Abstract
Obesity is a global epidemic associated with aging-like cellular processes; in both aging and obesity, resistance to hormones such as insulin and leptin can be observed. Leptin is a circulating hormone/cytokine with central and peripheral effects that is released mainly by subcutaneous white adipose tissue. Centrally, leptin controls food intake, energy expenditure, and fat distribution, whereas it controls (among several others) insulin sensitivity, free fatty acids (FFAs) oxidation, and lipolysis in the periphery. Aging is associated with important changes in both the distribution and the composition of adipose tissue. Fat is redistributed from the subcutaneous to the visceral depot and increased inflammation participates in adipocyte dysfunction. This redistribution of adipose tissue in favor of visceral fat influences negatively both longevity and healthy aging as shown in numerous animal models. These modifications observed during aging are also associated with leptin resistance. This resistance blunts normal central and peripheral functions of leptin, which leads to a decrease in neuroendocrine function and insulin sensitivity, an imbalance in energy regulation, and disturbances in lipid metabolism. Here, we review how age-related leptin resistance triggers metabolic disturbances and affects the longevity of obese patients. Furthermore, we discuss the potential impacts of leptin resistance on the decline of brown adipose tissue thermogenesis observed in elderly individuals.
Introduction
Obesity is a worldwide socioeconomic concern. In 2013, the World Health Organization estimated that more than 1.4 billion adults are overweight and that more than 500 million are obese.Citation1 This poses major economic and public health issues as some of the leading causes of mortality (ie, cardiovascular diseases and cancer) are strongly associated with obesity.Citation2 Obesity also leads to a reduced lifespan and accelerates cellular processes similar to aging such as oxidative stress and disturbance in homeostatic pathways.Citation3–Citation5 One important feature of obesity and aging in relation to morbidity and mortality is the dysregulation of both white adipose tissue (WAT) and brown adipose tissue (BAT).Citation6,Citation7 Adipose tissue has the capacity to secrete a large number of bioactive substances named adipokines.Citation8 These adipokines, such as leptin, adiponectin, tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6) have paracrine and/or endocrine functions and their dysregulation is a common ground for the development of insulin resistance, hypertension, and dyslipidemia.Citation9 Another important common feature of obesity and aging is the development of resistance to certain hormones such as insulin and leptin, which triggers metabolic dysregulations such as type 2 diabetes and failure to regulate food intake as well as fat distribution.Citation10 Since obese patients will make up an important proportion of the elderly population in the near future, it is important to determine the causes and effects of leptin resistance in age-related diseases.
Physiological role of leptin
Leptin, from the Greek “leptos” (thin), is a 16-kDa circulating protein with hormone/cytokine activities released by WAT, mainly subcutaneous fat.Citation11 However, other tissues such as ovaries, skeletal muscles, stomach, and BAT also secrete leptin.Citation12 There is a clear sexual dimorphism; women have higher circulating levels of leptin than men.Citation13 Although some studies have postulated that these increased circulating levels of leptin in women were associated with their higher percentage of body fat when compared to men, others have demonstrated that higher leptin levels in women were independent of fat mass.Citation14 These sex differences may be associated with a stimulating role of estrogen or a suppressing role of androgens on leptin production.Citation15,Citation16 Leptin circulates in the plasma as a free adipokine or bound to leptin-binding proteins, mainly its soluble receptor. In lean individuals, the great majority of leptin circulates in the bound form whereas it circulates in the free form in obese individuals.Citation17,Citation18 Physiologically, leptin is involved in regulating energy balance through central actions, which is of importance particularly in a context of fat accumulation and metabolic disorders. Leptin also has many peripheral actions, mainly on the circulatory and respiratory systems, glucose homeostasis, and reproduction ().Citation12
Table 1 Summary of the main central and peripheral actions of leptin
Leptin is a key regulator of energy balanceCitation19,Citation20 as it acts in the brain to decrease food intake and increase energy expenditure ().Citation21–Citation23 The actions of leptin in neurons are mediated by the long isoform of the leptin receptor (LEPR-B), a splicing variant member of the class I cytokine receptor family.Citation24 This variant is characterized by a long cytoplasmic regionCitation25 allowing the activation of the Jak-Stat signal transduction pathway, which is crucial for leptin action.Citation24 LEPR-B is predominantly located in hypothalamic nuclei known to be involved in the regulation of energy balance, including the arcuate nucleus (ARC).Citation26–Citation29 LEPR-B is expressed in at least two distinct neuronal populations of the ARC. One population cosynthesizes the orexigenic (appetite-stimulating) neuropeptide Y (NPY) and agouti-related peptide (AgRP), and the other synthesizes pro-opiomelanocortin (POMC).Citation30–Citation32 In addition to being an orexigenic peptide, NPY suppresses the central leptin-mediated growth and reproductive axes.Citation33 In turn, POMC is processed to produce α-melanocyte-stimulating hormone (αMSH), which leads to hypophagia by primarily activating the melanocortin-4 receptor (MC4R).Citation34,Citation35 AgRP is an antagonist of αMSH/MC4R signaling as well as an inverse agonist of MC4R activity itself.Citation36 NPY/AgRP neurons also release γ-aminobutyric acid (GABA) and thus, can also negatively modulate POMC by a direct GABAergic synaptic mechanism.Citation37 Leptin modulates energy balance by promoting several neuronal responses involving inhibition of the expression and secretion of NPYCitation28,Citation38 and stimulation of POMC synthesis.Citation39,Citation40 Consequently, microinjection of leptin into the ARC inhibits food intake.Citation41 The involvement of leptin in the control of energy balance goes beyond food intake since leptin is also involved in energy expenditure by promoting BAT thermogenesis.Citation21 A recent study showed a critical role for LEPR-B in the ARC in mediating the sympathetic nervous system (SNS) response to leptin.Citation42 The role of leptin-induced activation of SNS-mediated BAT thermogenesis will be discussed in a later section.
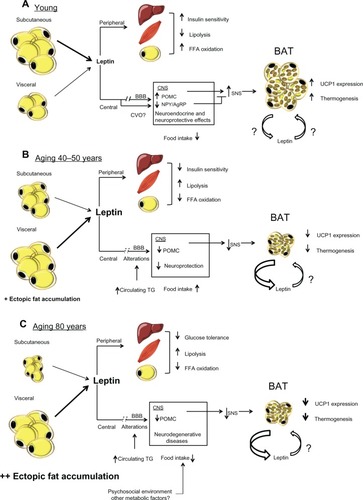
Figure 1 (A) Effects of leptin production in a young state. White adipocytes, mostly subcutaneous, secrete normal levels of leptin. Peripherally, leptin contributes to insulin sensitivity and free fatty acids oxidation in liver, muscle, and adipose tissue. Centrally, leptin reach its targets through its transport across the blood brain barrier provided by an active saturable transport system. Leptin is also transported through the circumventricular organs. Its binding to LEPRs expressed in the arcuate nucleus of the hypothalamus leads to an increase of POMC and a decrease of NPY/AgRP levels. This modulation of specific neuronal populations triggers SNS activation, which leads to an increase of UCP1 transcription and thermogenesis in BAT. (B) Effects of leptin production in middle-age condition. Subcutaneous fat begins to be redistributed and white adipocytes, mostly visceral, produce a high amount of leptin. Peripherally, leptin resistance develops in the liver, muscle, and adipose tissue and causes a decrease in insulin sensitivity and FFAs oxidation and an increase in lipolysis. Centrally, alterations in the blood brain barrier decrease leptin transport to the CNS, which leads to a reduction in the production of POMC. This diminution blunts SNS signaling and induces BAT atrophy and leads to a decrease in both UCP1 levels and thermogenesis. This BAT atrophy also contributes to an increase in leptin secretion. (C) Effects of old age on leptin secretion. The subcutaneous depot is atrophied and fat accumulates viscerally and mostly in ectopic depots. High levels of leptin are secreted by visceral adipose tissue, concomitantly with an increase in glucose intolerance peripherally probably attributed to a loss of leptin signaling. Centrally, levels of POMC are still decreased, which leads to a more important atrophy of BAT and quiescent thermogenesis. Since BAT is inactive, levels of secreted leptin by this tissue are increased.
Until recently and as described above, strong evidence suggested that the actions of leptin on energy balance were only due to its binding to LEPRs on AgRP/NPY and POMC neurons. However, since mice lacking LEPRs on either or both POMC and AgRP neurons develop very mild obesity,Citation43,Citation44 there are likely other leptin-responding neurons that contribute importantly to leptin’s effects on energy balance. It has been shown that the effects of leptin on food intake and energy expenditure are also mediated by GABAergic neurons, raising the possibility that modulation of GABAergic output is a key aspect of leptin actions.Citation45 Moreover, leptin action on presynaptic GABAergic neurons decreases the inhibitory tone to postsynaptic POMC neurons, explaining in part the orexigenic and thermogenic POMC-dependent effects of leptin.Citation45 LEPRs-expressing GABAergic neurons are located in the ARC, where a small fraction are AgRP neurons, as well as in other hypothalamic nuclei known to be involved in energy balance regulation. However, although the investigation of these new first-order leptin-responsive neurons undoubtedly represents research perspectives, current knowledge mainly revolves around ARC NPY/AgRP and POMC neurons, which will be the focus of the present review. In addition to its effect on energy balance, central leptin is also involved in regulating peripheral lipid and glucose metabolism.Citation46 Indeed, it has been shown that intracerebroventricular administration of leptin significantly increases insulin sensitivity, glucose utilization, and glucose uptake in peripheral tissue including BAT. Citation47 Leptin also has neuroendocrine/neuroprotective functions; LEPRs are highly expressed in brain areas involved in learning and memory and leptin levels are associated with lower risk of dementia.Citation48,Citation49
Initial knowledge with regards to leptin effects on energy balance derived from cross-circulation experiments called parabiosis in ob/ob and db/db mice, which showed that the ob gene was responsible for the generation of a circulating factor that regulates energy balance, and that the db gene encodes the receptor for this factor.Citation50 These mice weight up to three times more than normal mice and show remarkably high levels of body fat, an obese phenotype now known to be attributed to a deficiency in leptin production or its receptor respectively.Citation50,Citation51 Although human leptin gene mutations are relatively rare, some cases result in morbid obesity.Citation52 Administration of leptin either peripherally or centrally to ob/ob mice greatly reduces food intake and body weight.Citation53,Citation54 Moreover, a morbidly obese child who was found to have a mutation in the leptin gene was successfully treated with recombinant human leptin.Citation55 Therefore, in a context in which leptin is circulating at physiological levels, leptin-sensitive patients should have a normal body weight. Consequently, leptin acts as a feedback signal from body energy stores to the brain; circulating levels diminish during starvation, when fat depots are depleted to support the energy need of the organism, and increase during refeeding, when fat depots are replenished.Citation56 However, although absence of leptin leads to obesity, an excess of this hormone does not lead to a phenotype of leanness as theoretically expected. Indeed, leptin levels are found to be increased in obese humans and in several genetic and environmentally induced forms of rodent obesity,Citation57,Citation58 a state now commonly called leptin resistance, similar to the insulin resistance found in type 2 diabetes.
LEPR is also expressed in hemopoietic cells and studies showed that leptin could be linked to the proliferation and differentiation of hemopoietic precursors such as granulocyte-macrophage.Citation59 These effects of leptin have also been suggested to synergize with those of stem-cell factor (SCF) at least in primitive and progenitor cells.Citation59 Other reports propose a role for leptin in platelet aggregation, angiogenesis, and wound repair.Citation60–Citation62 Moreover, SNS activation by leptin goes beyond BAT thermogenesis, since leptin administration can increase heart rate and blood pressure in a dose-dependent manner.Citation63,Citation64 Elevated plasma leptin levels increase blood pressure in mice and plasma leptin concentration positively correlates with the sympathetic renal activation in humans, thus contributing to the development of hypertension.Citation65
Leptin is also implicated in respiratory control. The combination of leptin deficiency and profound weight gain in adult ob/ob mice can produce marked changes in the mechanics of respiration. In ob/ob mice, total lung capacity and lung compliance are 50% less than those of their wild-type counterparts.Citation66 Ob/ob mice develop a rapid breathing pattern when compared to age-matched wild-type mice and exhibit a depressed hypercapnic ventilatory response (HCVR).Citation67 These effects of leptin on ventilation were shown to be independent of obesity.Citation67 In humans, clinical studies provided some evidence supporting these animal data. For example, leptin is a predictor of lung function in various conditions, including asthma and heart failure and correlates negatively with lung volume in chronic obstructive pulmonary disease patients.Citation68 However, the pathophysiological implications of leptin in the respiratory function in humans remain to be clarified. In rodent skeletal muscle, both leptin mRNA and leptin receptor have been identified. Leptin triggers skeletal muscle FFAs oxidation by selectively activating 5′-AMP-activated protein kinase and attenuates insulin-mediated lipogenic effects by 50%.Citation69,Citation70 Additional studies are needed to specify the role of leptin on immune cells development and angiogenesis considering that these two processes are implicated in the metabolic dysregulations associated with obesity. Importantly, the effects of the high circulating levels of leptin found in leptin resistant states on immune cells differentiation and the implications of this process on low-grade inflammation should be determined.
In the liver, leptin inhibits both insulin binding and glucagon-activated cAMP production.Citation71–Citation73 Additionally, leptin was shown to have a profibrotic effect in hepatic stellate cells.Citation74 This profibrotic effect was also described in a mouse model of hepatic steatosis where leptin was associated with an increase of CD-14+ Kupffer cell content.Citation75 However, in leptin-deficient lipodystrophic patients suffering from nonalcoholic fatty liver disease (NAFLD), administration of leptin improved their condition dramaticallyCitation76 Taken together, these data suggest that the positive effects of leptin on triglycerides oxidation in the liver may overcome the effects of stellate and immune cells activation in the context of NAFLD. Since ectopic lipid storage is an important feature of aging, further studies are needed to better describe the effects of leptin on NAFLD. One important basic issue will be to determine whether mouse models of NAFLD are representative of an ectopic lipid storage disease and if findings can be translated to humans.
In WAT, leptin inhibits insulin binding and insulin-mediated effects on glucose transport, glycogen synthase activity, and lipogenesis.Citation77,Citation78 These effects can be observed when treating isolated rat adipocytes with recombinant leptin as it affects insulin sensitivity and alters important metabolic effects of insulin.Citation79 Leptin can stimulate lipolysis as well; these effects have been demonstrated both in vitro and in vivo in mouse models.Citation80,Citation81 In WAT, leptin also functions as a proinflammatory adipokine by acting as a chemoattractant for macrophagesCitation82 and by inducing the production of proinflammatory mediators from macrophages and T lymphocytes such as TNFα and IL-6.Citation83 TNFα interferes with preadipocyte differentiation and causes lipolysis, decreases size, and reduces insulin responsiveness in adipocytes.Citation84 Considering the similarity between some of their responses, differentiating between central and peripheral effect of leptin on adipose tissue can be difficult. Consistently, this subject is still a matter of debate. Some studies showed that leptin’s actions on lipolysis and insulin sensitivity in WAT are not mediated by the central nervous system since leptin can act on denervated fat.Citation85 Others have determined that leptin also regulates WAT via the central nervous system considering that intracerebroventricular leptin infusion leads to lipolysis in WAT, as shown by a decrease of stearoyl-coenzyme A-desaturase-1 expression and an increase in hormonesensitive lipase expression.Citation86,Citation87 At the whole-body level, leptin has a strong, positive influence on glycemia; this subject was recently thoroughly reviewed elsewhere.Citation88 Interestingly, leptin actions on lowering glycemia were suggested to be partly mediated via insulin-like growth factor (IGF)-binding protein 2, an established modulator of the IGF-1 pathway. Leptin also exerts peripheral effects on BAT since peripheral, but not central, administration of leptin in rats increases the insulin-stimulated utilization of glucose in BAT.Citation89
Finally leptin plays an important role in reproduction.Citation90 LEPR is expressed in both ovary and prostate and leptin can inhibit insulin-induced estradiol production by granulose cells from bovine follicles.Citation91 Moreover, a significant increase of circulating leptin levels is observed during ovarian hyperstimulation, which suggests that leptin plays a role in follicular growth and maturation.Citation92 These observations can also be translated to animal models since infertility is a phenotype observed in both male and female leptin-deficient ob/ob mice. In this model, circulating reproductive hormones are decreased in females, but it is possible to restore fertility with repeated injections of human leptin.Citation93,Citation94 Further studies will be necessary to determine the exact role of leptin in male fertility and the role of androgens in the control of leptin expression.
Aging and inflammation in adipose tissue
Aging is associated with an important redistribution of fat among the different depots. Body weight usually increases until middle age (30–50 years old) and declines thereafter.Citation95 In contrast, total fat mass peaks in early and middle old age (40–70 years old), which results in an increased percentage of body fat ().Citation96 This increased percentage of body fat is the consequence of both the increase in fat mass and the loss of fat-free mass encountered in the elderly, mainly in skeletal muscle and bone. Aging-associated redistribution of adipose tissue from the subcutaneous depot to the visceral compartment and ectopic sites namely muscle, liver, and bone marrow has been shown to happen in subjects over 85 years old.Citation97 This redistribution may potentially contribute to age-related dysfunction of these tissuesCitation98,Citation99 but also has strong metabolic implications, since visceral adiposity and intramuscular lipid accumulation are markers of impaired glucose metabolism independently of adiposity.Citation100 Aging is also associated with loss of BAT activity and brown preadipocyte dysfunction in rodents and humans.Citation101–Citation103 This process may contribute to energy imbalance since BAT is a thermoregulatory organ. Interestingly, leptin is also expressed in brown adipocytes. This expression seems to be only under conditions of inactivity and atrophyCitation104 considering that cold and activation of β3 adrenoreceptors both decrease leptin gene expression.Citation105 Ueno et al showed that BAT thermogenic capacity and activity are diminished in both lean and obese old mice, but particularly in the obese ones. These observations suggest a synergy between aging and obesity in the context of leptin secretion and BAT thermogenesis.Citation106
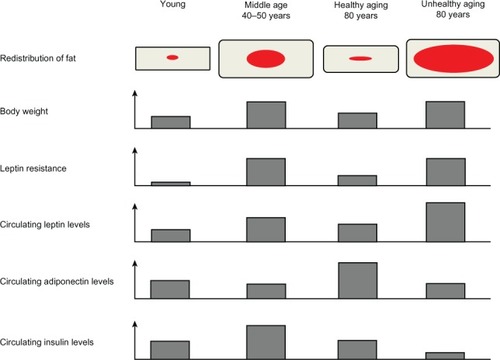
Figure 2 Representation of the different modifications regarding the redistribution of body fat, body weight, leptin resistance, and circulating levels of leptin, adiponectin, and insulin in conditions of youth, middle age, healthy aging, and unhealthy aging. Red and grey represent visceral and subcutaneous adipose tissue, respectively.
The different fat compartments undergo changes at different rates during aging ().Citation107 Retro-orbital and peripheral subcutaneous adipose tissue tend to be lost first during aging whereas visceral fat tends to be preserved.Citation108 Since subcutaneous adipose tissue is the principal source of leptin,Citation109 its loss reduces leptin’s capacity for β-oxidation of FFAs, which contributes to the increase of circulating FFAs upon aging. In turn, increased levels of circulating FFAs favor visceral and ectopic fat redistribution.Citation110 Age-associated decline in fat depot size appears to be the result of a decrease in cell size as opposed to a decline in cell number.Citation96 However, other reports have also noted a negative association between age and proliferation of subcutaneous preadipocytes, which may explain selective loss of subcutaneous adipose tissue with aging.Citation111 Adipogenesis is likely to occur throughout the lifespan since adipocyte numbers remain constant or even increase in old age.Citation107,Citation112 However, during aging, preadipocytes experience a decrease in their potential to differentiate and replicate.Citation113 Preadipocytes are precursor cells found in adipose tissue; they have the potential to differentiate, store triglycerides, respond to insulin, and secrete adipokines.Citation107 In normal adipose tissue, fat mass is regulated by apoptosis and differentiation of preadipocytes.Citation114 However, differentiation of preadipocytes isolated from aged rats and humans leads to a decrease of lipid accumulation and smaller fat cells when compared to preadipocytes from younger individuals.Citation115,Citation116 Since control for lipolysis is altered in fat cells upon aging,Citation117,Citation118 a decrease in lipid accumulation capacity could be a particularly important contributing factor for reduced adipocyte size. Thus, these age-related intrinsic factors could affect fat mass by influencing both preadipocytes and adipocytes.
Table 2 Modifications observed in different adipose tissue depot during aging
Changes in fat distribution associated with aging also lead to a modification of the secretion profile of adipokines. Circulating levels of inflammatory cytokines such as TNFα, C-reactive protein, and IL-6 are elevated in both aging and obesity.Citation119 High plasma leptin levels are observed in aging rodent modelsCitation120,Citation121 as well as in humansCitation122,Citation123 suggesting that aging is a leptin-resistant state. Although normal aging involves a decline in appetite,Citation124 hyperleptinemia observed in the elderly may serve to counterbalance the decrease in hypothalamic responsiveness found in both animal and human models of aging.Citation125 This decrease leads to a poor response to fasting and plays a causative role in the intolerance to catabolic situations.Citation124 Increased fat mass may also contribute to elevated leptin in aging, but the increase is often disproportionate to the amount of fat. Consistently, leptin resistance during aging has been shown to be independent of fat mass in rats.Citation126 Via its proinflammatory adipokine function, leptin also plays an important role in adipose tissue inflammation through preadipocytes activation. When preadipocytes are activated, they likely make a greater contribution to age-related adipose tissue dysfunction because of their number (15%–50% of cells in fat).Citation127 They have full innate immune response capability, considering that they constitutively express toll-like receptor (TLR) 4 as adipocytes, but express TLR2 in a specific manner. This may contribute in part for the greater inflammatory responsiveness of preadipocytes compared to that of fat cells.Citation128 Consistent with those findings, other reports suggest that cytokines and chemokines are expressed predominantly in preadipocytes rather than in adipocytes.Citation128,Citation129 Activated preadipocytes can even acquire a macrophage-like phenotype.Citation130 This plasticity predisposes them to contribute to adipose tissue inflammation and dysfunction.Citation130 Stressed preadipocytes may promote an inflammatory response leading to dysfunctionalities in adipose tissue as well as metabolic abnormalities.
The release of various cytokines and chemokines in adipose tissue attracts immune cells such as proinflammatory T lymphocytes and macrophages. Infiltration of visceral adipose tissue by macrophages is increased in both obese and lipodystrophic subjects.Citation131,Citation132 The macrophages also experience a shift of their activation: anti-inflammatory IL-10-producing M2 macrophages are replaced by proinflammatory M1 macrophages.Citation133 Macrophage infiltration is preceded by the infiltration of proinflammatory T lymphocytes and mast cells, and the decrease of anti-inflammatory T lymphocytes subtypes. This infiltration of immune cells also contributes to the production of proinflammatory cytokines, leading to a vicious circle. Although adipose tissue modifications caused by aging and obesity lead to highly similar metabolic abnormalities, the underlying mechanisms differ between the two conditions.Citation134,Citation135 Age-related metabolic dysfunctions cannot be explained completely by either the lipotoxicity associated with impaired lipid storage or by the increase in immune effectors. A combination of different mechanisms including, but not limited to, lipotoxicity caused by FFAs, inflammation and incapacity to store lipids may be responsible for the dysfunction of adipose tissue during aging.
Association of leptin, insulin sensitivity, and longevity
Adipokines from centenarians have been investigated and, in general, the subjects studied were lean (body mass index [BMI] of 19.4 ± 3.3) and exhibited low levels of plasma leptin which was an indicator of their low adipose tissue mass.Citation136 However, the lowest tertile of circulating leptin was surprisingly associated with higher all-cause mortality and, remarkably, these effects of low leptin levels on mortality were emphasized in patients with higher BMI.Citation136 Thus, maintaining adipose tissue mass and function, mainly by keeping a functional leptin signaling, seems to be essential for normal physiological functions under energy-deprived conditions associated with aging.Citation137 The role of leptin in sexual maturation makes associations between circulating leptin levels and longevity difficult since there is a positive correlation between age of reproduction and lifespan.Citation138 In rodents, caloric restriction delays both sexual maturity and aging.Citation139 Consistently, delayed sexual maturity and aging are observed in most animal models carrying a genetic mutation in the genes of the IGF1 pathway.Citation140 These studies suggest that genes implicated in the regulation of sexual maturation include a subset of pleiotropic genes that mediate the trade-off between development and aging.Citation141 The expression of other adipokines, like adiponectin, has also been associated with healthy aging and increased lifespan. Adiponectin, a hormone secreted exclusively by WAT, has circulating levels inversely associated with percent body fat in adults.Citation142 Centenarians have higher plasma adiponectin concentration than BMI–matched younger adults.Citation143 This hyperadiponectinemia in centenarians is also associated with a positive metabolic profile.Citation143 Furthermore, two common variants of the adiponectin gene have been associated with higher adiponectin levels and longevity.Citation143 Adiponectin levels were also inversely correlated with BMI, waist circumference, the percentage of body fat and with the homeostasis model assessment for insulin resistance, a marker of insulin resistance.Citation143,Citation144 In addition to adiponectin levels, insulin sensitivity has also been identified as an important component of longevity and healthy aging.
Several studies have been conducted on centenarians to better characterize the healthy aging phenotype.Citation145,Citation146 Numerous pathways have been identified and insulin sensitivity has been considered a key factor for healthy aging.Citation147 In humans, insulin sensitivity decreases with advancing age and, accordingly, prevalence of type 2 diabetes increases with aging.Citation148 When compared to elderly individuals over 75 years of age, centenarians have better preserved glucose tolerance and insulin sensitivity.Citation149 Certain epidemiological evidence may provide a mechanistic insight into insulin sensitivity in centenarians: healthy centenarians with preserved insulin sensitivity have been shown to have a lower waist-to-hip ratio and a more favorable body fat content than their elderly controls.Citation150 To test this hypothesis, numerous models have shown that improving insulin sensitivity leads to an increased longevity. Modifying adipose tissue either genetically, surgically or by caloric restriction has important effects on lifespan and age-associated diseases onset. Mice with fat-specific disruption of the insulin receptor gene (FIRKO mice) have decreased adiposity and lower fasting insulin levels, which lead to an extended lifespan. Concomitantly, FIRKO mice also have elevated serum adiponectin levels.Citation151
Modification of adiposity is also possible without genetic modifications through caloric restriction. For more than 75 years, caloric restriction without malnutrition has been noted to prolong lifespan.Citation152 This modification of caloric intake leads to an important loss of adipose tissue, especially visceral fat.Citation153 The Barzilai group showed that surgical removal of visceral adipose tissue in rats increased lifespan and improved both insulin resistance and glucose intolerance.Citation154,Citation155 Comparatively, genetic interventions aimed at reducing fat mass are also associated with increased lifespan. For example, replacing the adipogenic transcription factor CCAAT/enhancer-binding protein α (C/EBPα) with C/EBPβ in mice results in an increase in FFAs oxidation and reduced fat mass which leads to an extension of mean and maximal lifespan.Citation156 These animal models reveal that lifespan is closely related to adiposity. Taken together, these data show that reduced adiposity combined with hyperadiponectinemia and insulin sensitivity constitutes an important conserved pathway implicated in prolonged lifespan (). However, aging has often been associated with leptin and insulin resistance. These conditions have been linked to metabolic disorders and associated with age-related diseases, which increase morbidity and mortality.
Leptin resistance and development of disease in old age
The discovery of leptin has brought the long-awaited dream for a potential cure, which could induce satiety and weight loss in obese humans. This hope faded after the first observations of leptin resistance.Citation157–Citation159 Indeed, the magnitude of the weight loss achieved with leptin therapy in most obese individuals was modest compared to expectations, as most obese patients already exhibited elevated circulating levels of leptin as a consequence of their increased fat mass.Citation56 Generally, leptin resistance is described as a reduced sensitivity with respect to the anorectic response to exogenously administrated leptin,Citation160 but a universally accepted definition of this condition is currently under consideration by the National Institutes of Health.Citation161 The identity of the underlying mechanisms of leptin resistance still remains unclear, but at least three possibilities have been postulated to underlie it: (1) a failure of circulating leptin to reach its targets in the brain; (2) a decrease in the expression of LEPR; and/or (3) an inhibition of the signaling events within selected neurons in specific brain regions.Citation33,Citation56,Citation162,Citation163 The former hypothesis in itself establishes a debate, as the mechanisms by which leptin achieves its targets in the brain are controversial. Leptin is transported across the blood–brain barrier (BBB) by an active saturable transport system, but some suggest an action also attributed to the BBB-exempted circumventricular organs.Citation164,Citation165 Decreased leptin transport across the BBB has been demonstrated in diet-induced obesity rodentsCitation166 and leptin resistance may be partly overcome by bypassing the BBB with intracerebroventricular injection of leptin.Citation167 Moreover, cerebrospinal fluid levels of leptin are lower in obese individuals, in spite of high serum leptin levels, reinforcing the view of an impaired transport into the brain.Citation168 Triglycerides have also been shown to mediate resistance to leptin by impairing the transport across the BBB.Citation169 In addition to the contribution of a defective leptin transport, it is also suggested that a decreased number of LEPR-B, impaired signal transduction with old age, as well as a decrease in POMC with aging could also contribute to leptin resistance.Citation170–Citation172
Aged obese rats exhibit little or no anorectic or weight loss responses to peripherally infused leptin whereas young rats respond more robustly to leptin administration.Citation121,Citation172,Citation173 Moreover, plasma leptin levels are high in aged animals and humans, thus suggesting that a failure in leptin action may represent one of the primary defects in the metabolic dysfunctions observed in aging.Citation10,Citation126 Failure of action of leptin with aging is also evidenced in the transgenic mouse model that overexpressed leptin since despite continuous secretion of leptin, aged mice showed an increase in body weight.Citation174 Furthermore, a decreased number of LEPR-B has been demonstrated in the hypothalamic nuclei of old rats.Citation170,Citation172 Consistently, leptin injections failed to decrease body weight and hyperglycemia adequately in middle-aged obese and diabetic subjects.Citation175,Citation176 This suggests that leptin resistance also affects glycemic control. One other interesting way to determine the effects of leptin resistance during aging is to study the leptin receptor deficient db/db mice longitudinally. In this model, young animals (5–6 weeks) are normoglycemic because their peripheral insulin resistance is overcome by an increase in insulin secretion. This hyperinsulinemia usually occurs for 2 to 3 months and is then followed by a rapid increase in glycemia, refecting a defect in β-cells secretion.Citation177 Aging in db/db mice is also characterized by an important increase in plasma FFAs,Citation178 higher mean arterial pressure,Citation179 and lower hepatic insulin-binding capacity.Citation180 Associated with the already well described important weight gain of db/db mice, these metabolic factors contribute to the alteration of cardiac metabolism in favor of fatty acid oxidation and the progressive development of a cardiomyopathy.Citation178 Whether these important age-related metabolic effects are directly mediated by leptin resistance or triggered by the consequent WAT accumulation still needs to be determined. Taken together, these data suggest that youth is associated with leptin sensitivity and that resistance to leptin occurs with aging. Aging is thus associated with failure in leptin’s action, independent of obesity or changes in body fat distribution.Citation126 The failure of leptin to regulate food intake, body fat and its distribution, and insulin action suggests that leptin resistance plays a major role in the metabolic syndrome that is typical to aging.
Leptin also regulates the excitability of inter alia hippocampal neurons as well as synaptic plasticity,Citation49 demonstrating its involvement in neuroendocrine functions. Moreover, LEPR-B has been identified in neurons of the hippocampus, which are particularly vulnerable in Alzheimer disease (AD).Citation181 AD is a progressive neurodegenerative disease that involves a variety of symptoms such as progressive impairment of cognitive function, and impaired orientation and attention.Citation182 Several reports from various clinical studies support the idea of a negative correlation between obesity and cognitive function, as well as a positive association between reduced levels of circulating leptin and risk for AD.Citation182,Citation183 Moreover, higher leptin levels are associated with lower risk of dementia and AD and less cognitive decline.Citation184,Citation185 Mice with targeted disruption of LEPR-B show a decreased synaptic plasticity associated with a poorer performance on spatial memory tasks,Citation186 reinforcing the view that leptin has an undoubted neuroprotective implication. Since obesity worsens cognitive functions and leptin protects against neurodegeneration, higher prevalence of AD in obese elderly patientsCitation187 might be explained, in addition to low-grade inflammation and factors contributing to amyloidogenesis, by the presence of leptin resistance. Indeed, a large prospective study showed that aged individuals with the lowest leptin levels had a greater decline in their cognitive ability than those with the highest levels.Citation185 Moreover, central obesity in midlife increases the risk of dementia later in life independently of comorbidities such as type 2 diabetes or cardiovascular diseases, indicating that leptin deficiency is commonly observed in AD and is someway associated with obesity characterized by leptin resistance.Citation183 Leptin also raises many research interests regarding its possible involvement in Parkinson’s disease, epilepsy, and ischemia.Citation181 All these lines of evidence suggest that leptin protects, at least indirectly, against age-related neuroendocrine/cognitive functions decline.Citation182
Leptin resistance and the loss of BAT activity upon aging
BAT is a specialized fat depot with incredible thermogenic potential.Citation188,Citation189 It is found in relative abundance in small eutherian mammals, allowing them to live in cold environments without relying on the shivering process to produce heat.Citation190 Brown adipocytes are characterized by multilocular lipid droplets and a high density of mitochondria, which contain uncoupling protein 1 (UCP1). UCP1 is located in the inner membrane of the mitochondria and uncouples substrate oxidation from adenosine triphosphate (ATP) synthesis, thereby providing the BAT capacity for nonshivering thermogenesis by dissipating energy as heat instead of generating ATP.Citation188
Until recently, BAT was thought to disappear rapidly after birth in humans and play only a minimal physiological role in adults.Citation191,Citation192 However, recent evidence involving imaging procedures has brought a whole new perspective to the involvement of BAT in adults.Citation193,Citation194 In nuclear medicine, since fat and tumors capture the radioisotope Citation18fluorodeoxyglucose in the same way, brown fat has long eluded researchers.Citation195 The use of combined positron emission tomography and computed tomography scanning has revealed the presence of a metabolically active fat depot in adult humans.Citation195 BAT presence and activity are prevalent at a young age and remain present throughout adulthood, before declining robustly at an older age.Citation196,Citation197 Accordingly, BAT prevalence is higher in people younger than 50 years of age compared to patients older than 64 years.Citation194 A prospective study also showed that adolescents exhibit a higher prevalence of BAT (40%) compared to adults (5%–10%).Citation198 Thereby, all these lines of evidence suggest a protective role of BAT against age-related obesity. This age-related decline in BAT is thought to be the result of a gradual reduction of the amount of active BAT, followed by a reduction in the function and sensitivity at older age.Citation199 Unfortunately, the exact timing of the age-related decline in BAT is still undetermined as well as the possible impact that age-related leptin resistance could exert on it. Different assumptions suggesting that age-related leptin resistance could affect the decline of BAT observed in elderly individuals are discussed below.
The age-associated decline in thermoregulation ()Citation195,Citation200 has been related to BAT atrophy associated with a loss of UCP1 activity and has been shown to be influenced by sex, as females show a more important loss of thermogenic capacity with advancing age than males.Citation200 In this view, ob/ob mice have lower metabolic rate and a lower body temperature than their lean counterparts and are unable to survive for more than a few hours when exposed to cold due to a failure in thermogenesis.Citation201 BAT of the ob/ob mouse is usually thermogenically inactive, relatively atrophied, and has little UCP1, indicating that leptin deficiency leads to reduced BAT activity.Citation106 Furthermore, BAT thermogenesis is triggered by the activation of the SNS, which abundantly innervates BAT.Citation188,Citation202 SNS nerve endings in BAT release noradrenaline, which activates β-adrenergic receptors and a downstream cascade of events leading to BAT cell proliferation, mitochondrial biogenesis, and increased expression and activation of UCP1.Citation188 In addition, BAT-mediated thermogenesis induced by noradrenaline is significantly decreased in old rats compared to their younger controlsCitation103 and impairment of brown adipocytes function with increasing age appears to be mediated, at least in part, by a disturbance in the intracellular adrenergic signaling.Citation203,Citation204 It is possible that a selective reduction in the SNS activity/sensitivity of BAT occurs with aging, even if paradoxically human SNS activity is generally increased in older individuals, at least in specific context such as at the cardiovascular level.Citation205 The hypothesis of a selective reduction in SNS–BAT activity comes from the observations that selective neuronal circuits control SNS-mediated BAT thermogenesis in rodents.Citation206 However, although it remains unclear whether such selectivity in SNS–BAT activity also exists in humans, it is known that leptin regulates SNS outflow to peripheral organs including BAT, suggesting that age-related leptin resistance could be related to this SNS–BAT activity reduction.Citation207
In addition to stimulating food intake, NPY is known to suppress thermogenesis in BAT.Citation208,Citation209 Leptin increases energy expenditure through increasing thermogenesis in BAT and inhibits NPY, suggesting that age-related decrease in BAT activity could be the result of the inability of leptin to inhibit NPY-suppressed BAT thermogenesis. Moreover, aging is characterized by a significant decrease in POMC,Citation171 which is activated by leptin and implicated in the activation of BAT thermogenesis mainly through αMSH/MC4R signaling, suggesting another plausible mechanism that can attribute the decline of BAT activity to leptin resistance. Absence of MC4R has also been shown to compromise the ability of leptin to increase UCP1 expression in BAT.Citation210 Several studies have demonstrated that changes in circulating levels of sex and thyroid hormones may contribute to age-related decline in BAT.Citation211,Citation212 Indeed, thyroid hormones have direct stimulatory effects on UCP-1 expression and enhance the adrenergic signaling to BAT.Citation213 However, the possible involvement of age-related leptin resistance in thyroid receptor hormone-mediated BAT activity needs further studies. This avenue represents a promising future for a better comprehension of the effect of leptin resistance associated with age-induced reduction of BAT thermogenesis. Age-related leptin resistance could selectively affect the SNS-BAT activity, as well as the NPY and/or POMC/αMSH/MC4R modulation of BAT thermogenesis.
Conclusion
Leptin resistance and aging seem to be strongly intertwined. Both in the brain and in the periphery, the physiological actions of leptin experience a decrease during advancing age. The redistribution of adipose tissue and increased percent body fat observed during middle and old age contribute however to an increase in circulating leptin. This suggests that leptin resistance in aging is not overcome by an increase in leptin levels. Once established, leptin resistance increases adipose tissue inflammation through preadipocytes activation and seems to negatively impact cognitive function. When comparing the effects of leptin resistance to the metabolic dysfunctions observed in aging, leptin resistance appears to be an early contributor to the development of metabolic abnormalities in old age. Leptin resistance also exerts important functional impairment on BAT; this decrease in thermogenesis may act in synergy with the robust age-associated decline of BAT prevalence to contribute to the negative metabolic changes associated with unhealthy aging.
Studies on leptin show that this hormone is central to the dysregulations observed in aging and obesity. Whether leptin plays an important role in longevity is still to be determined, but since leptin resistance and aging share common metabolic alterations, its role in morbidity is very likely. Taking into account the important fat redistribution associated with aging, numerous questions are still unanswered considering leptin secretion and autocrine actions, particularly in BAT. Although it is now recognized that BAT remains present during adulthood, available data do not allow a causal relationship between BAT atrophy and increasing body weight with aging. A role for leptin resistance has not yet been defined in this weight-gain process. Preventing leptin resistance and/or counteracting the effects of aging on BAT decline represent a plausible therapeutic approach to prevent age-related metabolic alterations. To our knowledge, no drug is currently available for these applications. However, exercise could be a large-scale and affordable manner of maintaining leptin signaling and BAT functionality. Importantly, understanding the mechanisms underlying age-related leptin resistance and involved in modulating BAT activity is an unavoidable prerequisite before trying to treat metabolic disorders that life reserves.
Acknowledgments
Research related to the subject of this paper in F Picard laboratory was funded by grants from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research. F Picard holds a Senior Scholar Award from the Fond de recherche du Québec-Santé (FRQ-S). S Carter holds a FRQ-S PhD studentship. A Caron holds a Canadian Institutes of Health Research training program PhD studentship.
Disclosure
The authors declare no conflicts of interest in this work.
References
- World Health OrganizationObesity and overweight fact sheet2012Geneva, Switzerland Retrieved from: http://www.who.int/mediacentre/factsheets/fs311/en/
- MokdadAHFordESBowmanBAPrevalence of obesity, diabetes, and obesity-related health risk factors, 2001JAMA20032891767912503980
- RussellSJKahnCREndocrine regulation of ageingNat Rev Mol Cell Biol20078968169117684529
- AhimaRSConnecting obesity, aging and diabetesNat Med200915999699719734871
- PetoRWhitlockGJhaPEffects of obesity and smoking on US life expectancyN Engl J Med20103629855856 author reply, 856–85720200394
- MillerRAAging and immune functionInt Rev Cytol19911241872152001916
- MauryEBrichardSMAdipokine dysregulation, adipose tissue inflammation and metabolic syndromeMol Cell Endocrinol2010314111619682539
- KershawEEFlierJSAdipose tissue as an endocrine organJ Clin Endocrinol Metab20048962548255615181022
- ScarpelliniETackJObesity and metabolic syndrome: an inflammatory conditionDig Dis201230214815322722429
- MaXHMuzumdarRYangXMGabrielyIBergerRBarzilaiNAging is associated with resistance to effects of leptin on fat distribution and insulin actionJ Gerontol A Biol Sci Med Sci2002576B225B23112023258
- MasuzakiHOgawaYIsseNHuman obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissueDiabetes19954478558587789654
- MargeticSGazzolaCPeggGGHillRALeptin: a review of its peripheral actions and interactionsInt J Obes Relat Metab Disord200226111407143312439643
- SaadMFDamaniSGingerichRLSexual dimorphism in plasma leptin concentrationJ Clin Endocrinol Metab19978225795849024258
- HassinkSGSheslowDVde LanceyEOpentanovaIConsidineRVCaroJFSerum leptin in children with obesity: relationship to gender and developmentPediatrics1996982 Pt 12012038692618
- HubeFLietzUIgelMDifference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humansHorm Metab Res199628126906939013743
- RosenbaumMNicolsonMHirschJEffects of gender, body composition, and menopause on plasma concentrations of leptinJ Clin Endocrinol Metab1996819342434278784109
- SinhaMKOpentanovaIOhannesianJPEvidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fastingJ Clin Invest1996986127712828823291
- BrabantGNaveHMayrBBehrendMvan HarmelenVArnerPSecretion of free and protein-bound leptin from subcutaneous adipose tissue of lean and obese womenJ Clin Endocrinol Metab20028783966397012161541
- HalaasJLGajiwalaKSMaffeiMWeight-reducing effects of the plasma protein encoded by the obese geneScience199526952235435467624777
- ZhangGTanejaKLSingerRHGreenMRLocalization of pre-mRNA splicing in mammalian nucleiNature199437265088098127997273
- ElmquistJKMaratos-FlierESaperCBFlierJSUnraveling the central nervous system pathways underlying responses to leptinNat Neurosci19981644545010196541
- KalraSPDubeMGPuSXuBHorvathTLKalraPSInteracting appetite-regulating pathways in the hypothalamic regulation of body weightEndocr Rev19992016810010047974
- ZhangYScarpacePJCircumventing central leptin resistance: lessons from central leptin and POMC gene deliveryPeptides200627235036416274846
- TartagliaLAThe leptin receptorJ Biol Chem199727210609360969102398
- FriedmanJMHalaasJLLeptin and the regulation of body weight in mammalsNature199839567047637709796811
- FeiHOkanoHJLiCAnatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissuesProc Natl Acad Sci USA19979413700170059192681
- MercerJGHoggardNWilliamsLMLawrenceCBHannahLTTrayhurnPLocalization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridizationFEBS Lett19963872–31131168674530
- SchwartzMWSeeleyRJCampfieldLABurnPBaskinDGIdentification of targets of leptin action in rat hypothalamusJ Clin Invest1996985110111068787671
- ElmquistJKBjorbaekCAhimaRSFlierJSSaperCBDistributions of leptin receptor mRNA isoforms in the rat brainJ Comp Neurol199839545355479619505
- ElmquistJKEliasCFSaperCBFrom lesions to leptin: hypothalamic control of food intake and body weightNeuron199922222123210069329
- BaskinDGBreiningerJFSchwartzMWLeptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamusDiabetes199948482883310102700
- BaskinDGSchwartzMWSeeleyRJLeptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleusJ Histochem Cytochem199947335336210026237
- MyersMGCowleyMAMunzbergHMechanisms of leptin action and leptin resistanceAnn Rev Physiol20087053755617937601
- HuszarDLynchCAFairchild-HuntressVTargeted disruption of the melanocortin-4 receptor results in obesity in miceCell19978811311419019399
- ButlerAAConeRDThe melanocortin receptors: lessons from knockout modelsNeuropeptides2002362–3778412359499
- OllmannMMWilsonBDYangYKAntagonism of central melanocortin receptors in vitro and in vivo by agouti-related proteinScience199727853351351389311920
- MercerAJHentgesSTMeshulCKLowMJUnraveling the central proopiomelanocortin neural circuitsFront Neurosci201371923440036
- StephensTWBasinskiMBristowPKThe role of neuropeptide Y in the antiobesity action of the obese gene productNature199537765495305327566151
- MizunoTMKleopoulosSPBergenHTRobertsJLPriestCAMobbsCVHypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptinDiabetes19984722942979519731
- SchwartzMWSeeleyRJWoodsSCLeptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleusDiabetes19974612211921239392508
- SatohNOgawaYKatsuuraGThe arcuate nucleus as a primary site of satiety effect of leptin in ratsNeurosci Lett199722431491529131658
- HarlanSMMorganDAAgassandianKAblation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activationCirc Res2011108780881221311043
- BalthasarNCoppariRMcMinnJLeptin receptor signaling in POMC neurons is required for normal body weight homeostasisNeuron200442698399115207242
- van de WallELeshanRXuAWCollective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestionEndocrinology200814941773178518162515
- VongLYeCYangZChoiBChuaSJrLowellBBLeptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neuronsNeuron201171114215421745644
- NogueirasRWilsonHRohner-JeanrenaudFTschopMHCentral nervous system regulation of adipocyte metabolismRegul Pept20081491–3263118453013
- MinokoshiYHaqueMSShimazuTMicroinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in ratsDiabetes199948228729110334303
- MarwarhaGGhribiOLeptin signaling and Alzheimer’s diseaseAm J Neurodegener Dis20121324526523383396
- OomuraYHoriNShiraishiTLeptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in ratsPeptides200627112738274916914228
- ColemanDLObese and diabetes: two mutant genes causing diabetes-obesity syndromes in miceDiabetologia1978143141148350680
- ZhangYProencaRMaffeiMBaroneMLeopoldLFriedmanJMPositional cloning of the mouse obese gene and its human homologueNature199437265054254327984236
- MontagueCTFarooqiISWhiteheadJPCongenital leptin deficiency is associated with severe early-onset obesity in humansNature199738766369039089202122
- PelleymounterMACullenMJBakerMBEffects of the obese gene product on body weight regulation in ob/ob miceScience199526952235405437624776
- CampfieldLASmithFJGuisezYDevosRBurnPRecombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networksScience199526952235465497624778
- FarooqiISJebbSALangmackGEffects of recombinant leptin therapy in a child with congenital leptin deficiencyN Engl J Med19993411287988410486419
- MunzbergHBjornholmMBatesSHMyersMGJrLeptin receptor action and mechanisms of leptin resistanceCell Mol Life Sci200562664265215770417
- StunkardAJHarrisJRPedersenNLMcClearnGEThe body-mass index of twins who have been reared apartN Engl J Med199032221148314872336075
- MaffeiMHalaasJRavussinELeptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjectsNat Med1995111115511617584987
- GainsfordTAlexanderWSA role for leptin in hemopoieses?Mol Biotechnol199911214915810464769
- NakataMYadaTSoejimaNMaruyamaILeptin promotes aggregation of human platelets via the long form of its receptorDiabetes199948242642910334326
- Sierra-HonigmannMRNathAKMurakamiCBiological action of leptin as an angiogenic factorScience19982815383168316869733517
- RingBDScullySDavisCRSystemically and topically administered leptin both accelerate wound healing in diabetic ob/ob miceEndocrinology2000141144644910614668
- HaynesWGMorganDAWalshSAMarkALSivitzWIReceptor-mediated regional sympathetic nerve activation by leptinJ Clin Invest199710022702789218503
- ShekEWBrandsMWHallJEChronic leptin infusion increases arterial pressureHypertension1998311 Pt 24094149453337
- ShankarAXiaoJPositive relationship between plasma leptin level and hypertensionHypertension201056462362820713919
- TankersleyCGO’DonnellCDaoodMJLeptin attenuates respiratory complications associated with the obese phenotypeJ Appl Physiol1998856226122699843551
- O’DonnellCPTankersleyCGPolotskyVPSchwartzARSmithPLLeptin, obesity, and respiratory functionRespir Physiol20001192–316317010722859
- MalliFPapaioannouAIGourgoulianisKIDaniilZThe role of leptin in the respiratory system: an overviewRespir Res20101115221040518
- MinokoshiYKimYBPeroniODLeptin stimulates fatty-acid oxidation by activating AMP-activated protein kinaseNature2002415686933934311797013
- MuoioDMDohmGLFiedorekFTJrTapscottEBColemanRALeptin directly alters lipid partitioning in skeletal muscleDiabetes1997468136013639231663
- NowakKWMackowiakPNogowskiLSzkudelskiTMalendowiczLKAcute leptin action on insulin blood level and liver insulin receptor in the ratLife Sci19986315134713529768872
- ZhaoAZShinoharaMMHuangDLeptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytesJ Biol Chem200027515113481135410753948
- AbbatecolaAMRizzoMRBarbieriMPostprandial plasma glucose excursions and cognitive functioning in aged type 2 diabeticsNeurology200667223524016864814
- SaxenaNKIkedaKRockeyDCFriedmanSLAnaniaFALeptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob miceHepatology200235476277111915021
- ImajoKFujitaKYonedaMHyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signalingCell Metab2012161445422768838
- JavorEDGhanyMGCochranEKLeptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophyHepatology200541475376015791619
- WalderKFilippisAClarkSZimmetPCollierGRLeptin inhibits insulin binding in isolated rat adipocytesJ Endocrinol19971553R5R79488006
- ZierathJRFrevertEURyderJWBerggrenPOKahnBBEvidence against a direct effect of leptin on glucose transport in skeletal muscle and adipocytesDiabetes1998471149421367
- MullerGErtlJGerlMPreibischGLeptin impairs metabolic actions of insulin in isolated rat adipocytesJ Biol Chem19972721610585105939099705
- FruhbeckGGomez-AmbrosiJModulation of the leptin-induced white adipose tissue lipolysis by nitric oxideCell Signal2001131182783311583918
- FruhbeckGAguadoMMartinezJAIn vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptinBiochem Biophys Res Commun199724035905949398609
- Santos-AlvarezJGobernaRSanchez-MargaletVHuman leptin stimulates proliferation and activation of human circulating monocytesCell Immunol1999194161110357875
- La CavaAMatareseGThe weight of leptin in immunityNat Rev Immunol20044537137915122202
- HubeFHaunerHThe role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance?Horm Metab Res1999311262663110668912
- WangZWZhouYTLeeYHigaMKalraSPUngerRHHyperleptinemia depletes fat from denervated fat tissueBiochem Biophys Res Commun1999260365365710403821
- LinJChoiYHHartzellDLLiCDella-FeraMABaileCACNS melanocortin and leptin effects on stearoyl-CoA desaturase-1 and resistin expressionBiochem Biophys Res Commu20033112324328
- TajimaDMasakiTHidakaSKakumaTSakataTYoshimatsuHAcute central infusion of leptin modulates fatty acid mobilization by affecting lipolysis and mRNA expression for uncoupling proteinsExp Biol Med (Maywood)2005230320020615734723
- CoppariRBjorbaekCLeptin revisited: its mechanism of action and potential for treating diabetesNat Rev Drug Discov201211969270822935803
- Siegrist-KaiserCAPauliVJuge-AubryCEDirect effects of leptin on brown and white adipose tissueJ Clin Invest199710011285828649389752
- ChehabFFMounzihKLuRLimMEEarly onset of reproductive function in normal female mice treated with leptinScience1997275529688908974400
- SpicerLJFranciscoCCThe adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian functionEndocrinology19971388337433799231790
- LindheimSRSauerMVCarminaEChangPLZimmermanRLoboRACirculating leptin levels during ovulation induction: relation to adiposity and ovarian morphologyFertil Steril200073349349810689001
- HoggardNHunterLTrayhurnPWilliamsLMMercerJGLeptin and reproductionProc Nutr Soc19985734214279794000
- MounzihKLuRChehabFFLeptin treatment rescues the sterility of genetically obese ob/ob malesEndocrinology19971383119011939048626
- BaumgartnerRNStauberPMMcHughDKoehlerKMGarryPJCross-sectional age differences in body composition in persons 60+ years of ageJ Gerontol A Biol Sci Med Sci1995506M307M3167583802
- CartwrightMJTchkoniaTKirklandJLAging in adipocytes: potential impact of inherent, depot-specific mechanismsExp Gerontol200742646347117507194
- KukJLSaundersTJDavidsonLERossRAge-related changes in total and regional fat distributionAgeing Res Rev20098433934819576300
- RosenCJBouxseinMLMechanisms of disease: is osteoporosis the obesity of bone?Nat Clin Pract Rheumatol200621354316932650
- UngerRHLongevity, lipotoxicity and leptin: the adipocyte defense against feasting and famineBiochimie2005871576415733738
- GoodpasterBHKrishnaswamiSResnickHAssociation between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and womenDiabetes Care200326237237912547865
- PfannenbergCWernerMKRipkensSImpact of age on the relationships of brown adipose tissue with sex and adiposity in humansDiabetes20105971789179320357363
- GabaldonAMMcDonaldRBHorwitzBAEffects of age, gender, and senescence on beta-adrenergic responses of isolated F344 rat brown adipocytes in vitroAm J Physiol19982744 Pt 1E726E7369575835
- McDonaldRBHorwitzBABrown adipose tissue thermogenesis during aging and senescenceJ Bioenerg Biomembr199931550751610653478
- CannonBNedergaardJBrown adipose tissue: function and physiological significancePhysiol Rev200484127735914715917
- BuyseMViengchareunSBadoALombesMInsulin and glucocorticoids differentially regulate leptin transcription and secretion in brown adipocytesFASEB J20011581357136611387233
- UenoNOh-ishiSSegawaMEffect of age on brown adipose tissue activity in the obese (ob/ob) mouseMech Ageing Dev1998100167769509396
- KirklandJLTchkoniaTPirtskhalavaTHanJKaragiannidesIAdipogenesis and aging: does aging make fat go MAD?Exp Gerontol200237675776712175476
- HughesVARoubenoffRWoodMFronteraWREvansWJFiatarone SinghMAAnthropometric assessment of 10-y changes in body composition in the elderlyAm J Clin Nutr200480247548215277173
- Van HarmelenVReynisdottirSErikssonPLeptin secretion from subcutaneous and visceral adipose tissue in womenDiabetes19984769139179604868
- EckelRHGrundySMZimmetPZThe metabolic syndromeLancet200536594681415142815836891
- Van HarmelenVRohrigKHaunerHComparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjectsMetabolism200453563263715131769
- ArnerPBernardSSalehpourMDynamics of human adipose lipid turnover in health and metabolic diseaseNature2011478736711011321947005
- KaragiannidesIThomouTTchkoniaTIncreased CUG triplet repeat-binding protein-1 predisposes to impaired adipogenesis with agingJ Biol Chem200628132230252303316754681
- SpaldingKLArnerEWestermarkPODynamics of fat cell turnover in humansNature2008453719678378718454136
- DjianPRoncariAKHollenbergCHInfluence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in cultureJ Clin Invest1983724120012086630508
- KirklandJLHollenbergCHGillonWSAge, anatomic site, and the replication and differentiation of adipocyte precursorsAm J Physiol19902582 Pt 1C206C2102305864
- KirklandJLDaxEMAdipocyte hormone responsiveness and aging in the rat: problems in the interpretation of aging researchJ Am Geriatr Soc19843232192286321584
- GregermanRIAging and hormone-sensitive lipolysis: reconciling the literatureJ Gerontol1994494B135B1398014384
- FriedSKBunkinDAGreenbergASOmental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoidJ Clin Endocrinol Metab19988338478509506738
- AhrenBManssonSGingerichRLHavelPJRegulation of plasma leptin in mice: influence of age, high-fat diet, and fastingAm J Physiol19972731 Pt 2R113R1209249540
- LiHMathenyMNicolsonMTumerNScarpacePJLeptin gene expression increases with age independent of increasing adiposity in ratsDiabetes19974612203520399392492
- WangFNMaCGZhangNXSongHYGlucose and Insulin Regulate Leptin Expression in 3T3-F442A AdipocytesSheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai)199931335035212136196
- Sanchez-RodriguezMGarcia-SanchezARetana-UgaldeRMendoza-NunezVMSerum leptin levels and blood pressure in the overweight elderlyArch Med Res200031442542811068088
- EngineerDRGarciaJMLeptin in anorexia and cachexia syndromeInt J Pept2012201228745722518191
- ScarpacePJMathenyMMooreRLTumerNImpaired leptin responsiveness in aged ratsDiabetes200049343143510868965
- GabrielyIMaXHYangXMRossettiLBarzilaiNLeptin resistance during aging is independent of fat massDiabetes20025141016102111916920
- MackIBelAibaRSDjordjevicTGorlachAHaunerHBaderBLFunctional analyses reveal the greater potency of preadipocytes compared with adipocytes as endothelial cell activator under normoxia, hypoxia, and TNFalpha exposureAm J Physiol Endocrinol Metab20092973E735E74819549791
- ChungSLapointKMartinezKKennedyABoysen SandbergMMcIntoshMKPreadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytesEndocrinology2006147115340535116873530
- HarkinsJMMoustaid-MoussaNChungYJExpression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob miceJ Nutr2004134102673267715465765
- CousinBMunozOAndreMA role for preadipocytes as macrophage-like cellsFASEB J19991323053129973318
- WeisbergSPMcCannDDesaiMRosenbaumMLeibelRLFerranteAWJrObesity is associated with macrophage accumulation in adipose tissueJ Clin Invest2003112121796180814679176
- SevastianovaKSutinenJKannistoKHamstenARistolaMYki-JarvinenHAdipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophyAm J Physiol Endocrinol Metab20082951E85E9118430964
- LumengCNBodzinJLSaltielARObesity induces a phenotypic switch in adipose tissue macrophage polarizationJ Clin Invest2007117117518417200717
- MiardSPicardFObesity and aging have divergent genomic fingerprintsInt J Obes (Lond)200832121873187418982010
- MiardSDombrowskiLCarterSBoivinLPicardFAging alters PPARgamma in rodent and human adipose tissue by modulating the balance in steroid receptor coactivator-1Aging Cell20098444945919485965
- AraiYTakayamaMGondoYAdipose endocrine function, insulinlike growth factor-1 axis, and exceptional survival beyond 100 years of ageJ Gerontol A Biol Sci Med Sci200863111209121819038836
- AraiYTakayamaMAbeYHiroseNAdipokines and agingJ Atheroscler Thromb201118754555021551960
- HarveyPHZammutoRMPatterns of mortality and age at first reproduction in natural populations of mammalsNature198531560173193204000262
- MerryBJHolehanAMOnset of puberty and duration of fertility in rats fed a restricted dietJ Reprod Fertil1979572253259513013
- Brown-BorgHMHormonal control of aging in rodents: the somatotropic axisMol Cell Endocrinol20092991647118674587
- YuanRMengQNautiyalJGenetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strainsProc Natl Acad Sci USA2012109218224822922566614
- UkkolaOSantaniemiMAdiponectin: a link between excess adiposity and associated comorbidities?J Mol Med (Berl)2002801169670212436346
- AtzmonGPollinTICrandallJAdiponectin levels and genotype: a potential regulator of life span in humansJ Gerontol A Biol Sci Med Sci200863544745318511746
- BikWBaranowska-BikAWolinska-WitortEThe relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese womenNeuro Endocrinol Lett200627449350016891987
- FranceschiCMontiDSansoniPCossarizzaAThe immunology of exceptional individuals: the lesson of centenariansImmunol Today199516112167880382
- HittRYoung-XuYSilverMPerlsTCentenarians: the older you get, the healthier you have beenLancet1999354917965210466675
- AraiYKojimaTTakayamaMHiroseNThe metabolic syndrome, IGF-1, and insulin actionMol Cell Endocrinol2009299112412818672019
- FordESGilesWHDietzWHPrevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination SurveyJAMA2002287335635911790215
- PaolissoGGambardellaAAmmendolaSGlucose tolerance and insulin action in healty centenariansAm J Physiol19962705 Pt 1E890E8948967479
- PaolissoGGambardellaABalbiVAmmendolaSD’AmoreAVarricchioMBody composition, body fat distribution, and resting metabolic rate in healthy centenariansAm J Clin Nutr19956247467507572703
- BluherMKahnBBKahnCRExtended longevity in mice lacking the insulin receptor in adipose tissueScience2003299560657257412543978
- MehtaLHRothGSCaloric restriction and longevity: the science and the ascetic experienceAnn N Y Acad Sci20091172283319735237
- BarzilaiNGuptaGRevisiting the role of fat mass in the life extension induced by caloric restrictionJ Gerontol A Biol Sci Med Sci1999543B89B96 discussion B97–B8810191831
- GabrielyIBarzilaiNSurgical removal of visceral adipose tissue: effects on insulin actionCurr Diab Rep20033320120612762966
- MuzumdarRAllisonDBHuffmanDMVisceral adipose tissue modulates mammalian longevityAging Cell20087343844018363902
- ChiuCHLinWDHuangSYLeeYHEffect of a C/EBP gene replacement on mitochondrial biogenesis in fat cellsGenes Dev200418161970197515289464
- FogtelooAJPijlHFrolichMMcCamishMMeindersAEEffects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humansDiabetes Nutr Metab200316210911412846450
- McDuffeJRRiggsPACalisKAEffects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiencyJ Clin Endocrinol Metab20048994258426315356018
- OralEASimhaVRuizELeptin-replacement therapy for lipodystrophyN Engl J Med2002346857057811856796
- ScarpacePJZhangYLeptin resistance: a prediposing factor for diet-induced obesityAm J Physiol20092963R493R500
- MyersMGJrHeymsfieldSBHaftCChallenges and opportunities of defining clinical leptin resistanceCell Metab201215215015622326217
- EnrioriPJEvansAESinnayahPDiet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neuronsCell Metab20075318119417339026
- MunzbergHMyersMGJrMolecular and anatomical determinants of central leptin resistanceNat Neurosci20058556657015856064
- BanksWAThe many lives of leptinPeptides3200425333133815134858
- FryMFergusonAVThe sensory circumventricular organs: brain targets for circulating signals controlling ingestive behaviorPhysiol Behav200791441342317531276
- LevinBEDunn-MeynellAABanksWAObesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onsetAm J Physiol20042861R143R150
- MuzumdarRHMaXYangXAtzmonGBarzilaiNCentral resistance to the inhibitory effects of leptin on stimulated insulin secretion with agingNeurobiol Aging20062791308131416122839
- SchwartzMWPeskindERaskindMBoykoEJPorteDJrCerebrospinal fuid leptin levels: relationship to plasma levels and to adiposity in humansNat Med1996255895938616722
- BanksWACoonABRobinsonSMTriglycerides induce leptin resistance at the blood-brain barrierDiabetes20045351253126015111494
- Fernandez-GalazCFernandez-AgulloTCampoyFDecreased leptin uptake in hypothalamic nuclei with ageing in Wistar ratsJ Endocrinol20011711233211572787
- GruenewaldDAMatsumotoAMAge-related decrease in proopiomelanocortin gene expression in the arcuate nucleus of the male rat brainNeurobiol Aging19911221131211711159
- ScarpacePJMathenyMTumerNHypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese ratsNeuroscience200110441111111711457594
- ShekEWScarpacePJResistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged ratsRegul Pept2000921–3657111024567
- QiuJOgusSLuRChehabFFTransgenic mice overexpressing leptin accumulate adipose mass at an older, but not younger, ageEndocrinology2001142134835811145598
- HeymsfieldSBGreenbergASFujiokaKRecombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trialJAMA1999282161568157510546697
- MittendorferBHorowitzJFDePaoliAMMcCamishMAPattersonBWKleinSRecombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetesDiabetes20116051474147721411512
- WyseBMDulinWEThe influence of age and dietary conditions on diabetes in the db mouseDiabetologia1970632682734914664
- AasumEHafstadADSeversonDLLarsenTSAge-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db miceDiabetes200352243444112540618
- SenadorDKanakamedalaKIrigoyenMCMorrisMElasedKMCardiovascular and autonomic phenotype of db/db diabetic miceExper Physiol200994664865819218356
- KodamaHFujitaMYamaguchiIDevelopment of hyperglycaemia and insulin resistance in conscious genetically diabetic (C57BL/KsJ-db/db) miceDiabetologia19943787397447988774
- FolchJPedrosIPatracaINeuroprotective and anti-ageing role of leptinJ Mol Endocrinol2012493R149R15622967480
- NaderaliEKRatcliffeSHDaleMCObesity and Alzheimer’s disease: a link between body weight and cognitive function in old ageAm J Alzheimers Dis Other Demen200924644544919801534
- TezapsidisNJohnstonJMSmithMALeptin: a novel therapeutic strategy for Alzheimer’s diseaseJ Alzheimers Dis200916473174019387109
- LiebWBeiserASVasanRSAssociation of plasma leptin levels with incident Alzheimer disease and MRI measures of brain agingJAMA2009302232565257220009056
- HoldenKFLindquistKTylavskyFARosanoCHarrisTBYaffeKSerum leptin level and cognition in the elderly: Findings from the Health ABC StudyNeurobiol Aging20093091483148918358569
- LiXLAouSOomuraYHoriNFukunagaKHoriTImpairment of long-term potentiation and spatial memory in leptin receptor-deficient rodentsNeuroscience2002113360761512150780
- Singh-ManouxACzernichowSElbazAObesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort studyNeurology201279875576222915175
- SellHDeshaiesYRichardDThe brown adipocyte: update on its metabolic roleInt J Biochem Cell Biol200436112098210415313455
- RichardDCarpentierACDoreGOuelletVPicardFDeterminants of brown adipocyte development and thermogenesisInt J Obes (Lond)201034Suppl 2S59S6621151149
- RichardDPicardFBrown fat biology and thermogenesisFront Biosci2011161233126021196229
- LeanMEBrown adipose tissue in humansProc Nutr Soc19894822432562678120
- HeatonJMThe distribution of brown adipose tissue in the humanJ Anat1972112Pt 135395086212
- ZingarettiMCCrostaFVitaliAThe presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissueFASEB J20092393113312019417078
- CypessAMLehmanSWilliamsGIdentification and importance of brown adipose tissue in adult humansN Engl J Med2009360151509151719357406
- RichardDMonge-RoffarelloBChechiKLabbeSMTurcotteEEControl and physiological determinants of sympathetically mediated brown adipose tissue thermogenesisFront Endocrinol2012336
- OuelletVRouthier-LabadieABellemareWOutdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humansJ Clin Endocrinol Metab201196119219920943785
- LeePGreenfieldJRHoKKFulhamMJA critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humansAm J Physiol Endocrinol Metab20102994E601E60620606075
- DrubachLAPalmerEL3rdConnollyLPBakerAZurakowskiDCypessAMPediatric brown adipose tissue: detection, epidemiology, and differences from adultsJ Pediatr2011159693994421839465
- TamCSLecoultreVRavussinEBrown adipose tissue: mechanisms and potential therapeutic targetsCirculation2012125222782279122665886
- SaelyCHGeigerKDrexelHBrown versus white adipose tissue: a mini-reviewGerontology2012581152321135534
- Himms-HagenJDesautelsMA mitochondrial defect in brown adipose tissue of the obese (ob/ob) mouse: reduced binding of purine nucleotides and a failure to respond to cold by an increase in bindingBiochem Biophys Res Comm1978832628634212061
- BartnessTJVaughanCHSongCKSympathetic and sensory innervation of brown adipose tissueInt J Obes (Lond)201034Suppl 1S36S4220935665
- LanginDTavernierGLafontanMRegulation of beta 3-adrenoceptor expression in white fat cellsFundam Clin Pharmacol199592971067628838
- ScarpacePJTseCMathenyMThermoregulation with age: restoration of beta(3)-adrenergic responsiveness in brown adipose tissue by cold exposureProc Soc Exp Biol Med199621143743808618944
- SealsDREslerMDHuman ageing and the sympathoadrenal systemJ Physiol2000528Pt 340741711060120
- MorrisonSFMaddenCJTuponeDCentral control of brown adipose tissue thermogenesisFront Endocrinol201235
- DunbarJCLuHLeptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) productsBrain Res Bull199950321522110566984
- LevineASMorleyJENeuropeptide Y: a potent inducer of consummatory behavior in ratsPeptides198456102510296549409
- BillingtonCJBriggsJEGraceMLevineASEffects of intracerebroventricular injection of neuropeptide Y on energy metabolismAm J Physiol19912602 Pt 2R321R3271996719
- ZhangYKilroyGEHenaganTMTargeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptinFASEB J200519111482149116126916
- MattsonMPPerspective: Does brown fat protect against diseases of aging?Ageing Res Rev201091697619969105
- ValleAGuevaraRGarcia-PalmerFJRocaPOliverJCaloric restriction retards the age-related decline in mitochondrial function of brown adipose tissueRejuv Res2008113597604
- SilvestriESchiavoLLombardiAGogliaFThyroid hormones as molecular determinants of thermogenesisActa Physiol Scand2005184426528316026419