseases, such as hypertension or diabetes mellitus. In addition, there may be some sampling errors. For instance, diabetes mellitus was significantly more common in patients with low-grade CSO-EPVS. Second, it is too difficult to completely place ROIs on EPVS areas since they are too small to be accurately identified in the postprocessed images. We have tried our best to place ROIs on EPVS, but there could still be some errors. Third, this is a cross-sectional study and is therefore unable to observe whether the BBB breakdown will cause EPVS progress. Longitudinal studies are needed to verify the causal relationship between the increased BBB permeability and EPVS progression. Fourth, there is a lack of reliable biological markers to verify the increased permeability of BBB.
Conclusion
In conclusion, we found that BBB permeability varied with the severity of BG-EPVS, which suggests that BBB breakdown may be part of the pathogenesis of BG-EPVS. CSO-EPVS and BG-EPVS may have distinct pathophysiology. More information on brain tissue alterations is crucial for understanding EPVS pathogenesis.
Data sharing statement
All coauthors have agreed to the submission and publication of this manuscript. The authors confirm that all data underlying the findings described in this manuscript are fully available to all interested researchers upon request.
Acknowledgments
This study was supported by National Natural Science Foundation of China (Grant No. 81271309, 81301016) and Beijing Municipal Administration of Hospitals‘ Youth Programme (QML20150303).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary material
Table S1 Laboratory tests in participants with different severity of EPVS
References
- Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi:10.1016/S1474-4422(13)70124-823867200
- Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:289–299. doi:10.1007/s10571-016-0343-626993511
- Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–454. doi:10.1161/STROKEAHA.109.56491420056930
- Ding J, Sigurethsson S, Jonsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment Susceptibility-Reykjavik study. JAMA Neurol. 2017. doi:10.1001/jamaneurol.2017.1397
- Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi:10.1136/jnnp.2009.17207219710048
- Li Y, Li M, Zhang X, et al. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol. 2017;264:1474–1481. doi:10.1007/s00415-017-8550-828653212
- Zhang CE, Wong SM, van de Haar HJ, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi:10.1212/WNL.000000000000355628031395
- Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi:10.1161/STROKEAHA.110.61173121719768
- Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci (Lond). 2017;131:635–651. doi:10.1042/CS2016038028351960
- Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi:10.1002/ana.2154919260033
- Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces–MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–2340. doi:10.1093/brain/awn17118676439
- Wong SM, Jansen JFA, Zhang CE, et al. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: test-retest reproducibility and its influencing factors. J Magn Reson Imaging. 2017;46:159–166. doi:10.1002/jmri.2554028160347
- Heye AK, Thrippleton MJ, Armitage PA, et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage. 2016;125:446–455. doi:10.1016/j.neuroimage.2015.10.01826477653
- Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi:10.1161/STROKEAHA.110.60825721940972
- Lavini C, Verhoeff JJ. Reproducibility of the gadolinium concentration measurements and of the fitting parameters of the vascular input function in the superior sagittal sinus in a patient population. Magn Reson Imaging. 2010;28:1420–1430. doi:10.1016/j.mri.2010.06.01720817379
- Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83:1228–1234. doi:10.1212/WNL.000000000000083725165388
- Li Y, Li M, Zuo L, et al. Compromised blood-brain barrier integrity is associated with total magnetic resonance imaging burden of cerebral small vessel disease. Front Neurol. 2018;9:221. doi:10.3389/fneur.2018.0022129681883
- Schreiber S, Bueche CZ, Garz C, Braun H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease?-New insights from a rat model. Exp Transl Stroke Med. 2013;5:4. doi:10.1186/2040-7378-5-423497521
- Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry. 2014;85:522–525. doi:10.1136/jnnp-2013-30581524249785
- Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–381. doi:10.1111/ijs.1205423692610
- Muller K, Courtois G, Ursini MV, Schwaninger M. New insight into the pathogenesis of cerebral small-vessel diseases. Stroke. 2017;48:520–527. doi:10.1161/STROKEAHA.116.01288828082670
- Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi:10.1016/j.neuint.2003.11.00615186921
- Satizabal CL, Zhu YC, Dufouil C, Tzourio C. Inflammatory proteins and the severity of dilated Virchow-Robin Spaces in the elderly. J Alzheimers Dis. 2013;33:323–328. doi:10.3233/JAD-2012-12087422976070
- Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab. 2016;36:72–94. doi:10.1038/jcbfm.2015.11626058695
- Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36:6–25. doi:10.1038/jcbfm.2015.17226198175
- Gutierrez J, Rundek T, Ekind MS, Sacco RL, Wright CB. Perivascular spaces are associated with atherosclerosis: an insight from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2013;34:1711–1716. doi:10.3174/ajnr.A349823557952
- Ostergaard L, Engedal TS, Moreton F, et al. Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36:302–325. doi:10.1177/0271678X1560672326661176
- Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25:44–50. doi:10.1111/bpa.1222425521176
- Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–S52. doi:10.1161/STROKEAHA.108.53770419064767
- Kuribara T, Mikami T, Komatsu K, et al. Prevalence of and risk factors for enlarged perivascular spaces in adult patients with moyamoya disease. BMC Neurol. 2017;17:149. doi:10.1186/s12883-017-0935-x28778183
- Arba F, Quinn TJ, Hankey GJ, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke. 2018;13:47–56. doi:10.1177/174749301666609127543501
- Huijts M, Duits A, Staals J, Kroon AA, de Leeuw PW, van Oostenbrugge RJ. Basal ganglia enlarged perivascular spaces are linked to cognitive function in patients with cerebral small vessel disease. Curr Neurovasc Res. 2014;11:136–141.24606607
- Hansen TP, Cain J, Thomas O, Jackson A. Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. AJNR Am J Neuroradiol. 2015;36:893–898. doi:10.3174/ajnr.A423725698626
- Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Invest Radiol. 2014;49:307–313. doi:10.1097/RLI.000000000000002724473365
- Charidimou A, Hong YT, Jager HR, et al. White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke. 2015;46:1707–1709. doi:10.1161/STROKEAHA.115.00909025908461
- Charidimou A, Jaunmuktane Z, Baron JC, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi:10.1212/01.wnl.0000438225.02729.0424285616
- Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi:10.1212/WNL.0b013e31828f187623553482
