?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The purpose of this study was to determine the relationship between hypothesized pain behaviors in the elderly and a measurement model of pain derived from the Minimum Data Set-Resident Assessment Instrument (MDS-RAI) 2.0 items.
Methods
This work included a longitudinal cohort recruited from Medicare-certified longterm care facilities across the United States. MDS data were collected from 52,996 residents (mean age 83.7 years). Structural equation modeling was used to build a measurement model of pain to test correlations between indicators and the fit of the model by cognitive status. The model evaluates the theoretical constructs of pain to improve how pain is assessed and detected within cognitive levels.
Results
Using pain frequency and intensity as the only indicators of pain, the overall prevalence of pain was 31.2%; however, analysis by cognitive status showed that 47.7% of the intact group was in pain, while only 18.2% of the severely, 29.4% of the moderately, and 39.6% of the mildly cognitively impaired groups were experiencing pain. This finding supports previous research indicating that pain is potentially under-reported in severely cognitively impaired elderly nursing home residents. With adjustments to the measurement model, a revised format containing affective, behavioral, and inferred pain indicates a better fit of the data to include these domains, as a more complete measure of the pain construct.
Conclusion
Pain has a significant effect on quality of life and long-term health outcomes in nursing home residents. Patients most at risk are those with mild to severe cognitive decline, or those unable to report pain verbally. Nursing homes are under great scrutiny to maintain standards of care and provide uniform high-quality care outcomes. Existing data from federally required resident surveys can serve as a valuable tool to identify indicators of pain and trends in care. Great responsibility lies in ensuring pain is included and monitored as a quality measure in long-term care, especially for residents unable to communicate their pain verbally.
Introduction
Pain affects 49%–83% of 1.8 million residents living in long-term care facilities in the United States.Citation1–Citation4 The outcome of pain and long-term suffering influences psychological, physiological, and social aspects of an individual’s life. Chronic pain is associated with symptoms of anxiety and depression,Citation5 and can have a serious adverse impact on quality of life. This may result in an inability to sleep, clinical depression, weight loss, disturbances in gait, immune suppression, decreased socialization, increased morbidity,Citation6,Citation7 and burgeoning health care costs.Citation5,Citation7,Citation8
Behavioral and psychosocial factors play an important role in understanding the experience, continuation, and exacerbation of pain.Citation9 Individuals display many different behavioral cues, making it difficult for the clinician to comprehend the nursing home resident’s needs. Research indicates specific verbal, behavioral, and facial expressions as being representative of pain.Citation10,Citation11 Because pain is an individual subjective experience, the complexity of assessing and determining patient pain may increase with cognitive decline. Cognitive decline progressively hampers the individual’s ability to anticipate and verbalize pain.Citation12 Decades of research indicate pain is poorly assessed and managed in long-term care, especially for those with moderate to severe cognitive impairment.Citation13–Citation18 Looking at underlying common characteristics of pain could clarify our understanding of how to measure and better identify pain. Basing detection of pain only on self-reports from patients fails to take into account other indicators that an individual could be expressing.
Research to date lacks a large-scale analysis of pain in long-term care that evaluates a multidimensional construct of pain. The aims of this study were to determine the magnitude of the relationship between pain behaviors and a hypothesized measurement model, to compare theoretical models to existing pain scales, and to examine the construct validity of a pain measurement model. The research question was: can a theoretically derived model of pain aid in detecting pain across all cognition levels?
Multiple smaller-scale studies have evaluated specific pain tools, while recommending additional research using larger samples to increase the generalizability across longterm care settings and to include a more comprehensive analysis of residents most at risk, ie, the severely cognitively impaired.Citation19–Citation23 Data from existing nationwide assessment instruments, like the minimum data set (MDS), are a source for evaluating resident pain and other quality initiatives.Citation24 The goal of evaluating the dimensions and theoretical constructs of pain is to clarify the validity of measures and the reliability of existing quality indicators from the MDS to be able to detect pain across all cognitive states more accurately.
Significance
Nursing homes are under great scrutiny for adherence to regulations, quality improvement actions, and public reporting. Stakeholders and researchers have raised concerns about the accuracy, usefulness, and timeliness of reports to describe care in skilled nursing settings.Citation25,Citation26 The Joint Commission calls for the close monitoring of pain management in health care settings and evaluates the appropriateness of interventions.Citation27,Citation28 The American Health Quality Association reports on health care entities that strive to improve pain management through quality initiatives, and the Centers for Medicare and Medicaid Services encourage ongoing quality improvement in skilled care settings through resident assessment surveys.Citation29 Multiple entities are working towards improving care for the elderly, but large-scale research is needed to understand pain behaviors better and ensure pain treatment is effective and ongoing in this population.
Pain has a significant impact on quality of life and outcomes in nursing home residents. Higher levels of comorbidities are reported with severe pain, along with increased depressive symptoms, reduced activity and significant physical effects.Citation30 Chronic pain is attributed to diseases like osteoarthritis, cancer, and facture, and neuropathies, with arthritis being the most common.Citation5
The study of pain, especially among those residents who are non-communicative, could significantly improve quality of life and the quality of care in nursing homes.Citation31 Residents with advanced cognitive decline are at the highest risk for under-treatment because of an inability to verbalize pain. Incorrectly assessing pain leads to a higher incidence of inappropriate medication use, medication side effects, and residents remaining in discomfort. These outcomes fail to apportion health care resources correctly, provide optimal treatment, or resolve the target issue of pain. Using evaluation tools to include a broader context of resident symptoms might help recognize patterns and methods to improve care.
Evaluating aggregate resident care at points over time can highlight successes or failures, and opportunities to improve treatments and outcomes. The integration and mechanisms of information technology/information systems are a helpful tool to combine health care delivery networks to improve resident outcomes. Analysis of data sets can reveal statistical relationships between symptoms, diagnoses, treatments, and outcomes.Citation32 Using existing data lessens the difficulties in recruiting and retaining those with increasing inability to assent or comprehend informed consent, offering important insights into resident care.
Background
Chronic pain in the elderly is most often felt in the feet, legs, back, and major joints.Citation5,Citation33 Other types of pain, like headache or visceral aches, are less reported in the elderly. It is estimated at least one in four older individuals suffers with chronic musculoskeletal pain.Citation5 Pain is an expression of underlying body damage, or peripheral nociceptive stimulation.Citation34,Citation35
Pain is often communicated via behaviors.Citation34,Citation36 Cohen- Mansfield and CreedonCitation31 define pain behaviors as “observable nonverbal behaviors” to indicate pain to others. Broader definitions include all forms of behaviors displayed by an individual thought to reflect the existence of nociception, including facial expressions, speech, posturing, patterns of medication use, seeking health care intervention, or changes in socialization.Citation35 Current studies suggest four clusters of pain behaviors, ie, altered ambulation (gait) or posture, negative affect, facial/audible expressions, and avoidance of activities.Citation37 A research study of nurses’ perceptions of key indicators of pain state that changes in behaviors, repetitive movements, repetitive vocalizations, and physical symptoms are indicative of pain.Citation31 Patients with severe dementia do not experience less pain intensity, less painful sites, or have a lower incidence of pain-causing diseases, but pain often goes unassessed and untreated in this population.Citation22
The responsiveness of caregivers with regard to intervention is a primary quality of care concern, especially for those institutionalized who rely upon others to interpret and meet their individual needs. A challenge to an understanding of pain is how to differentiate between pain behaviors and the behaviors expected from progression of a disease, such as memory impairment or the inability to communicate needs. If we use unique domains or categories to explain concepts of pain, this can broaden how pain is recognized, especially in the elderly who are cognitively impaired.
Cognition
Cognition describes how individuals differentiate, encode, store, retrieve, and use information.Citation38 The patient’s ability to reason, remember, and think describes cognitive status. Cognitive status influences the resident’s ability and how he/she communicates with others. A distinction in increasing cognitive decline is how behaviors are communicated. In dementia, wandering may involve an interruption in the individual’s ability to follow sequential mental tasks to reach a destination or goal.Citation39 The cognitively impaired resident has increased difficulty staying on task and remaining attentive to reach the goal of their activities. Cognitive impairment in conjunction with pain is a significant factor in explaining why certain verbal or nonverbal behaviors occur, and how the clinician could incorrectly interpret cues. Residents with severe cognitive impairment, as with dementia, are at high risk of suffering from pain because of an inability to report their pain verbally.Citation22
Affect
Affect and cognition are thought to be inextricably intertwined; however, some see emotion completely independent of cognition.Citation40 Beyond culture-bound expressions of affect, the elderly resident with severe cognitive impairment might have a flattened affect, or have limited verbal capacity with increased moodiness and crying. Affective domains include emotions and feelings. When evaluating mood in nursing home residents, depression may present as generalized aches and pains without a source of injury or disease, while chronic untreated pain may cause depression. Citation41 This makes discernment of pain especially difficult in residents with depression. The existence of multiple pain conditions is associated with anxiety and mood disorders across cultures.Citation42 Patient mood is an important concept of the pain construct in modeling whether depressed mood is an indicator of pain or a consequence of long-term untreated pain. A seminal workCitation37 demonstrated dimensions of pain behaviors including a negative affect and facial expressions of distress consistent with a pain behavior construct. Multiple studies have found significant associations between pain and grimacing.Citation43 Research on facial action coding systems has been used to confirm the existence of pain with different levels of cognitive impairment.Citation43,Citation44 Findings indicate facial expressions to noxious stimulation are significantly increased in residents with dementia in comparison with cognitively intact residents. Citation45 Research of facial expressions indicates that basic primordial expressions occur across cultures, gender, and age, along with learned “socially acceptable” emotions and expressions of mood. If the resident reverts to lower cognitive functioning, making facial expressions instinctive and not a culturally-bound expected reaction, universal expressions of pain could exist. Considering a severe decline in cognition, this might explain facial grimacing as a universal expression of pain.
Behavioral
A significant determinant of pain behavior is the severity of pain.Citation46 Behaviors like verbal complaints/negative vocalizations, sighing, moaning, agitation, crying, grimacing, rapid blinking, shifting/fidgeting, rubbing, resistance, bracing, guarding, and rigidity are common indicators of pain from the literature.Citation47–Citation49 Aggressive behaviors in cognitively impaired residents are also indicated as a sign of pain.Citation50 Behavioral science indicates that pain behaviors are subject to the same changes and influences which alter actions, as are other types of behaviors.Citation41 Much of the research into pain describes learned behaviors and operant conditioning as factors involved in continuation of pain behaviors.Citation9,Citation35 This assumption might hold true for cognitively intact residents, but is inadequate in explaining repetitive behaviors in the cognitively impaired resident. If pain needs are not being met, what would be the drivers for continuing the behavior?
Behaviors that are not followed by positive consequences but have neutral or adverse responses should diminish and end unwanted behaviors, thus describing the process of operant conditioning. The behavior should be deterred if these actions are not eliciting the desired response. Alternative behaviors would be attempted. The mechanism of operant conditioning does not explain repetitive behaviors, or why pain behaviors would not be eliminated if pain needs were being ignored. This behavioral perspective makes it difficult to attribute behaviors to progression of a disease and those of pain. Essential to an understanding of pain in the elderly are those variables that correlate with actual behaviors, ie, the outcome (consequence) of the behaviors, rather than just isolation of certain affective characteristics.
Disruptive behaviors common in dementia may lead to negative consequences, like continued untreated pain and the use of physical or chemical restraints to control the behavior. Citation51 Because one set of signs or behaviors do not uniformly detect pain at all cognitive levels, examining the association of behaviors according to cognitive group would be valuable in advancing research in this field. A comprehensive reviewCitation37 characterizes common problems in attempting to assess pain behaviors accurately as insufficient attention to the attributes of the construct and precision and consistency in the characteristics of the methods of assessment (ie, are the measures comprehensive and reliable?)
Inferred pain
Pain can be inferred from existing diseases (ie, osteoarthritis, osteoporosis, neuropathies, cancer) that are known to cause pain, and existing pain sites. Having multiple sites of pain causes more severe and disabling effects than having a single site of pain.Citation52 Pain assessment tools most commonly ask residents to rate pain and/or report its frequency and intensity. This aspect of pain assessment is essential, because even residents with cognitive impairment should be engaged with eye contact and inquiries into their level of comfort and not discounted as a unreliable source.Citation53,Citation54 Additionally, for cognitively impaired residents, direct observation of behaviors is the strongest evidence for ensuring pain is appropriately assessed and treated.Citation55 Inferred pain can be another valuable clue to examine and capture pain better. When clinicians use reported pain as the only assessment tool, ie, as a one-dimensional measure, assessments often fall short of being able to detect pain accurately. Application of the Nonverbal Pain Scale to the cognitively impaired person may help increase the accuracy of assessment, detection, and treatment of pain.
Theoretical framework
The theoretical foundation for this research incorporates the concept of need-driven behaviors and consequences of need-driven, dementia-compromised behaviors to frame a person-centered approach to careCitation39,Citation51,Citation56–Citation59 (see for definitions). Need-driven, dementia-compromised behaviors are actions displayed to communicate an underlying need.Citation39 Optimally, the immediate identification of primary need-driven behaviors would result in an action and resolution to decrease disruptive behaviors. Need-driven behaviors produce behavioral symptoms and explain how certain interventions could lessen disruptive behaviors.Citation60
Table 1 Theoretical construct definitionsCitation38,Citation63
The concept of dementia-compromised behaviors aids in explaining why continued behaviors are not lessened via the mechanisms of operant conditioning. Pain is one aspect of the framework. The framework is helpful in identifying the primary problem (pain) and developing antecedent and resulting consequences of unmet needs. The initial portion of the theoretical framework is used in this study to identify pain. The remaining structure of the framework is integral to the evaluation of other aspects of the model, including cognitive status and outcomes of untreated pain, like depression, social isolation, comorbidities, effective/non-effective interventions, and the cost-effectiveness of actions taken.Citation61
The construct of pain is thought to be multidimensional. Citation36,Citation37 How need-driven, dementia-compromised behaviors are expressed is specific to the individual and dependent upon proximal and background factors. Proximal factors are defined as “current situational issues or events”,Citation56,Citation62 varying greatly and dependent upon personal and environmental cues like staffing level or pain with movement. Background factors involve cognitive, psychosocial, neurological, and general health causes. These factors tend to be more constant. Need-driven behaviors aid in explaining why individuals display certain behaviors, especially those with cognitive impairment from dementia.Citation39 Need-driven behaviors provide a foundational framework for this study to draw theoretical links between unique indicators obtained from research, state of the science, and clinical practice.
Materials and methods
Design and sample
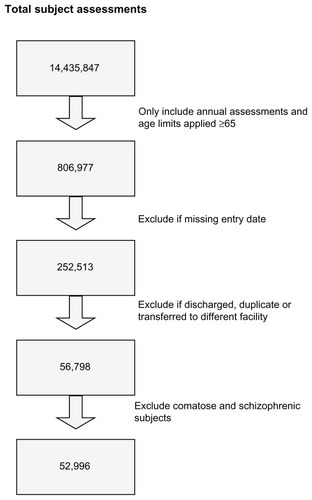
A secondary analysis of data from the Minimum Data Set- Resident Assessment Instrument (MDS-RAI) was conducted. A cross-sectional analysis was used to determine pain prevalence. The first-year records of a longitudinal data collection were used for the study. A combined total of 14,435,847 subject observations was reduced to 806,977 () by using annual assessments and applying the inclusion criterion of age ≥65 years. Unconfirmed entry dates into the system were also excluded, resulting in 252,513 subjects. Residents discharged over a 3-year span were dropped, reducing the total to 56,798. Individuals coded as being comatose were excluded, because the behavioral sections of B–F in the MDS are omitted as per the MDS-RAI instructions. The behavioral indicators evaluated in this research are contained in this section. Residents with schizophrenia were excluded to gain baseline cognitive levels, enabling reduction of the probability of fluctuating mental states due to psychosis. Data cleaning rules yielded a final sample of 52,996 residents to evaluate trends in pain behaviors and associations between cognitive, affective, behavioral, and inferred pain domains.
Instruments
The MDS is both mandated and the most commonly used resident assessment document in nursing home facilities. The MDS is not a comprehensive assessment, but a preliminary screening tool to help identify potential problems, strengths, and preferences for care. The MDS is a core set of items, definitions, and response categories composed of two parts, ie, the MDS and the resident assessment protocol. The resident assessment protocol is a section of the MDS-RAI providing a problem-oriented framework for additional assessment.Citation63 Key items that are problem-specific, trigger assessment for specific conditions. The resident assessment protocol items provide a critical link with care planning. The MDS-RAI 2.0 version has 18 resident assessment protocol items covering the majority of areas addressed by a typical skilled nursing care facility in the care planning process. These items help staff to look for causal or confounding factors that may be reversible. Goals are set to improve deficits where possible, and to maintain and prevent avoidable decline. The current updated MDS is version 3.0 and was introduced after this study was completed.
The MDS has demonstrated good reliability and validity.Citation64–Citation66 MDS items have excellent inter-rater and test-retest reliability in the key areas of cognition and activities of daily living, with an average weighted kappa of 0.80. MDS-RAI items met a standard for superb reliability (ie, intraclass correlation of 0.7 or higher) in key categories of functional status, such as cognition, activities of daily living, continence, and diagnoses.Citation67
The Cognitive Performance Scale (CPS)Citation68,Citation69 was used to assess resident cognitive status. The CPS instrument is a MDS-RAI item scale derived from sections B, C, and G of the resident assessment form. Seven levels of cognitive functioning can be determined, ranging from a score of 0 (intact) to 6 (severely cognitively impaired). The scores are obtained from five MDS items, ie, one communication item (ability to make self understood), three cognitive items (short-term memory if comatose and decision-making), and one item for activities of daily living (eating). The CPS measure correlates highly (r ≥ 0.70) with the frequently used Folstein Mini-Mental Status Examination (MMSE),Citation70 a tool used systematically to assess mental status.Citation71 Validation testing of CPS scoring against the MMSE shows a sensitivity of 0.94 and a specificity of 0.94. MMSE scores range from 0 to 30. A score of 0–9 denotes severe impairment, 10–18 is moderate, 19–24 is mild, and scores >24 indicate that the individual’s cognitive status is intact. The MMSE scores are converted into CPS scores. A CPS score of 5–6 correlates with severe impairment, 3–4 with moderate impairment, 2 with mild impairment, and 0–1 with borderline intact to intact. The CPS scores are converted into average MMSE values, ie, 3 is a mean MMSE of 15.4 (moderate impairment) and a CPS score of 4–5 is a mean MMSE of 5–6 (severe cognitive impairment).Citation72
The Pain Scale devised by Fries et al uses two items from the MDS instrument, ie, item J2a for pain frequency and item J2b for pain intensity. If pain frequency is marked as “no pain”, subsequent pain intensity and pain sites are not scored. The Pain Scale devised by Fries et alCitation73 was validated against a standardized pain instrument, ie, the Visual Analog Scale, and has shown validity in detecting pain in intact to moderately cognitively impaired residents. The Pain Scale was not performed in a validation sample for severely cognitively impaired residents, because these residents were unable to perform the Visual Analog Scale. The limitation of using this tool in significantly cognitively impaired residents was also indicated in the instrument validation study of Fries et al, indicating that the percentage of residents reporting no pain increased with increasing cognitive impairment.Citation73 The potential to use the Pain Scale in addition to other indicators was the impetus for testing a theoretical construct to improve pain detection in residents with severe cognitive impairment, because pain frequency and intensity alone might not fully capture the pain spectrum in those with limited capacity to verbalize pain.
Data collection
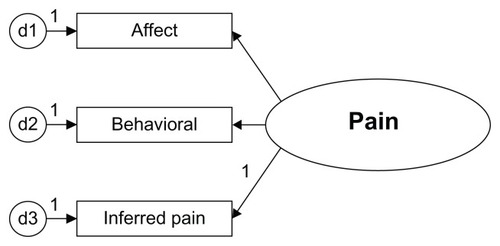
Data from 2001, 2002, and 2003 were collected from annual assessment of deidentified residents in Medicare-certified nursing homes from across the United States (http://www.resdac.umn.edu/MDS/data_available.asp). At the time of analysis, 2001–2003 were the latest data sets available and ready for analysis. A proposed model panel was evaluated for model fit by a series of steps using MDS-RAI data. The goal was to identify the dimensions (indicators) of the measurement instrument, clarify the order of the measurement levels, and examine the integrity of the measurement instruments. The study was conducted to compare statistical models of pain, while grouping residents by cognitive status. The model contains affective, behavioral, and inferred pain traits grouped by cognitive status (see ). The model was compared for utility with the existing pain instrument devised by Fries et al. The Pain Scale is widely used as a secondarily derived tool using MDS data.
Statistical analysis
Descriptive statistics and factor analyses were run with SPSS 14.0 (SPSS Inc, Chicago, IL). Advanced multivariate techniques were used to build a measurement model and to test the model fit with structural equation modeling. A measurement model of pain was hypothesized based on current research and literature of the domains and dimensions of pain in the elderly. Ordinal level correlations were run with Spearman’s rho. A latent model of pain was built with AMOS 6.0 to determine how well 12 indicators from the MDS-RAI represented the latent construct of pain. Equality constraints were applied to compare four cognitive levels of residents, ie, intact, mild, moderate, and severe cognitive impairment. Construct validity was evaluated by the extent to which the measurement of pain accurately represents the construct and assumes a theoretical basis.
A critical step in building the model was hypothesizing associations based on conceptual relationships, not simply based on the data available. Content validity or logical validity was evaluated in the model to determine if indicators represent all dimensions of the construct of pain. The Pain Scale devised by Fries et alCitation73 contains only two indicators, ie, pain frequency (J2a) and pain intensity (J2b) in an ordinal scale. These two indicators yield an underidentified model and cannot run as a stand-alone model in AMOS. These items were highly correlated (r = 0.977, P = 0.01, one-tailed), indicating that one of these items could be dropped, because they closely measure the same aspect of the inferred pain dimension. These core indicators of pain are included in the hypothesized model for testing to define the dimension of inferred pain.
Confirmatory analysis was undertaken to review factor loadings. Confirmatory factor analysis was used to reduce the factors and confirm factor groupings, ie, inferred pain, affect, and behaviors. The measurement model was evaluated for validity and goodness of fit statistics to improve the model to ensure the final prototype is parsimonious. Indicators with a probability of 0.01 were included, and non-significant items were not included in the model. The specification of free and fixed elements represents the initial hypothesis that presumes indirect or direct effects among latent variables.Citation74 The assessment of power in structural equation modeling is complex, because there are substantially more parameters beyond a straightforward procedure like the t-test and analysis of variance, which contain only a few parameters.Citation74 The sample size was considerable (n = 52,996), so power analysis was not critical to determining an appropriate sample size prior to the study to ensure statistical significance of the findings.
Results
The selected MDS items were collected for 52,996 residents. Overall, 80% of the sample was female and the average age was 84 years (see ). Of the medical conditions selected, arthritis was the most prevalent (34.2%), with diabetes affecting around 20.9% (see ). The most common pain site was the joints (14.9%).
Table 2 Demographic table of resident characteristics
Table 3 Diseases/events with potential pain symptoms
contains an index of behaviors, which with additional models could clarify the antecedents and consequences of pain. The Pain Scale items (see ) indicated that 68.8% reported no pain, while only 12.8% experienced pain daily. Pain frequency and intensity declined as cognitive status declined, indicating that only 18.2% of residents with cognitive severe impairment were experiencing pain, while 47.7% of the intact group experienced pain daily or less than daily. This suggests that underdetection of pain was more likely as cognition declined, assuming the same relative comorbidities between cognitively intact and impaired residents.
Table 4 Behavioral index
Table 5 Fries pain scale (PS) ratings
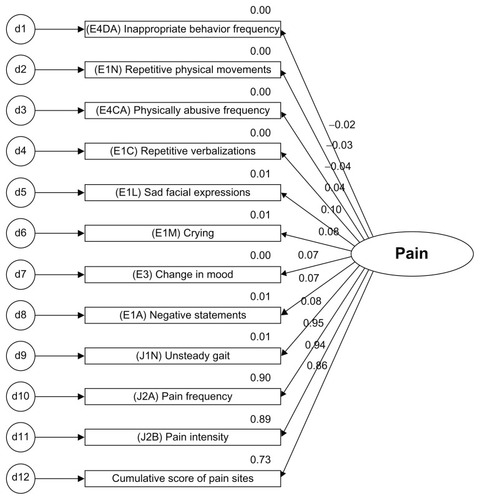
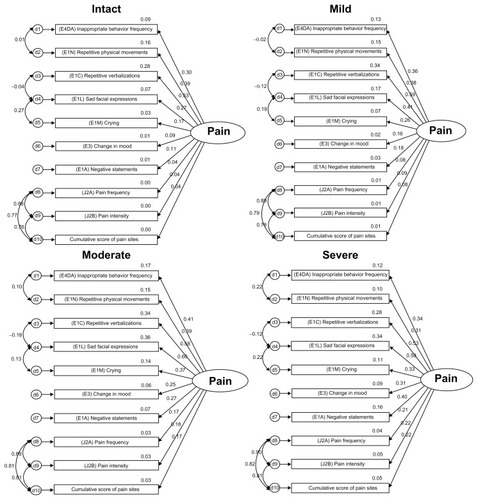
Initial and final models were built from the original pain model using the dimensions of affective, behavioral, and inferred pain grouped by cognitive status. Careful consideration was given to what items to include in the initial model (see , and ) based on current empirical findings of reported pain symptoms and behaviors. All of the indicators in the measurement model were statistically significant (P < 0.01, see ). Correlations are used to test for association but not for causality. The inferences made should have a logical connection with each other. It is important to examine both the degree of the relationship and the P value. Research has a tendency to disregard weak correlations, but a linear relationship may have meaning in terms of current knowledge when examined in the context of other variables.
Table 6 Preliminary model factoring loadings
Table 7 Definitions of the indicators
Table 8 Correlation matrix of the indicators of pain
Cumulative scores of five potential pain-causing diseases (arthritis, hip fracture, cancer, pneumonia, and urinary tract infection) were evaluated as an indicator for pain. While cumulative pain diagnoses were significant at the P < 0.01 level, the correlation was low (r = 0.182). In efforts to build a parsimonious model, the indicators of pain frequency, intensity, and cumulative pain site scores were retained, and potential pain diagnosis scoring were not included in the preliminary model.
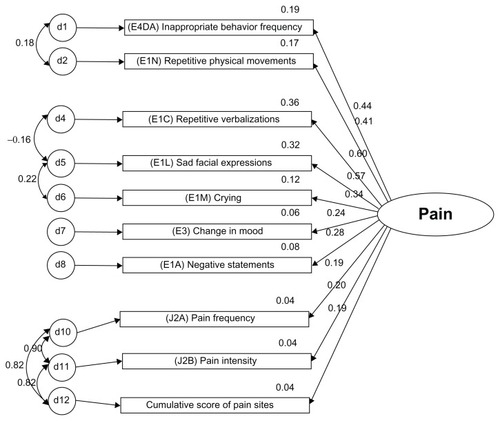
Both models were recursive. The modification indices were examined for correlating measurement errors to reduce the chi-square and degrees of freedom from χ2 = 305889.3, df = 249, P < 0.01 in the original model to χ2 = 4933.4, df = 143, P < 0.01 in the corrected model (, ).
Table 9 Final model factor loadings
The differences between the chi-square (Δχ2) and the degrees of freedom (df) of the two models were compared to assess the model improvement from the initial model with 12 indicators to the final model with ten indicators:
Comparing the original model with the final model shows a large gap, and therefore increases the probability that the changed model is improved. The behavioral item “physically abusive” (E4CA) was dropped due to weak correlations and a non-significant factor loading (P = 0.288). The inferred pain component, unsteady gait (J1N), was also dropped due to weak correlations and to improve the model parsimony for the inferred dimension of pain. The final revised model allows measurement errors to be correlated with each other and better capture shared measurement errors for more correlated items. Chi-square values of the model were expected to be large, because of the sample size. Model fit statistics are found in (see for definitions of goodness of fit statistics).
Table 10 Goodness of fit statistics for the measurement models
Table 11 Goodness of fit statistical terms
The model fit was greatly improved from the initial to the final model. Reduced root mean square residuals were achieved and the goodness of fit further approached 1.0 with the adjustments made. The Tucker-Lewis index values should be between 0 and 1, and the adjusted model indicates a value of 0.965. Values close to 1.0 indicate a very good fit. Scores for root mean square error of approximation are ideally below 0.05, and the changes made reduced this value to 0.025.
In comparison, the model fit by cognitive status with a side-by-side evaluation (), notable variations in correlations occur within inferred pain domains, especially comparing intact/mild with moderate/severe cognitive states. The intact/mild groups and the moderate/severe groups showed similar values for associations and correlated errors for inferred pain items (ie, J2a pain frequency, J2b pain intensity, and cumulative score of pain sites). This information is helpful in understanding the relationship between resident cognition and how further dimensions (eg, behavioral, affective, and cognitive) add further detail to clarifying the pain construct. The overall model fit indicates utility across all cognitive levels (). Pain scores could be converted to a standardized score, including all of the indicators to a converted t-score, the factorial scores could be retained using a weighted score, or pain indicators could simply be added for a cumulative score.
Discussion
The findings of this study support the pragmatic utility of additional measures to detect pain in the elderly beyond self-reports of pain intensity and frequency. Research working towards further defining dimensions of pain in the elderly increases our ability to understand and assess pain characteristics in this population. Findings of primary concern substantiate research to dateCitation75 on pain in those residents with severe cognitive impairment, along with the contribution of behavioral indicators to identification of pain beyond self-report measures.
The Pain Scale items () indicated that the majority of the sample (68.8%) were not experiencing pain. When this total was broken down by cognitive status, as the cognitive state declined, pain frequency and intensity also appeared to decline. Forty-eight percent of the cognitively intact group was reported as experiencing pain, while only 18.2% of those with severe cognitive impairment were assessed as having pain. These findings support other research to date indicating that pain is potentially under-reported in this population.Citation4,Citation22,Citation75–Citation79
Prior models of pain have included cognitive, affective, and behavioral components.Citation20,Citation33,Citation80–Citation82 The latent construct of pain could include these three dimensions as a discrete measure in a model. Because this study was used as a stacked comparison, cognitive items were used as the grouping variable and not as a separate measure in the pain model. The goal was to gain an understanding of the overall fit of the model by cognitive state. Future studies could examine this construct using cognition, affect, and behavior as separate measures.
Self-reported measures of pain could be further validated by more objective assessment. From a theoretical perspective, evaluation of the proposed models and indicators is not exhaustive of all the potential cues within the dimensions of cognition, affect, behavioral, and inferred pain indicators that could explain the construct of pain. The research was limited to the available items from the MDS. Important in the use of large data sets is having a clear clinical and evidential base to substantiate why certain indicators are used and not others.Citation83 Hypothesized indicators chosen from the MDS were based on knowledge and research conducted to this point. Theoretical modeling can start a dialog concerning other indicators which are potentially useful and shown from previous smaller-scale studies to indicate pain beyond self-reports from the resident, such as the use of nonverbal pain scales. Correlations between indicators can clarify the degree of association between the dimensions and unique relationships between behaviors. As our understanding of pain increases, clinicians will be better equipped to measure quality initiatives in the assessment, treatment, and prevention of pain.
Focusing interventions only on severely cognitively impaired residents, ie, those at high risk for untreated pain, fails to take into account factors at the population level, and would limit options to reduce the burden of chronic pain for all residents in longterm care.Citation84 A need exists for continued quality improvement and additional research to increase our understanding of pain behaviors and the effect of treatments on the elderly. The goal is improving pain control at all cognitive levels.Citation22 Using existing data, we can target specific behaviors and evaluate outcomes to determine if uniformity of care is being applied across long-term care settings. In addition, when constructing federally required assessments, it is important to assess what standards are being applied in the use of key items as quality measures.
This study adds insight into additional domains/dimensions that can be used to improve pain assessment, and to re-evaluate efforts to detect pain and improve pain outcomes. Further evaluation of concomitance between pain and cognitive status longitudinally would provide an additional perspective on the long-term relationship between these two constructs. Future directions for research should include the persistence of pain behaviors. The MDS 2.0 contains alterability of selected behavioral items in section E4. Persistence of behaviors could indicate progression of the disease process, effectiveness of interventions to change behaviors, or an unknown factor in behavioral response to multiple stimuli.
Limitations of this study concern the data distribution. The data were positively skewed. Normality and equal group distribution were not assumed. Mahanalobis distance was not used to eliminate outliers, because the majority (70%) of the population was initially reported as not experiencing pain and was not evenly distributed. Removing these cases would have removed a full spectrum of pain presentation of atypical symptoms of pain, ie, the target of the study. Previously published studies question the reliability of mood and behavioral sections from rater to rater when using the MDS.Citation67,Citation85 Further, the majority of residents needing skilled nursing care have some level of cognitive impairment, so intact groups were not proportionate to the mild, moderate, and severe cognitively impaired groups. This seminal work will build towards supporting change in current clinical practice, but additional clinical studies are needed to evaluate the assessment items as a clinical intervention from a translational research perspective.
Conclusion
A comprehensive plan for pain management should evaluate staffing patterns, staff education, and examine differences in pain policies and procedures to use pain management ultimately as a primary quality indicator in long-term care settings.Citation86 Modeling theoretical constructs can serve as a valuable tool to determine the fit between clinical knowledge, the health care context, and individual needs. Additional research examining a covariance model of the relationship between pain and cognitive status over the long term could reveal if concomitant relationships exist. Evaluating covariance models, including antecedents and consequences of longterm suffering from unresolved pain, would further support the significance of understanding indicators and accurately assessing, documenting, and treating pain.
Acknowledgments
This research could not have been possible without the assistance of Seung Chun Paek, Research Associate of the Korean Health Insurance Corporation, Seoul, Korea, who ran the dataset parameters and queries. Thanks are extended to Steven Talbert and Diane Andrews, at the University of Central Florida, College of Nursing, for their reviews and edits of this manuscript. This research was completed at the University of Central Florida, College of Nursing in partnership with the College of Health and Public Affairs, Orlando, FL, and funded by the University of Central Florida Provost Fellowship.
Disclosure
The authors report no conflicts of interest in this work.
References
- EppsCDRecognizing pain in the institutionalized elder with dementiaGeriatr Nurs200122717911326213
- American Health Care AssociationOSCAR data reports: Patient characteristics Available at: http://www.ahca.org/research/oscar_patient.htmAccessed April 15, 2006
- LoweSCensus Bureau releases new data on residents of adult correctional facilities, nursing homes and other group quarters annual data also pain diverse portrait of nation’s race, ethnic and ancestry group Available at: http://www.census.gov/Press-Release/www/releases/archives/american_community_survey_acs/010709.htmlAccessed November 27, 2007
- SawyerPLillisJPBodnerEVAllmanRSubstantial daily pain among nursing home residentsJ Am Med Dir Assoc2007815816517349944
- FrondiniCLandfranchiaGMinardiMCucinottaDAffective, behavior and cognitive disorders in the elderly with chronic musculoskeletal pain: the impact on an aging populationArch Gerontol Geriatr20074416717117317450
- HorgasALElliotAFPain assessment and management in persons with dementiaNurs Clin North Am20043959360615331304
- HuffmanJCKunikMEAssessment and understanding of pain in patients with dementiaGerontologist20004057458111037936
- BalfourJEO’RourkeNOlder adults with Alzheimer disease, co-morbid arthritis and prescription of psychotropic medicationsPain Res Manag200319819820414679414
- TurkDCSwansonKSTunksERPsychological approaches in the treatment of chronic pain patients – when pills, scalpels, and needles are not enoughCan J Psychiatry20085321322318478824
- DaviesEMaleMReimerVTurnerMPain assessment and cognitive impairment: part 2Nurs Stand200419334015624384
- DaviesEMaleMReimerVTurnerMWylieKPain assessment and cognitive impairment: part 1Nurs Stand200419394215620035
- BenedettiFArduinoCVighettiSAsteggianoGTarenziLRaineroIPain reactivity in Alzheimer patients with different degrees of cognitive impairment and brain electrical activity deteriorationPain2004111222915327805
- BachinoCSnowALKunikMECodyMWristersKPrinciples of pain assessment and treatment in non-communicative demented patientsClin Gerontol2001297115
- BrignellAAssessment of pain in non-cognizant elderlyCanadian Nursing Home Feb-Mar20031417174
- CadoganMPAssessing pain in cognitively impaired nursing home residents: the state of the science and the state we’re inJ Am Med Dir Assoc20034505112807599
- CavalieriTAPain management in the elderlyJ Am Osteopath Assoc200210248148512361180
- MillerLLTalericoKAPain in older adultsAnnu Rev Nurs Res200220638812092519
- WeinerDKRudyTEAttitudinal barriers to effective treatment of persistent pain in nursing home residentsJ Am Geriatr Soc2002502035204012473018
- KovachCRLoganBRNoonanPEEffects of the serial trial intervention on discomfort and behavior of nursing home residents with dementiaAm J Alzheimers Dis Other Demen20062114715516869334
- HorgasALNicholsALSchapsonCAVietesKAssessing pain in persons with dementia: Relationships among the non-communicative patient’s pain assessment instrument, self-report, and behavioral observationsPain Manag Nurs20078778517544127
- Cohen-MansfieldJPain assessment in noncommunicative elderly persons – PAINEClin J Pain20062256957516788345
- HuseboBSStrandLIMoe-NilssenRHuseboSBAarslandDLjunggrenAEWho suffers most? Dementia and pain in nursing home patient’s: a cross-sectional studyJ Am Med Dir Assoc2008942743318585645
- KrulewitchHLondonMRSkakelVJLundstedtGJThomasonHBrummel-SmithKAssessment of pain in cognitively impaired older adults: a comparison of pain assessment tools and their use by nonprofessional caregiversJ Am Geriatr Soc2000481607161111129750
- RyanJStoneRIRaynorCRUsing large data sets in long-term care to measure and improve qualityNurs Outlook200452384415014378
- ArlingGKaneRLLewisTMuellerCFuture development of nursing home quality indicatorsGerontologist20054514715615799979
- ReichardJSerious deficiencies found at almost one in every five nursing homesWashington Health Policy Available at: http://www.commonwealthfund.org/healthpolicyweek/healthpolicyweek_show.htm?doc_id=709233&#doc709237Accessed on October 6, 2008
- JCAHO Joint CommissionPain: Current understanding of assessment, management, and treatments Available at: http://www.jcaho.org/news+room/health+care+issues/pain_mono_npc.pdfAccessed January 16, 2006
- AcelloBPain assessment in patients with dementia and how to use pain scalesHome Healthc Nurse20011947347611982182
- American Health Quality AssociationImproving quality of care in nursing homesFact sheet: Q10 success stories Available at: http://www.ahqa.org/pub/media/159_766_3906.cfmAccessed January 20, 2006
- LeongIYFarrellMJHelmeRDGibsonSJThe relationship between medical comorbidity and self-rated pain, mood disturbance, and function in older people with chronic painJ Gerontol B Biol Sci Med Sci200762A550555
- Cohen-MansfieldJCreedonMNursing staff member’s perceptions of pain indicators in persons with severe dementiaClin J Pain200218647311803305
- WarrenJBoltonPData mining for on-line support of general practiceTop Health Inf Manage200122516411680277
- HelmeRDGibsonSJThe epidemiology of pain in elderly peopleClin Geriatr Med20011741743111459713
- FordyceWEPain and suffering: a reappraisalAm Psychol1988432762832968063
- FordyceWEA behavioral perspective on chronic painBr J Clin Psychol1982213133206216937
- FordyceWEPain measurement and pain behaviorPain19841853696709379
- TurkDCWackJTKernsRDAn empirical examination of the “pain-behavior” constructJ Behav Med19858921191304032472
- BuffumMDHuttEChangVTCraineMHSnowALCognitive impairment and pain management: Review of issues and challengesJ Rehabil Res Dev20074431532917551882
- AlgaseDLBeckCKolanowskiANeed-driven dementia-compromised behavior: An alternative view of disruptive behaviorAm J Alzheimers Dis Other Demen1996111219
- RatnerCA social constructionist critique of naturalistic theories of emotionJournal of Mind and Behavior198910211230 Available at: http://www.humboldt1.com/~cr2/emotions.htmAccessed November 13, 2008
- FordyceWEEvaluating and managing chronic painGeriatrics1978335962620926
- GurejeOKorffMVKolaLThe relation between multiple pains and mental disorders: results from the World Mental Health surveysPain2008135829117570586
- ShegaJWRudyTKeefeFJPerriLCMenginOTWeinerDKValidity of pain behaviors in persons with mild to moderate cognitive impairmentJ Am Geriatr Soc2008561631163718662203
- Lints-MartindaleACHadjistavropoulosTBarberBGibsonSJA psychophysical investigation of the facial action coding system as an index of pain variability among older adults with and without Alzheimer’s DiseasePain Med2007867868918028046
- KunzMScharmannSHemmeterUSchepelmannKLautenbacherSThe facial expression of pain in patients with dementiaPain200813322122817949906
- ThibaultPLoiselPDurandM-JCatchloveRSullivanMJLPsychological predictors of pain expression and activity intolerance in chronic pain patientsPain2008139475418430518
- HorgasALAssessing pain in persons with dementiaMedsurg Nurs20071620720817849932
- KaasalainenSCokerEDolovichLPain management decisions making among long-term care physicians and nursesWest J Nurs Res20072956158017548894
- HurleyACVolicerBJHanrahanPAHoudeSVolicerLAssessment of discomfort in advanced Alzheimer patientsRes Nurs Health1992153693771529121
- OhHEomMKwonYA study on aggressive behavior among nursing home residents with cognitive impairmentTaehan Kanho Hakhoe Chi20043415411459 Korean
- KolanowskiAMGarrMRelation of premorbid factors to aggressive physical behavior in dementiaJ Neurosci Nurs19993127828410633304
- KamaleriYNatvigBIhlebaekCMBenthJSBruusgaardDChange in the number of musculoskeletal pain sites: a 14-year prospective studyPain2009141253018977088
- PautexSMichonAGuediraMPain in severe dementia: self-assessment or observational scales?J Am Geriatr Soc2006541040104516866673
- FisherSEBurgioLDThornBEHardinJMObtaining self-report data from cognitively impaired elders: methodological issues and clinical implications for nursing home pain assessmentGerontologist200646818816452287
- MurdochJLarsenDAssessing pain in cognitively impaired older adultsNurs Stand200418333915199708
- KovachCRNoonanPESchlidtAMWellsTA model of consequences of need-driven, dementia-compromised behaviorJ Nurs Scholarsh20053713414015960057
- PenrodJYuFKolanowskiAFickDMLoebSJHupceyJEReframing person-centered nursing care for persons with dementiaRes Theory Nurs Pract200721577217378465
- CollingKBPassive behaviors in dementia: clinical application of the need-driven dementia-compromised behavior modelJ Gerontol Nurs199925273210776141
- MittyEFloresSAssisted living nursing practice: the language of dementia: theories and interventionsGeriatr Nurs20072828328817923285
- KolanowskiALitakerMBuettnerLEfficacy of theory-based activities for behavioral symptoms of dementiaNurs Res20055421922816027564
- CollingKBBuettnerLSimple pleasures: interventions from the need-driven dementia-compromised behavior modelJ Gerontol Nurs2002281019
- EllisJ[Commentary] A model of consequences of need-driven, dementia-compromised behaviorJ Nurs Scholarsh2005372140
- Centers for Medicare and Medicaid ServicesMDS 2.0 for Nursing HomesCMS Available at: http://www.cms.hhs.gov/NursingHomeQualityInits/20_NHQIMDS20.aspAccessed June 23, 2008
- WonAMorrisJNNonemakerSLipsitzLAA foundation for excellence in long-term care: the minimum data setAnn Longterm Care199979297
- SnowdenMMcCormickWRussoJValidity and responsiveness of the minimum data setJ Am Geriatr Soc199935172178
- LawtonMPCastenRParmeleePAVan HaitsmaKCornJKlebanMHPsychometric characteristics of the Minimum Data Set II: ValidityJ Am Geriatr Soc1998467367449625190
- HawesCMorrisJNPhillipsCDMorVFriesBENonemakerSReliability estimates for the minimum data set for nursing home resident assessment and care screening (MDS)Gerontologist1995351721787750773
- MorrisJNFriesBEMehrDRMDS Cognitive Performance ScaleJ Gerontol199449M1741828014392
- FriesBENew opportunities for research in cognitive aging: the National Nursing Home Resident Assessment InstrumentDivision 20 American Psychological AssociationUniversity of Michigan Available at: http://apadiv20.phhp.ufl.edu/fries.htmAccessed June 12, 2008
- Gruber-BaldiniALZimmermanSIMortimoreEMagazinerJThe validity of the minimum data set in measuring the cognitive impairment of persons admitted to nursing homesJ Am Geriatr Soc2000481601160611129749
- FolsteinMFFolsteinSEMcHughPRMini-mental state: a practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res1975121891981202204
- CarpenterGIHastieCLMorrisJNFriesBEAnkriJMeasuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairmentBMC Geriatr Available at: http://www.biomedcentral.com/1471-2318/6/7Accessed October 18, 2006
- FriesBESimonSEMorrisJNFlodstromCBooksteinFLPain in U.S. nursing homes: Validating a pain scale for the Minimum Data SetGerontologist20014117317911327482
- KaplanDStatistical power in structural equation modelsDepartment of Educational Studies, University of Delaware Available at: http://www2.gsu.edu/~mkteer/power.htmlAccessed October 21, 2008
- CipherDCliffordPARoperKDBehavioral manifestations of pain in the demented elderlyJ Am Med Dir Assoc2006735536516843236
- ZanocchiMMaeroBNicolaEChronic pain in a sample of nursing home residents: prevalence, characteristics, influence on quality of life (QOL)Arch Gerontol Geriatr20084712112818006088
- ReynoldsKSHansonLCDeVellisRFHendersonMSteinhauserKEDisparities in pain management between cognitively intact and cognitively impaired nursing home residentsJ Pain Symptom Manage20083538839618280101
- LeongIYNuoTHPrevalence of pain in nursing home residents with different cognitive and communicative abilitiesClin J Pain20072311912717237660
- NygaardHAJarlandMAre nursing home patients with dementia diagnosis at increased risk for inadequate pain treatment?Int J Geriatr Psychiatry20052073073716035124
- SnowALO’MalleyKJCodyMA conceptual model of pain assessment for noncommunicative persons with dementiaGerontologist20044480781715611217
- RuzickaSSanchez-ReilySMeghanGHolistic assessment of chronic pain among eldersAm J Hosp Palliat Med200724291299
- IezziTDuckworthMPVuongLNArchibaldYMKlinckAPredictors of neurocognitive performance in chronic pain patientsInt J Behav Med200411566115194520
- MageeTLeeSMGiulianoKKMunroBGenerating new knowledge from existing data: The use of large data sets for nursing residentsNurs Res200655S505616601635
- BlythFMChronic pain – is it a public health problem?Pain200813746546618485599
- CastenRLawtonMPParmeleePAKlebanMHPsychometric characteristics of the minimum data set I: confirmatory factor analysisJ Am Geriatr Soc1998467267359625189
- KeeneyCEScharfenbergerJAO’BrienJGLooneySPfeiferMPHermannCPInitiating and sustaining a standardized pain management program in long-term care facilitiesJ Am Med Dir Assoc2008934735318519117