Abstract
Purpose
This study compared the effects of a combination of soy peptide supplementation and exercise with those of exercise only, on the cognitive function of elderly adults.
Patients and methods
This randomized, non-blinded, controlled clinical trial included 67 participants aged 60 years or more with non-cognitive dysfunction who were divided into two groups according to the intervention method: an exercise group (Ex group, n = 36) and an exercise plus nutrition group (Ex+Nt group, n = 31). The Ex group completed a memory training activity for 15 mins and aerobic exercise for 45 mins once a week for 90 days. The Ex+Nt group completed the same training plus received soy peptide for 90 days. The Mini-Mental Status Examination score, trail-making test A/B score, skeletal muscle mass index, grip strength, gait speed, and geriatric depression scale were measured at baseline and post intervention. For comparison between the pretest and posttest measurements to determine the intervention effects, a two-way analysis of variance was performed. The significance level was set at < 5%.
Results
A two-way analysis of variance revealed significant time effects on trail-making test-A score, skeletal muscle index, grip strength, and gait speed in both groups. There were significant time x group interactions for greater increase in calculation score.
Conclusion
A combination of exercise and soy peptide supplementation was effective in improving a portion of cognitive function.
Introduction
There has been a rapid increase in the prevalence of dementia owing to societal aging, and this poses a very serious problem.Citation1 According to the World Alzheimer’s Association, the proportion of people with dementia is increasing at a rate of one person every 3 seconds. This value is likely to reach 74.7 million people worldwide in 2030, accompanied by increasing medical and nursing care expenses, at an estimated cost of 818 billion USD.Citation2 Risk factors for dementia include the presence of the apoE4 protein,Citation3 high blood pressure,Citation4–Citation6 diabetes,Citation7 lipid abnormality,Citation8 depression,Citation9 inactivity,Citation10 low income,Citation11,Citation12 and a decrease in the frequency of interpersonal exchanges.Citation13 Conversely, protective factors include the presence of higher education,Citation14 physical activity,Citation15 participation in cognitive activities,Citation15 antioxidant food intake,Citation16,Citation17 moderate alcohol consumption,Citation18 and increased frequency of social participation and interpersonal exchanges.Citation19 Since effective drugs for this disease have not yet been developed, it is important to reduce the number of risk factors through lifestyle modifications to include as many protective factors as possible.
According to previous studies,Citation20 the risk of dementia is halved in people who exercise more than three times a week, compared to the risk in those without exercise habits. Amyloid β can start accumulating in the brain more than 20 years before the onset of Alzheimer-type dementia, and exercise is known to prevent this type of accumulation.Citation21 Previous studies have shown that the maintenance of strong motor function can help reduce future risk of dementia at older age.Citation22 One previous study has suggested that the promotion of brain derived neurotrophic factor (BDNF) expression due to exercising inhibits cognitive decline.Citation23
In terms of nutrition, recent studies have demonstrated the properties of certain food components that might potentially prevent cognitive impairment and dementia.Citation17 Among them, we focused on soy peptide, which is obtained by hydrolyzing soy protein isolate (SPI) with food processing enzymes. Soy peptide is classified as a functional food with diverse physiological functions attributed in the form of bioactive peptides that are rapidly absorbed from the small intestine.Citation24 One study demonstrated that daily supplementation with soy peptide improved cognitive abilities, including sustained attention and short- and long-term memory in healthy middle-aged and elderly adults.Citation25 In addition, the oral administration of soy peptide for 6 months has been reported to suppress age-related cognitive decline and result in an upregulation of BDNF expression in senescence-accelerated mouse brains.Citation26
However, to our knowledge, the effect of a combination of exercise and soy peptide intake on cognitive function has not yet been investigated. To this aim, we compared the effects of an exercise program including cognitive training in combination with soy peptide intake and those of the exercise program alone on the cognitive function of community-dwelling older individuals.
Materials and Methods
Trial Design and Participants
This non-blind randomized controlled trial (RCT) compared the effects of a multicomponent exercise program (multiple forms of exercise combined with songs and cognitive training) supplemented with soy protein on cognitive function in elderly adults with the effects of the exercise program alone. The Ethics Committee of the Osaka Kawasaki Rehabilitation University (Reference No. OKRU29-A021) approved the trial protocol and the trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000030404). Written informed consent for study participation was obtained from all the participants in accordance with the tenets of the Declaration of Helsinki, and no stipend was provided.
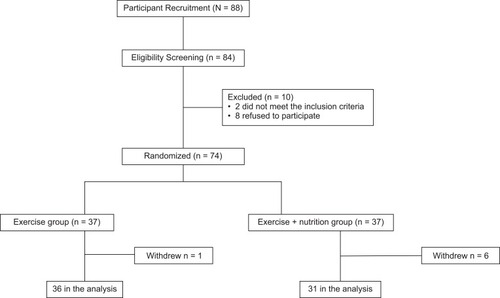
Participants included community-dwelling elderly adults living in Osaka, Japan. People aged 60 years or older who could independently perform the activities of daily living were enrolled. Those with collagen disease, depression, a history of cardiovascular disease, medical contraindications to exercise, and Parkinson’s disease were excluded. Using a computerized 1:1 randomization scheme, the eligible participants (n = 74) were assigned to the exercise (Ex) (n = 37) or the exercise plus nutrition (Ex+Nt) groups (n = 37).
Interventions
Multicomponent Exercise
Multicomponent exercise sessions were conducted at the Kaizuka City Welfare Center. Participants engaged in a 15-min memory training and 45-min aerobic exercise session once a week for 3 months. As part of the memory training, participants were required to listen to a novel story and repeat its contents, and perform the dual tasks of clapping their hands in multiples of three and five while stepping on the spot. The aerobic exercise session included self-stretching for 10 min, followed by an aerobic exercise regimen of 15 min. This was followed by conditioning for 10 min such that the heart rate normalized. The aerobic exercise regimen included gymnastics using songs featuring the locally popular character “Tsugasan.” Gymnastics were practiced on yoga mats (60 cm × 173 cm). The regimen included lower-limb steps, rhythmic exercises of the upper limbs, and deft movement of the fingers. Finally, mindfulness exercises were performed in the recumbent position for 10 min. All exercises were taught by two physical therapists and several staff volunteers.
Nutrition
A commercial drink (Peptide Athleeta 4000, Fuji Oil Co., Osaka, Japan) was used for soy peptide supplementation. shows the nutrition facts label of Peptide Athleeta 4000. The net weight of Peptide Athleeta 4000 that contains 4 g soy peptide was 190 g.
Table 1 Nutrition Facts Label of Peptide Athleeta 4000
Cognitive Status Measures
The Japanese language version of the Mini-Mental State Examination (MMSE) is widely used for the evaluation of cognitive function and cognitive impairment screening.Citation27 The MMSE score ranges from 0 to 30, with lower scores representing weaker cognitive function.
The trail making test (TMT)—a well-known psychomotor testCitation28—was used to evaluate attentiveness. In Part A of the TMT, participants are instructed to draw lines to connect 25 encircled numbers distributed on a page. Part B of TMT is completed by alternately linking the sequential numbers and letters on a page. This provides information on an individual’s cognitive flexibility.Citation29
Assessment of Body Composition
Physiological parameters measured using bioelectrical impedance analysis (InBody270; InBody, Tokyo, Japan) with 20 and 1000 kHz frequencies were obtained from the participants’ electronic medical records.Citation30 Participants were instructed to grasp the handles of the analyzer and stand on electrodes contacting the lower surface of their feet while they were wearing normal indoor clothing without socks or shoes. The surface of the hand electrode was placed in contact with each of the five fingers, while the participants’ heels and forefoot were placed on the circular foot electrode. Measurements were recorded within 30 sec by the staff. Participants were required to fast and avoid vigorous exercise for at least one hour before the assessment. Body mass index (BMI) was calculated by dividing the body weight (kg) by height squared (m2). The appendicular skeletal muscle index (SMI) was derived from the appendicular muscle mass (kg) divided by height squared (m2).
Muscle Strength
Handgrip strength is a well-known measure of muscle strength and is significantly associated with whole-body muscle strength.Citation31 The maximum voluntary isometric strength of the handgrip was measured with the dominant hand while in a standing position, by means of a hand dynamometer Grip-D (Takei, Niigata, Japan). Other bodily movements were not permitted.
Gait Speed
Participants were instructed to walk 6.4 m (divided into two 2.0-m zones at each end and a 2.4-m middle-zone) at a speed they found comfortable.Citation32 The time needed (in sec) to pass the 2.4-m middle zone was measured for the calculation of gait speed (m/s). Participants could use a cane or walker if they were unable to perform the gait test independently. An average of five gait tests was used for evaluation.
Geriatric Depression Scale 15
The Japanese version of the Geriatric Depression Scale 15 (GDS-15)Citation33 is a self-report evaluation. The scale comprises 15 items: 10 items confirm depression if the answers are positive, while the remaining 5 items confirm depression if the answers are negative. Normal scores range from 0–4, depending on factors such as age, education, and complaints; a score of 5–8 indicates mild depression, 9–11 moderate depression, and 12–15 severe depression. In this study, GDS-15 was measured with permission from the copyright holder.
Sample Size
To determine the sample size required for the detection of a significant interaction effect between intervention and time (pre/post), we performed a power calculation using the following parameters: a moderate effect size (f = 0.25), α error = 0.05, β error = 0.20. The required number of cases was 33 in each group. We estimated a 10% dropout rate, bringing the target number of participants to 37 per group.
Statistical Analyses
Analysis of variance was used for within- and between-group comparisons of continuous variables and a chi-square test was used for between-group comparisons of categorical variables at baseline. In addition, the effect of each intervention on outcome measurements was analyzed using a mixed 2 × 2 group (Ex, Ex+Nt group) × time (pre, post) two-way analysis of variance (ANOVA).
In the sub-analysis, the sub-items of the MMSE—orientation time, orientation place, registration, attention and calculation, recall and language, and praxis— were analyzed separately in the Ex and Ex+Nt group using two-way ANOVA. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, New York). P < 0.05 was considered to be statistically significant.
Results
Of the 74 individuals selected for the study, 67 (76.1%) completed the 3-month follow-up (; Ex group: 36 [97.3%] and Ex + Nt group: 31 [83.8%]). The baseline characteristics of the participants were matched and found to be comparable (). The median relative adherence rate was 90% with nutritional supplementation. No health problems, musculoskeletal complications, muscle pain, or fall incidents occurred during the study period.
Table 2 Baseline Characteristics of the Participants in the Two Groups
presents a comparison of the cognitive function and physical function test values before and after the intervention. The pre- and post-intervention group statistics and group-time interactions are shown in . Significant time-specific effects were observed for TMT-A, gait speed, grip strength, and SMI.
Table 3 Comparisons of Changes in Exercise and Exercise + Nutrition Groups
presents the results of comparisons of MMSE total and subdivision changes in the Exercise and Exercise + Nutrition groups. There was a statistically significant interaction between time and group (p < 0.05).
Table 4 Comparisons of MMSE Total and Subdivision Changes in Exercise and Exercise + Nutrition Groups
Discussion
There is no absolute method to prevent dementia. However, a healthy lifestyle that includes regular physical activity, cognitive activities, and a well-balanced diet reduces the risk of dementia.Citation34 Conventional exercise programs, such as those involving walking, have shown cognitive benefits in community-dwelling elderly people.Citation35 However, the beneficial effects of exercise cannot be experienced without continued participation.Citation36 In this study, therefore, we designed a multicomponent exercise program using songs featuring a locally popular character with cooperation from the local government to ensure a high rate of participation (final participation rate: 76.1%). Moreover, the program included a memory training session for the prevention of dementia. The 3-month multicomponent exercise intervention had beneficial effects on physiological function such as gait speed, grip strength, and on SMIin community-dwelling older adults.
In addition, 3-month multicomponent exercise intervention improved cognitive function as evidenced by the improved TMT-A, but not TMT-B. This result is consistent with those of a previous dance intervention study targeting mild cognitive impairment, in that the TMT-A score improved while the TMT-B score did not.Citation37 These results suggest a selective beneficial effect of exercise on cognitive function.
Soy peptide is generated by the enzymatic digestion of SPI. SPI has a Protein Digestibility Corrected Amino Acid Score of 1.0, the highest possible score.Citation38 This means that soy peptide derived from SPI has the ideal amino acid balance. Soy peptide ingestion can maintain and promote the synthesis and metabolism of noradrenaline in the brain.Citation39 Several studies have reported an alleviation of peripheral fatigue following its rapid absorption.Citation24 A recent clinical study also demonstrated the positive effects of soy peptide on brain function.Citation40–Citation42 Furthermore, another study showed that a soy peptide diet suppressed age-related cognitive decline via the upregulation of BDNF in a mouse model with accelerated senescence.Citation26 Therefore, we focused on soy peptide as the nutrition of choice combined with multicomponent exercise.
The 3-month soy peptide supplemented with exercise intervention showed a significant effect on calculation function compared with exercise alone. Previous research indicates that exercise and soy peptide intake for 2 weeks in young people improved their computational ability.Citation43 In this study, the interaction of time and group for the computational ability was not observed, but the significant beneficial effect for the total score change combined with the recall ability was recognized in soy intake group. Previous research indicates that there is no relationship between typical soy isoflavone intake and the maintenance of cognitive function.Citation44 However, in this study, it is speculated that an increase in soy intake compared to typical use concentrated on a three-month period had an effect on calculation improvement.
It has been demonstrated that physical exercise increases BDNF levels.Citation45 Thus, the synergistic effect of soy and exercise shown in our study might be due to increased BDNF levels that promote several aspects of brain function. In addition, we predicted a synergistic effect of soy and the multicomponent intervention on physical performance and skeletal muscle mass given that one previous report demonstrates that soy peptide intake after exercising enhances the levels of serum growth hormone involved in muscle synthesis.Citation46 However, those synergistic effects were not observed in this study.
The present study has some limitations. First, we did not record the dietary intake of liquids. There may have been changes in dietary intake owing to the consumption of the nutritional supplement. Second, the participants were of Japanese nationality and, hence, caution must be exercised while generalizing the study’s results to other ethnic groups. Finally, the persistence of the effects of exercise and nutrition remains unclear. Further studies are also needed to measure serum levels of soybean peptide-derived substances in patients to explain the physiological outcomes better. Future interventional trials using soy supplementation in different populations and over longer periods of time have been scheduled.
Conclusions
In summary, this non-blinded controlled trial showed that participation in a three-month weekly program comprising multicomponent exercise both alone and with the addition of soy peptide supplementation effectively improved a portion of cognitive performance and physical function in elderly adults. A combination of exercise and nutrition may be effective in improving calculation function in such populations.
Research Ethics and Informed Consent
The Ethics Committee of the Osaka Kawasaki Rehabilitation University (Reference No. OKRU29-A021) approved the trial protocol and the trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000030404). Written informed consent for study participation was obtained from all the participants in accordance with the tenets of the Declaration of Helsinki, and no stipend was provided.
Data Sharing Statement
The datasets available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgment
The authors would like to thank Ms Kanako Kodama, Ms Masako Kawano, Ms Saeko Araki, Ms Kaori Hamamura, and senior volunteer staff for their contributions to data collection.
Disclosure
M Maebuchi and M Ibuki are employees of Fuji Oil Co. Ltd., which produces Peptide Athleeta 4000. The authors report no other conflicts of interest in this work.
References
- Prince M, Guerchet M, Prina M, Alzheimer’s Disease International. Policy Brief for Heads of Government: The Global Impact of Dementia 2013-2050. London: Alzheimer’s Disease International; 2013 Accessed 65, 2019:1–8. Available from: https://www.alz.co.uk/research/GlobalImpactDementia2013.pdf.
- Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2015:22–23. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed 65, 2019.
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–777. doi:10.1097/00005768-200105000-0001511323547
- Peila R, White LR, Petrovich H, et al. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: the Honolulu-Asia aging study. Stroke. 2001;32(12):2882–2889. doi:10.1161/hs1201.10039211739991
- Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137(3):149–155. doi:10.7326/0003-4819-137-3-200208060-0000612160362
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia aging study. JAMA. 1995;274(23):1846–1851. doi:10.1001/jama.1995.035302300320267500533
- Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107(15):7036–7041. doi:10.1073/pnas.100064510720231468
- Tan ZS, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham study. Arch Intern Med. 2003;163(9):1053–1057. doi:10.1001/archinte.163.9.105312742802
- Dobie DJ. Depression, dementia, and pseudodementia. Semin Clin Neuropsychiatry. 2002;7(3):170–186. doi:10.1053/scnp.2002.007017012111672
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi:10.1016/S1474-4422(11)70072-221775213
- Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Melzer D. Neighbourhood deprivation and incident mobility disability in older adults. Age Ageing. 2008;37(4):403–410. doi:10.1093/ageing/afn09218487260
- Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the english longitudinal study of ageing. J Am Geriatr Soc. 2008;56(2):191–198. doi:10.1111/(ISSN)1532-541518179489
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131(3):165–173. doi:10.7326/0003-4819-131-3-199908030-0000210428732
- Karp A, Kareholt I, Qiu C, Bellander T, Winblad B, Fratiglioni L. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol. 2004;159(2):175–183. doi:10.1093/aje/kwh01814718220
- Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. doi:10.1056/NEJMoa02225212815136
- Gray SL, Hanlon JT, Landerman LR, Artz M, Schmader KE, Fillenbaum GG. Is antioxidant use protective of cognitive function in the community-dwelling elderly? Am J Geriatr Pharmacother. 2003;1(1):3–10. doi:10.1016/S1543-5946(03)80011-915555461
- Nicolas A-S, Nourhashemi LF, Lanzmann-Petithory D, Vellas B. Nutrition and cognitive function. Nutr Clin Care. 2001;4(3):156–167. doi:10.1046/j.1523-5408.2001.00137.x
- Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542–555. doi:10.1097/JGP.0b013e3181a2fd0719546653
- Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi:10.1001/jama.287.6.74211851541
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi:10.1001/archneur.58.3.49811255456
- Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: new cues into the human disease? Neurobiol Aging. 2006;27(11):1618–1631. doi:10.1016/j.neurobiolaging.2005.09.03916274856
- Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763–1769. doi:10.1001/archneur.63.12.176317172617
- Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi:10.1073/pnas.101595010821282661
- Maebuchi MYN, Furuya S. Soy peptide as functional food component. Med Biol. 2011;155:566–576.
- Maebuchi M, Kishi Y, Koikeda T, Furuya S. Soy peptide dietary supplementation increases serum dopamine level and improves cognitive dysfunction in subjects with mild cognitive impairment. Jpn Pharmacol Ther. 2013;41(1):67–73.
- Katayama S, Imai R, Sugiyama H, Nakamura S. Oral administration of soy peptides suppresses cognitive decline by induction of neurotrophic factors in SAMP8 mice. J Agric Food Chem. 2014;62(16):3563–3569. doi:10.1021/jf405416s24678753
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-61202204
- Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi:10.1016/S0887-6177(03)00039-815010086
- Kortte KB, Horner MD, Windham WK. The trail making test, part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9(2):106–109. doi:10.1207/S15324826AN0902_512214820
- Nakamura M, Tazaki F, Nomura K, et al. Cognitive impairment associated with locomotive syndrome in community-dwelling elderly women in Japan. Clin Interv Aging. 2017;12:1451–1457. doi:10.2147/CIA28979107
- Makizako H, Shimada H, Doi T, et al. Age-dependent changes in physical performance and body composition in community-dwelling Japanese older adults. J Cachexia Sarcopenia Muscle. 2017;8(4):607–614. doi:10.1002/jcsm.v8.428597612
- Shimada H, Suzuki T, Suzukawa M, et al. Performance-based assessments and demand for personal care in older Japanese people: a cross-sectional study. BMJ Open. 2013;3(4):e002424. doi:10.1136/bmjopen-2012-002424
- Burke WJ, Roccaforte WH, Wengel SP. The short form of the geriatric depression scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4(3):173–178. doi:10.1177/0891988791004003101953971
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi:10.1016/S0140-6736(15)60461-525771249
- Scherder E, Scherder R, Verburgh L, et al. Executive functions of sedentary elderly may benefit from walking: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2014;22(8):782–791. doi:10.1016/j.jagp.2012.12.02623636004
- Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102(2a):43–49. doi:10.1016/S0002-9343(97)00467-19217586
- Doi T, Verghese J, Makizako H, et al. Effects of cognitive leisure activity on cognition in mild cognitive impairment: results of a randomized controlled trial. J Am Med Dir Assoc. 2017;18(8):686–691. doi:10.1016/j.jamda.2017.02.01328396179
- Mathai JK, Liu Y, Stein HH. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. 2017;117(4):490–499. doi:10.1017/S000711451700012528382889
- Imai H, Moriyasu K, Nakahata A, Maebuchi M, Ichinose T, Furuya S. Soy peptide ingestion augments the synthesis and metabolism of noradrenaline in the mouse brain. Biosci Biotechnol Biochem. 2017;81(5):1007–1013. doi:10.1080/09168451.2017.128280728137184
- Nakamoto M, Otsuka R, Nishita Y, et al. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur J Clin Nutr. 2018;72(10):1458–1462. doi:10.1038/s41430-017-0061-229348624
- Moré MI, Freitas U, Rutenberg D. Positive effects of soy lecithin-derived phosphatidylserine plus phosphatidic acid on memory, cognition, daily functioning, and mood in elderly patients with Alzheimer’s disease and dementia. Adv Ther. 2014;31(12):1247–1262. doi:10.1007/s12325-014-0165-125414047
- Lin HC, Peng CH, Huang CN, et al. Soy-based foods are negatively associated with cognitive decline in Taiwan’s elderly. J Nutr Sci Vitaminol. 2018;64(5):335–339. doi:10.3177/jnsv.64.33530381623
- Parker AG, Gordon J, Thornton A, et al. The effects of IQPLUS Focus on cognitive function, mood and endocrine response before and following acute exercise. J Int Soc Sports Nutr. 2011;8(21):16. doi:10.1186/1550-2783-8-1622017963
- Talaei M, Feng L, Yuan JM, et al. Dairy, soy, and calcium consumption and risk of cognitive impairment: the Singapore Chinese Health Study. Eur J Nutr. 2019. doi:10.1007/s00394-019-02010-8
- Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A, et al. L-lactate promotes adult hippocampal neurogenesis. Front Neurosci. 2019;13:403. doi:10.3389/fnins.2019.0040331178678
- Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling japanese older adults: the INE (Intervention by Nutrition and Exercise) study. J Am Med Dir Assoc. 2015;16(8):654–660. doi:10.1016/j.jamda.2015.02.01725858281