 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The main goal of the present study is to examine to what extent age and cognitive impairment contribute to learning performance (cognitive plasticity, cognitive modifiability, or learning potential). To address this question, participants coming from four studies (Longitudinal Study of Active Aging, age range, 55–75 years, N = 458; Longitudinal Study in the very old [90+], age range, 90–102, N = 188, and Cognitive Plasticity within the Course of Cognitive Impairment, 97 “Normal”, 57 mild cognitive impairment [MCI], and 98 Alzheimer’s disease [AD] patients) were examined through a measure of verbal learning (developed from Rey). The results show that all age, MCI, and AD groups learned across the five learning trials of that test, but significant differences were found due to age, pathology, and education. The effects of pathology (MCI and AD) can be expressed in a metric of “years of normal decline by age”; specifically, being MCI means suffering an impairment in performance that is equivalent to the decline of a normal individual during 15 years, whereas the impact of AD is equivalent to 22.7 years. Likewise, the improvement associated with about 5 years of education is equivalent to about 1 year less of normal aging. Also, the two pathological groups significantly differed from “normal” groups in the delayed trial of the test. The most dramatic difference is that between the “normal” group and the AD patients, which shows relatively poorer performance for the AD group in the delayed trial than in the first learning trial. The potential role of this unique effect for quick detection purposes of AD is assessed (in the 75–89 years age range, sensitivity and specificity equal 0.813 and 0.917, respectively).
Introduction
Cognitive aging research shows a clear picture characterized by a gradual decline in function over time, starting early in life.Citation1–Citation4 From the last decades of the 20th century, learning potential,Citation5 cognitive modifiability,Citation6 or cognitive plasticity across the aging process (also called reserve capacity or testing-the-limit) has been a central issue in gerontology since a stereotypic trait in general population said that “older people are unable to learn.”Citation7 Cognitive plasticity is operationalized as the extent to which an individual can improve his/her performance in a given cognitive task through training (this procedure has also been called “dynamic assessment” as opposed to the standard “static” measures of cognitive functioning).Citation8–Citation12 Although cognitive plasticity measures seem to be preserved during normal aging, many authors agree that they are associated with age; in other words, cognitive plasticity measures have shown a profile of decline in most of the studies.Citation13
In order to examine plasticity (through episodic memory tasks), Singer et alCitation14 trained a mnemonic skill to survivors of the Berlin Aging Study (N = 96; mean age, 84 years; range, 75–101 years). Gains after mnemonic training were modest and most individuals were unable to improve their performance after training; the authors concluded that in very old age, “biological factors are a prominent source of individual differences in plasticity.”
Taking as a basis these and other results, Baltes and SmithCitation15 considered that the oldest-old, called the “fourth age,” “entails a level of biocultural incompleteness, vulnerability, and unpredictability that is distinct from the positive views of the third age (young-old)”; the authors concluded that the oldest-olds are at the limits of their functional capacity.
Nevertheless, a somewhat different conclusion was reached by Yang et al,Citation16 using a self-guided retest paradigm. This paradigm allows the study of a basic form of cognitive plasticity, called retest learning, as reflected in improvements of performance through retest practice in five trials. They investigated whether cognitive plasticity could be extended from the young-old to the oldest-old. The results showed evidence for continued plasticity until age 80 and above; substantial improvements in performance, comprising one standard deviation from the pre-test to the sixth trial, were observed.
However, research on cognitive functioning in individuals aged 65–90 years who have abnormal memory functioning, but do not meet formal criteria for the diagnosis of dementia, supported Petersen’s proposal that the syndrome Mild Cognitive Impairment (MCI) seems to be a transition between normal aging and dementia.Citation17 As the author claims: “The concept of the boundary between normal aging and early Alzheimer’s disease is a focus of a great deal of research in the field of aging and dementia.” In an attempt to determine whether MCI occurred in the oldest-old and whether neuropsychometric performance based on clinical diagnosis was working well in this age group, Boeve et alCitation18 examined with neuropsychometric testing a sample of individuals aged 90–100 years (N = 111). Results yielded 56 normal (50.45%), 13 MCI (11.7%), and 42 with dementia (37.8%). The authors concluded that it was possible to observe the full cognitive continuum from normal to MCI to dementia in the oldest-old, but it is important to emphasize that they found significant differences only between the MCI and normal groups in delayed recall. Individuals with MCI were more similar to normal than to patients with dementia.
On the other hand, cognitive plasticity is severely impaired in individuals with dementia, as Baltes and her group pointed out,Citation2,Citation19 the assessment of cognitive plasticity (or cognitive reserve capacity) could be a tool for early diagnosis of dementia and an expression of cognitive reserve. In the context of the Berlin Aging Study, Lindenberger and ReischiesCitation20 reported the results obtained through the Enhanced Cued Recall (ECR) test administered among several neuropsychological tests with the purpose of identifying dementia-specific cognitive impairments and learning potential. Six groups were distinguished: three age groups (N = 162; mean ages, 74.9, 84.7, and 94.5 years) and three groups with dementia patients (Mild, N = 32; Moderate, N = 30; Severe, N = 31). Persons without dementia showed a decreased level of performance in learning as age advanced but no significant differences were found in learning gains. In comparison with persons with dementia they found significant differences, both in performance levels and in learning gains. Within the dementia subsample, individuals with mild dementia differed from those with moderate or severe dementia with respect to both performance level and learning gain; only those patients with mild dementia obtained similar learning gains than the oldest “normal” groups. In sum, the results indicated that age and dementia had dissociable effects on the recall level and learning gains. Mild dementia patients showed a reduction in performance but they were capable of improvement in the post-test. Finally, individuals with moderate and severe dementia not only showed lower levels of initial recall performance, they did not show any gain after training.
During the last years we have studied cognitive plasticity (or learning potential) in healthy, MCI individuals, and mild Alzheimer’s disease (AD) patients. In our studies, cognitive plasticity was assessed through test-training-retest strategy administering several cognitive tasks (visuo-spatial memory, audio-verbal memory, executive function, and verbal fluency), among them the Verbal Memory Learning Potential testCitation21 (VMLPt, developed based on the “verbal learning test” from Rey).Citation1 Cognitive plasticity was assessed in three groups of older individuals: healthy (N = 100), MCI (N = 50), and AD patients (N = 50). In all tasks the three groups, similar in age and education, improved their performance when training was provided in each task, but healthy elders significantly obtained higher pre-test, post-test, and gain scores than the MCI and AD groups did. There was a gradient of modifiability from healthy to MCI and from MCI to AD, but it must be emphasized that mild AD patients benefited from training as well. Finally, the total score of cognitive plasticity correctly classified 89% of Healthy, MCI individuals, and AD patients, and it did better than other tests such as the Mini Mental State Examination (MMSE).Citation23 In sum, two important findings resulted from this study: (1) Although healthy individuals significantly showed better scores in all cognitive plasticity measures, both MCI individuals and mild AD patients were able to learn, that is, they improved their performance after training in those four cognitive tasks (dots memory, verbal memory, executive function, and verbal fluency),Citation21 and (2) AD patients improved their performance in cognitive plasticity measures after 6 months of a psychostimulation program.Citation24
Most of the studies in cognitive plasticity have been conducted in Western countries, where the level of education is quite high even in the oldest cohort. Nevertheless, in a previous study, Fernández-Ballesteros and CaleroCitation25 found significant differences in learning potential (cognitive plasticity) between elders with high and low levels of education. The effect of education in crystalized intelligence is very well known;Citation26 on the clinical field, lack of education is considered a risk factor for accelerated memory decline, on the contrary education is considered a protective factor for cognitive impairment and dementia.Citation27,Citation28 For example, Schmand et alCitation29 examined the decline across life span in the population density of neocortical synapses which do not reach the level found by AD patients. Also, they examined the broad effect of education in rising neocortical synaptic density; finally, after considering aging population projections, they concluded that it is important to protect cortical synapses through cognitive stimulation across life span. This panorama make education as a compensator and/or a protective factor for cognitive impairment.
In sum, cognitive plasticity seems to be a relatively new construct yielding useful information about normal and impaired mental functioning being influenced by age, level of pathology, and education. After this review, an important question remains: to what extent are age and pathology accounting for cognitive decline and/or impairment? Thus, considering the growing interest in the oldest-old and the relevance and increasing prevalence of cognitive impairment, dementia due to AD through very old age, the present article addresses three main questions: (1) to what extent cognitive plasticity is preserved but decline across different ages, including the very old, and to what extent this decline is mediated by education; (2) to what extent do MCI individuals and mild Alzheimer’s disease patients maintain a gradient of modifiability; and (3) to what differential extent age, pathology, and education influence plasticity.
Methods
Participants
The total sample came from four different research projects:
“Normal” older adults, 55–89 years old
From the baseline of the ELEA Project (Longitudinal Study of Active Aging),Citation30 in which people were assessed in the year 2006, individuals from 55 to 74 years of age were recruited. The criterion for inclusion in this population study was being in the age range 55–75 years. In order to have a varied sample, convenient subsamples were recruited from four contexts (a representative sample of the Madrid population, rural and urban citizen clubs, and a university program for the elderly).
Participants aged 75–89 years were assessed in 2002 from the Learning Potential Study (LPS).Citation21 In this clinical study, the criteria for inclusion of “normal” older sample were: absence of central nervous system pathological conditions and other health problems related to cognitive impairment (alcoholism, drug addiction, or systemic diseases, etc).
Therefore, a total of 601 participants 55–89 years old were included in the present study (mean age, 68.8 years; SD = 6.6; range, 55–89; 347 females and 254 males; mean years of education, 8.9 years; SD = 9.4)
“Normal” oldest-old, older than 90 years old
From the baseline of the “90+” Longitudinal Project, assessed during 2007, the data from 188 participants older than 90 years (mean age, 92.9; SD = 2.5; age range, 90–102; years of education, 9.3 years; SD = 14.5) were re-analyzed (all of them were born before 1917; 121 females and 67 males). The criteria for inclusion for this population study were the following: ≥90 years old, independence in basic activities of daily living (score > 60 on the Barthel scale),Citation31 and having preserved cognitive capacity (score > 56 in the Informant Questionnaire of Cognitive Impairment on the Elderly or have less than two errors in the Sort Portable Mental Status Questionnaire (SPMSQ)Citation32 or less than three if the level of education is low). This is also a convenience sample, recruited from several contexts.
It is important to mention that our “normal” age groups significantly differed (χ2[24] = 56.43, P < 0.001) in their level of education; younger cohorts had higher levels of education than older cohorts. These differences correspond to population differences in those cohorts due to socio-historical changes in the education system in Spain.Citation33
shows how the “normal” sample is distributed for age groups (55–64; 65–69; 70–74; 75–89; 90+ years) and gender.
Table 1 “Normal” age groups by gender
“Mild Cognitive Impairment” and Alzheimer’s disease patients
From the baseline of the Longitudinal Project “Cognitive Plasticity during the Course of Dementia CPCD” (baseline in 2007) data from 57 individuals diagnosed as “Mild Cognitive Impairment” (MCI; mean age, 76.11 years; SD = 5.20; range, 65–86 years) and 98 patients (mean age, 78.16; SD = 5.07; range, 64–88) diagnosed with Alzheimer’s disease (“mild” AD) were re-analyzed. shows the sample distribution by gender and age. Regarding education, no significant differences between groups were found; the mean years of education were 10.46 years for the MCI group (SD = 12.7) and 7.16 years for the AD group (SD = 5.32).
Table 2 Composition of the pathology groups (MCI and AD) according to gender and age group
MCI individuals were diagnosed in the Diagnostic Unit of the ACE Centre by independent experts according to the criteria from Petersen et al:Citation34 memory complaints, normal activities of daily living, normal general cognitive function, abnormal memory for age, and do not follow dementia criteria.
AD patients were included according to the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV);Citation35 criteria from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA; Protocol Attachment HGIV.3);Citation36 a Mini Mental State Examination (MMSE)Citation37 score 18–26 or a level 4 score in the Global Deterioration Scale (GDS);Citation38 a Hachinski Scale scoreCitation39 ≤4. Informed consent from a close relative was required. Subjects with psychiatric illness or primary neurological disorder or delirium, or with alcoholism or drug addiction history were excluded.
Instruments and procedures
In order to assess cognitive plasticity, all participants were evaluated under standard conditions by the Verbal Memory Learning Potential test VMLt (based on the “verbal learning test” from Rey;Citation22,Citation40 modified by Fernández-Ballesteros et al).Citation21 The task consisted of the auditory presentation of 15 common words which were to be immediately recalled (free recall). The number of words correctly recalled in the first trial was considered the pre-test score or baseline. Afterwards, five consecutive learning trials were performed using the same words; after the second, third, and fourth trials, feedback (number of words correctly recalled) and verbal reinforcement were provided (“good!; you did very well!”); in the fifth trial, a cognitive strategy (verbally described: “perhaps you can group the words”) was suggested. Trials 2–5 were considered the training phase. The sixth trial was considered the post-test. Finally, after the presentation of an interference task, delayed recall was included (seventh trial). The number of words correctly recalled in each trial was considered the raw score.
In all the studies, VMLt instructions were the same being administered for a trained psychologist expert on aging. In the two population studies (ELEA and 90+), the VMLt was placed inside an in-home interview, interference phenomena was a four-item Wellbeing Scale; in the two clinical studies (LPS and CPCD), the VMLt was placed into a Cognitive Battery, interference phenomena was a dots task.
Statistical analysis
In order to examine group differences (age group and pathology) among scores through trials, t-tests and between and/or repeated measures analysis of variances (ANOVAs) were conducted with post-hoc Bonferroni comparisons, when appropriate. ANOVAs were performed in order to test the effects of the level of education with the age groups. Finally, the relative weights of age and pathology group performance were assessed via logistic regression. Statistical analyses were carried out using SPSS software (v 15.0; SPSS, Inc, Chicago, IL).
Results
We first analyzed the learning performance of “normal” participants, and then we compared their performance to that of the pathological groups.
Performance of the “normal” group
shows performance levels of the “normal” participants, according to their age group. A 5 × 7 ANOVA (five between-group age levels, seven within-subject trials) was applied to the values (it could be argued that trial 7 is qualitatively different from the other six, and should not be included in this analysis; when repeated with only the first six trials, the pattern of results did not change in this group nor in the equivalent analyses for the other groups, in the sense that the same factors remained significant). There was a significant main effect of the age group (F [4,715] = 127.0; P < 0.001) and the trial (F [6,4290] = 812.6; P < 0.001). As revealed in the figure, performance was inversely related to age, and performance monotonically increased along the first six trials but decreased in the delayed trial (seventh trial). The interaction was also statistically significant (F [24,4290] = 15.3; P < 0.001); again, the functions in show that the rate of performance increase as a function of the trial number (until trial 6) was slower as age increased. When years of education was included as a covariate it explained a significant part of the variance (F [1,669] = 35.5; P < 0.001), and the interaction of trial number by years of education was also significant (F [6,4014] = 2.9; P = 0.01). However, the effects highlighted in the analysis above (age group, trial number, and the age group by trial interaction) remained significant when years of education was included as a covariate. Of course, years of education was positively associated with performance; performance was higher with increasing years of education.
Figure 1 Learning performance on (A) healthy elders, (B) MCI individuals, and (C) AD patients. The mean number of words correctly recalled (y-axis) is plotted as a function of the trial (1–7 in the x-axis), for each age group. The first trial is considered as the baseline, whereas trials 2–5 are the training phase, and trial 6 is considered the post-test; the 7th trial is a delayed trial performed (see the text).
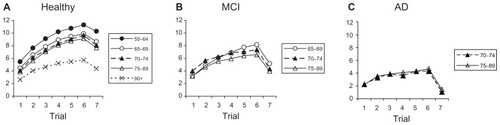
Regarding trial 7, the delayed recall trial, shows that all age groups demonstrated a decline. Although a one-way ANOVA of the five age groups on the differences (T6-T7) reached statistical significance (F [4,768] = 2.4; P = 0.048) there was no systematic pattern in the differences associated with age group. Bonferroni post-hoc comparisons showed that only one pair (55–64 versus 70–74 age groups) from ten possible comparisons differed significantly (P = 0.032). The result of this ANOVA is interpreted as a probable type I error, and we conclude that there is no systematic association between age and the amount of decline of performance in the delayed trial.
The upper panel of shows some specific comparisons of special interest to our purpose here: gain across the six trials (T6-T1), and decrease in the delayed trial as compared with the level reached in the sixth trial (T6-T7). Performance level at the first trial (T1) was compared across the groups by means of an ANOVA that showed a significant effect (F [4,779] = 65.8; P < 0.001)
Table 3 Learning comparison in gain score (Trial 6–Trial 1) and delayed scores (Trial 6–Trial 7), t-test
shows that, as expected, performance decreased monotonically as a function of age. Bonferroni post-hoc comparisons show significant differences between all pairs of age groups (P < 0.05) with two exceptions: 65–69 versus 70–74 and 70–74 versus 75–89.
MCI group
shows the performance levels of the MCI group according to their ages. A 2 × 2 × 7 ANOVA (two pathology between-groups – normal versus MCI, two between-group age levels, seven within-subject trials) was applied to the values (only the two age groups of 70–74 and 75–89 were included, given the small size of the 65–69 age group). There was a significant main effect of the pathology group (F [1,365] = 54.5; P < 0.001), the age group (F (1,365) = 4.8; P < 0.03), and the trial number (F [6,2190] = 275.1; P < 0.001); as revealed in the figure performance was lower for the MCI group than for normal participants, performance monotonically increased along trials 1–6 and decreased in the delayed (seventh) trial, and performance was worse for the older group. The first-order interaction between the pathology group and the trial was significant (F [6,2190] = 37.3; P < 0.001); the functions in show that the rate of performance increase as a function of trial was slower for the MCI group. However, the first-order interactions of age group were not significant, neither with trial number (F [6,2190}) = 0.524; P = 0.79), or the pathology group (F [1,365] = 0.675; P = 0.412); in the same vein, the second-order interaction (pathology group by age group by trial) was not significant (F [6,2190] = 0.437; P = 0.854).
In the previous section a significant interaction between age and trial was found. However, suggests that it could be due to the extreme age groups (55–64 and 90+) that were not present in the MCI group. Repeating the ANOVA of the previous section for the normal participants, with only the two age groups with enough data in the MCI group (70–74 and 75–89), it was found that the interaction between trial and age group was not significant (F (6,1374) = 1.4; P = 0.214). That is, the interaction of the previous analysis was due to the extended range of age groups.
When adding years of education as a covariate, a significant increase in the variance was explained (F [1,219] = 6.6; P = 0.011), and the interaction with trial was not significant (F [6,1314] = 0.79; P = 0.579). However, the effects high-lighted (pathology group, age group, trial, and pathology by trial interaction) remained significant when the covariate was years of education.
For the decline of performance as a dependent variable (difference between performance in the last learning trial and the delayed trial, T6-T7), a 2 × 2 ANOVA (two pathology groups, two age groups) was performed. The results showed no significant effect of the main factor age group (F [1,359] = 1.27; P = 0.261), nor of the interaction (F [1,359] = 1.58; P = 0.209), but a significant effect of the pathology group (F [1,359] = 28.0; P < 0.001). The decline was significantly greater for the MCI group than for the normal subjects (2.81 versus 1.62, on average).
The middle panel of shows the specific comparisons for the MCI age groups for which there were enough participants. Again, the two age groups showed significant gains and delayed decrease. Furthermore, alongside the first trial performance, a 2 × 2 ANOVA (two pathology groups, two age groups) was performed. It showed nonsignificant effects of pathology group (F [1,369] = 1.62; P = 0.424), the group (F [1,369] = 2.96; P = 0.335), and the interaction (F [1,369] = 2.69; P = 0.102).
In short, the results showed that when taking normal subjects of a wide age range, level of performance changes significantly as a function of age. The MCI group showed a level of performance comparable to that of the normal subjects in the first trial. However, the learning curves show slower rates of improvement for the MCI subjects, and a larger decrease in the delayed trial. Although the slope of the learning curve across age was smaller for the MCI group, this interaction was not significant.
AD group
shows the performance levels of the AD group, according to their age group; data were only available for subjects from the 70–74 and 75–89 age groups. Some analyses comparing the two pathological groups were run. A 2 × 2 × 7 ANOVA (two pathology between-group, two between-group age levels, seven within-subject trials) was applied to the values. There was a significant main effect of the pathology group (F [1,220] = 70.5; P < 0.001), and trial (F [6,1320] = 110.3; P < 0.001), but not of the age group (F [1,220] = 0.254; P = 0.615). As revealed in the figure, performance was lower for the AD group than for the MCI participants, and performance monotonically increased along trials 1–6 and decreased in the delayed (seventh) trial. The first-order interaction between pathology group and trial was significant (F [6,1320] = 6.62; P < 0.001); the functions in show that the rate of performance increase as a function of the trial was slower for the AD group. However, the first-order interactions of the age group were not significant, nor was the trial (F [6,1320] = 0.658; P = 0.684), or the pathology group (F [1,220] = 3.487; P = 0.063); in the same vein, the second-order interaction (pathology group by age group by trial) was not significant (F [6,1320] = 0.628; P = 0.708).
When adding the years of education as a covariate, it did not explain a significant part of the variance (F [1,137] = 0.018; P = 0.894), and the interaction with trial was not significant (F [6,822] = 1.683; P = 0.122). However, the effects highlighted (pathology group, trial, and the pathology group by trial interaction) remained significant when the covariate was years of education.
Again taking decline of performance as a dependent variable, a 2 × 2 ANOVA (two pathology groups, two age groups) was performed. The results show no significant effect of the main factors age group (F [1,220] = 0.318; P = 0.573), pathology group (F [1,220] = 1.617; P = 0.205), nor of the interaction (F [1,220] = 1.264; P = 0.262). The decline was statistically equivalent for the two pathology groups and age levels.
The lower panel of shows the specific comparisons for the age groups of AD for which we had enough participants. Again, the two age groups showed significant gain and delayed decrease. Furthermore, we ran with the first trial performance a 2 × 2 ANOVA (two pathology groups, two age groups). It did not show significant effects for either age group (F [1,181] = 2.60; P = 0.109) or the interaction (F [1,181] = 2.66; P = 0.105), but showed a significant effect of the pathology group (F [1,181] = 28.89; P < 0.001); as expected, the direction of the effect was that AD participants showed a lower performance than MCI participants (2.24 vs 3.59, respectively).
The Verbal Memory Learning Potential test as a diagnostic tool
The average values from suggest that the learning test employed could be a useful tool for quick classification or screening to discriminate between healthy individuals and AD patients. Especially interesting is the fact that the only group that showed lower performance in the delayed trial (trial 7) than in the first learning trial (trial 1) was the AD individuals. After checking other combinations, the best tool was found to be the difference between the levels of performance in the seventh and first trials (T7-T1). If individuals are categorized as AD with the criterion of having a difference of 0 or negative ((T7-T1) ≤0), the sensitivity for the 70–74 age group equals 0.824 (14/17), whereas the specificity equals 0.918 (168/183). These statistics are about the same in the 75–89 age group, as sensitivity is 0.813 (61/75) and specificity is 0.917 (88/96). However, there were insufficient participants for this same calculation to be done in other age groups.
In short, the difference between performance in the delayed (seventh) trial and the first trial can be used as a simple diagnostic tool, at least in the interval of ages between 70 and 89 years. Classifying as AD those who do not show better performance in trial 7 compared with their performance in trial 1 (T7-T1 ≤ 0) yields a good balance between sensitivity and specificity.
Assessing the impact of disease in terms of normal aging
This section considers how the association of impairment with pathology can be expressed in units that can be more easily understood. Specifically, a comparison between , allows us to ask the following question: can the performance of the MCI and AD groups be placed somewhere on a continuum of decline in normal individuals? Or, in other words, could the impairment associated with the pathology be converted (translated) into years of normal decline? How many years of normal impairment are equivalent to the impairment associated to the pathology, when both are reflected in a single measure?
In order to analyze the impact of pathology (MCI and AD) in terms of the impact of age, we adjusted some regression models. In the final model, performance in the 6th trial was the dependent variable, and age (not grouped) plus the years of education and two dummy variables that code the pathology group, were the independent variables. All independent variables were entered in the equation, as all explained a significant part of the variance. The final model, for which R2 (adjusted) = 0.467, was:
Some simple calculations allow estimation of the impact of having MCI in the 6th trial in terms of years of normal decline. Specifically, dividing the slopes (2.395/0.16) yield that having MCI means suffering an impairment in performance that is equivalent to the decline of a normal individual during 15 years; the same analysis for AD (3.627/0.16 = 22.7) yield that the impact of being AD is equivalent to 22.7 years of normal decline in performance. In other words, the regression model predicts (in trial 6) for an MCI individual the same performance as to a normal individual with 15 years more than them; it also predicts for an AD individual the same performance as to a normal individual with 22.7 years more than them. Of course, the years of education have a positive impact; expressed also in a “years of normal decline” metric, the impairment that in the average person has associated 1 year of normal aging (0.16/0.034) is equivalent to the improvement associated to about 5 years of education.
The same does not happen with the delay trial (T6-T7) or, in other words, with the interference phenomenon. The correlation between age and delay trial performance was not significant (R = 0.038; P = 0.287). That is, whereas age can account for a decreased gain across the six trials and the impact of MCI and AD can be assessed in terms of normal aging, the decline associated with the delayed trial is not related to normal aging, but both MCI and AD have an important impact on it. In other words, the interference phenomenon is not an age-based phenomenon but a sign of pathology.
Discussion
All age groups of normal individuals show plasticity as reflected in performance increases along the six trials of verbal learning. The effect of age is clear in the first trial, as performance is lower as age increases, and also in the rate of learning, as the interaction between trial and the age group indicates that people learn at a slower rate the older they are. In other words, in terms of plasticity theoryCitation1 or dynamic assessment,Citation9 “static” assessment of cognitive performance as well as learning performance (“dynamic” assessment) are both related to age. On the contrary, the drop-in performance from the 6th to the 7th (delayed) trial is not associated with age but to pathology.
In terms of this pathology, the AD group shows lower performance in the first trial than the MCI and normal individuals of the same age; this is a specific qualifier for AD persons. The rate of learning for the three groups follows a continuum (normal > MCI > AD). The drop-in performance in the delayed trial is significantly larger for the two pathological groups; there is no significant difference between them. This is a shared qualifier for the pathological groups. Differences between normal and AD individuals match the Baltes and RaykovCitation2 and Lindenberger and RicheisCitation20 results as well as our own previous studies regarding normal, MCI individuals, and AD patients.Citation21,Citation23
Regarding our research questions, the first one refers to what extent cognitive plasticity, assessed through verbal learning, declines across age, including the oldest-old. According to our results, all age groups from 55 through older than 90 learn from cognitive training and, therefore, they show cognitive plasticity as reflected in an increased performance along a learning curve of six trials. The effect of age is clear in the first trial, as baseline performance is lower as age increases, but there is an effect of age in the rate of learning as well, as the interaction between the trial and the age group indicates that people learn at a slower rate as age increases. On the contrary, the strong effect of interference showed in the delayed trial (difference of performance between trials 6 and 7) is not associated with age but is a specific characteristic of the impaired groups.
In sum, our findings provide evidence for differential learning performance across aging including the very old, and they converge with other studies with young-oldCitation1,Citation13,Citation25 and with the oldest-old.Citation16,Citation20 Regarding learning decline, it is important to mention that we did not find significant differences between two groups: 70–74 years and 75–89 years; that is, in our data there is no decline of learning from 70 to 89 years, as has been reported by Yang et al.Citation16
Regarding our very old nonagenarian participants, their decline in learning is significantly higher than that of the other age groups, but this age group continues to improve their learning performance. This supports the idea that cognitive plasticity as demonstrated in the young-old can be extended into the oldest-old, as has been reported by Lindenberger and Reschies.Citation20 Nevertheless, their performance in learning is similar to the decline of those younger individuals diagnosed as having MCI; that is, they show no significant differences in their learning curve with MCI individuals, with the exception of the 7th delayed trial. At this point we might conclude, as Baltes and SmithCitation15 do, that in spite of the fact that they constitute a group of independent nonagenarians, they perform as cognitive impaired individuals. Could it be considered at the limits of their biological capacity? From our point of view, we cannot reach that conclusion; given that our group of nonagenarians belongs to a cohort for whom mandatory education did not exist (almost half of them have no formal education). Even then, our analysis shows that when education is introduced as a covariant, age differences between nonagenarians and the other age groups continue to be significant. It can be stated that our nonagenarian sample is at the limits of their under-stimulated capacities. Education seems to be a trigger for learning and we must be aware that our very old participants had a very low education level, which is different from the younger cohorts.
As Terry and KatzmanCitation41 proposed, policies have to take into consideration the importance of education across lifespan. Moreover, the importance of life-long learning seems to be a new platform for improving plasticity along life and in old age. But it is also important to fight against social and group stereotypes which propagate the idea that older persons cannot learn or are unable to solve problems.
Also, it is important to investigate to what extent cognitive training across the lifespan and during old age can play a role in learning capacities, as pointed out by Mayr.Citation10 Additionally, in our data coming from the study of individuals of 90+ years, those nonagenarians who are cognitively active (that is, in the baseline performed cognitive activities such as “reading”, “playing chess”) show better cognitive functioning in the follow-up, after 6–14 months.Citation42 In other words, a new panorama is open for the aging mind.
Regarding our second goal, that is, to what extent MCI individuals and mild AD patients maintain a certain level of plasticity or gradient of modifiability, as in previous studies, both cognitive-impaired groups show genuine learning. Although there are significant differences in learning between “normal” and “impaired” groups, MCI and AD individuals can modify their performance through cognitive training. As expected, the baseline score was significantly lower in the AD than in MCI groups. The rate of learning is different for the three groups (normal > MCI > AD). But the decline of performance in the delayed trial is significantly larger for the two pathological groups. Moreover, there is no difference between them; this is a shared qualifier for the pathological groups. Finally, not showing higher performance in the delayed trial than in the first trial can be used as a quick diagnostic or screening tool for AD.Citation21,Citation23
Regarding cognitive plasticity shown by MCI and AD groups, it can be concluded that although they show lower performance, both in the pre-test and in the gain scores, than “normal” individuals (with the exception of the similarities between the oldest-old and the MCI group) both groups reach (after the training) twice the number of words recalled than on the first trial: both groups show the ability to learn. These results are in accordance to Schreiber and Schneider,Citation43 who indicate that plasticity-oriented information given in a pre-test-training-post-test-design is potentially useful for the purposes of early identification of dementia, and to Lindenberg and Reschies,Citation20 who found that mildly demented individuals, with a lower baseline that those “normal” individuals, show similar gains through learning.
Our results regarding the interference phenomenon show that a lower score in trial 7 than in trial 1 (a kind of “de-learning” effect), is a result specific to the AD group. It constitutes a clear and pathognomonic indicator of dementia, even though delay trial performance in the MCI group also showed significant differences with any other group (including the very old). This result is in accordance to the Petersen’s group,Citation18 who found differences between nonagenarian demented, with MCI and “normal,” just in the delay trials, which is an indicator of neuropsychological pathology. Nevertheless, it is important to emphasize that mild AD patients can take benefit from learning, as already indicated.Citation24 Any nihilistic position concerning these patients does not have empirical support; with balanced objectives, they can be trained and take benefit from learning.Citation44
Finally, taking into consideration that both age and pathology are accounting for cognitive plasticity variance, our third question refers to the relative weights of both independent variables. Since pathology is associated with age, it is difficult to disentangle both factors. Moreover, our MCI and AD samples are both younger (age range 55–90 years) than our total age range of “normal” sample (55–102 years) and have both nonsignificant but higher and homogenous levels of education. Nevertheless, our analyses support the hypothesis that the effect of having MCI or AD have weights equivalent to 15 and 22.7 years of normal aging, respectively. In the same vein, 1 year of reverse normal aging seems to be equivalent to the improvement associated with about 5 years of education.
Although theories of aging postulate a non-arithmetic aging decline process, at least in biological aging,Citation45,Citation46 our analysis assumes that the decline maintains a constant rate from 55 through 102 years, as also assumed by Terry and Katzman,Citation41 when they established synaptic density changes through normal aging. But we are aware of the weaknesses of our calculations, which must be considered as an attempt to disentangle age, pathology, and education in non-representative samples of “normal” individuals from 55 through 102 years (who were not assessed from neuropsychological perspectives), and clinical MCI and AD samples.
Nevertheless, it is important to emphasize the limit of these results, already mentioned in our ‘Participants’ section; since we are re-analyzing four studies (two population and two clinical studies), although measures and procedures are identical for assessing cognitive plasticity, our criteria for inclusion are not exactly the same (those “healthy” individuals and 90+ years and older). Perhaps this fact could justify the broad differences in cognitive plasticity between the 90+ and 75–89 years groups.
In conclusion, plasticity or learning capacities is present across old age, even in nonagenarian elders, but this plasticity declines through the normal aging process. MCI individuals and those with AD also show plasticity in the sense that they can improve their memory performance through learning. Nonagenarian individuals show a similar learning curve to those of MCI patients. “Normal” individuals from 55 through 89 years significantly differ in their learning curves from both pathological groups. Those pathological groups significantly differ from “normal” groups in the interference phenomenon. This effect is dramatic in AD patients; a “de-learning” effect appeared only in those individuals as a pathognomonic sign.
Acknowledgments
This study has been granted by the Research General Direction, MICINN: Project SEJ-2006-14438/PSIC; IMSERSO I+D+I Projects: 15-05 and 35-06. Our gratitude to Professor James Juola (University of Kansas) for his comments on this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BaltesPBSowarkaDKlieglRCognitive training research on fluid intelligence in old age: what can older adult achieve by themselves?Psychol Aging1989422172212789749
- BaltesMMRaykovTProspective validity of cognitive plasticity in the diagnosis of mental status: A structural equation modelNeuropsychology199610549556
- SalthouseTASpeed mediation of adult age differences in cognitionDev Psychol199629722738
- SchaieKWWhat can we learn from longitudinal studies of adult development?Res Hum Dev20052313315816467912
- VygotskyLSMind in Society. The Development of Higher Psychological ProcessesColeMJohn-SteinerSScriberSSoubermanECambridge, MAHarvard University Press1978
- EmbretsonSEMeasuring and validating cognitive modifiability as an abilityJEM19922912550
- CuddyANortonMIFiskeSTThis old stereotype: The pervasiveness and persistence of the elderly stereotypeJ Soc Issues2005612267283
- DoidgeNThe Brain That Changes ItselfNew YorkViking Press2009
- Fernández-BallesterosRCaleroMDThe assessment of learning potential: the EPA instrumentLidzCElliotJDynamic Assessment: Prevailing Models and ApplicationsGreenwich, CTJAI Press2000293323
- MayrUIntroduction to the special section on cognitive plasticity in the aging mindPsychol Aging200823468168319140639
- CaleroMDNavarroERelationships between plasticity, mild cognitive impairment and cognitive declineArc Clin Neuropsychol2004195653660
- Fernández-BallesterosRZamarrónMDCaleroMDTárragaLCognitive plasticity and cognitive impairmentFernández-BallesterosRGeroPsychology. European Perspectives for an Ageing WorldGöttingen, GermanyHogrefe and Huber2007145164
- KlieglRSmithJBaltesPBOn the locus and process of magnification of age differences during mnemonic trainingDev Psychol199026894904
- SingerTLindenbergerUBaltesPBPlasticity of memory for new learning in very old age: A story of major loss?Psychol Aging200318230631712825778
- BaltesPBSmithJNew frontiers in the future of aging: from successful aging of the young old to the dilemmas of the fourth ageGerontology200349212313512574672
- YangLKrampeRTBaltesPBBasic forms of cognitive plasticity extended into the oldest-old: retest learning, age and cognitive functioningPsychol Aging200621237237816768581
- PetersenRMild cognitive impairment: transition between aging and Alzheimer’s diseaseNeurología200015393101
- BoeveBMcCormickRNSmithGMild cognitive impairment in the oldest oldNeurology200360347748012578930
- BaltesMMKühlKPSowarkaDTesting the limits of cognitive reserve capacity a promising strategy for early diagnosis of dementia?J Gerontol1992473165167
- LindenbergerUReischiesFMLimits and potentials of intellectual functioning in old ageBaltesPBMayerKUThe Berlin Aging Study. Aging from 70 to 100Cambridge, UKCambridge University Press1999329360
- Fernández-BallesterosRZamarrónMDTárragaLMoyaRIniguezJLearning Potential in Healthy, Mild Cognitive Impairment subjects and in Alzheimer patientsEur Psychol20038148160
- ReyHClinical Examination in PsychologyParis, FrancePress Universitaire de France1964 French
- Fernández-BallesterosRZamarrónMDTárragaLLearning potential: a new method for assessing cognitive impairmentInt Psychogeriatr200517111912815945596
- ZamarrónMDTárragaLFernández-BallesterosRChanges in reserve capacity in Alzheimer’s disease patients attending psycho-stimulation training programsPsychology in Spain200913854
- Fernández-BallesterosRCaleroMDTraining effects on intelligence of older personsArch Gerontol Geriatr199520213514815374242
- ChristensenHKortenAEJormAFEducation and decline in cognitive performance: compensatory but not protectiveInt J Geriatr Psychiatry19971233233309152716
- KliegelMZimpricDRottCLife-long intellectual activities mediate the predictive effect of early education on cognitive impairment in centenarians: a retrospective studyAging Ment Health20048543043715511741
- NganduTvon StraussEHelkalaELEducation and dementia: what lies behind the association?Neurology200769141442145017909157
- SchmandBSmithJLindenboomJLow education is a genuine risk factor for accelerated memory decline and dementiaJ Clin Epidemiol1997509102510339363037
- Fernandez-BallesterosRZamarrónMDLópezMDSuccessful aging: criteria and predictorsPsychology in Spain201115194101 Spanish
- MahoneyFIBarthelDWFunctional evaluation: the Barthel IndexMd State Med J1965146465
- PfeifferAA short portable mental status questionnaire for the assessment of organic brain deficit in elderly patientsJ Am Geriatr Soc197523104334411159263
- Díez-NicolásJLos Mayores en la Comunidad de MadridMadrid, SpainObra Social Caja Madrid1996 Spanish
- PetersenRSmithGWaringSCIvnikRTangalosTGKokmenEMild cognitive impairment: clinical characterization and outcomeArch Neurol199956330330810190820
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders IVWashington, DCAmerican Psychiatric Association1994
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology19843479399446610841
- FolsteinMFFolsteinSEMcHughPRMinimental state. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res19751231891981202204
- ReisbergBFerrisSHde LeonMJCrookTThe Global Deterioration Scale for assessment of primary degenerative dementiaAm J Psychiatry19821399113611397114305
- HachinskiVCIliffLDZihlkaECerebral blood flow in dementiaArch Neurol19753296326371164215
- DelisDKramerJKaplanEOberBCalifornia Verbal Learning Test: Adult Version ManualSan Antonio, TXThe Psychological Corporation1987
- TerryRDKatzmanRLife span and synapses: will there be a primary senile dementia?Neurobiol Aging200122334734811378236
- MolinaMASchettiniRBravoMDZamarrónMDFernández-BallesterosRCognitive activies and cognitive functioning in the elderlyRev Esp Geriatr Gerontol2011 [Epub ahead of print.]
- SchreiberMSchneiderRCognitive plasticity in people at risk for dementia: optimising the testing-the-limits-approachAging Men Health20071117581
- TárragaLBoadaMModinosGA randomized pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer’s DiseaseJ Neurol Neurosurg Psychiatry200677101116112116820420
- MayrEThis is Biology The Science of the Living WorldCambridge, MAHarvard University Press1997
- MedinaJJThe Clock of AgesCambridge, UKCambridge University Press1997