Abstract
There is a growing population of older adults requiring admission to the intensive care unit (ICU). This population outpaces the ability of clinicians with geriatric training to assist in their management. Specific training and education for intensivists in the care of older patients is valuable to help understand and inform clinical care, as physiologic changes of aging affect each organ system. This review highlights some of these aging processes and discusses clinical implications in the vulnerable older population. Other considerations when caring for these older patients in the ICU include functional outcomes and morbidity, as opposed to merely a focus on mortality. An overall holistic approach incorporating physiology of aging, applying current evidence, and including the patient and their family in care should be used when caring for older adults in the ICU.
Introduction
Older Americans (aged 65 and older) are expected to double in population from 46 million to over 98 million by 2060, with the proportion of this age group increasing from 15% to almost a quarter of the total population.Citation1 Intensive care unit (ICU) providers are already seeing the average age of admitted critically ill patients increasing.Citation2 The growing geriatric population necessitates the training of all health care providers to implement care that reflects the unique needs and conditions of this population. Targeting training in the care of older patients may prove significant as current reports demonstrate that established collaboration between geriatricians and other clinicians improves outcomes by catering care specifically to older adults, particularly in the perioperative and acute care populations.Citation3,Citation4 Thus, geriatric specific knowledge and training among intensivists must be expanded to meet the complex needs of these older patients.Citation5 This review will describe ICU-specific concerns within the aging population by reviewing existing data, understanding the impact of aging on critical illness pathophysiology, and its impact on patient outcomes using current evidence and highlighting areas in need of future evaluation.
Geriatric Syndromes and Comorbidities
Older adults often experience several aging-related common clinical conditions outside of discrete disease processes that increase vulnerability to morbidity and poor outcomes. These conditions, termed “geriatric syndromes”, include pressure ulcers, incontinence, falls, functional decline, and delirium.Citation6 While the prevalence of geriatric syndromes prior to ICU admission can be as high, up to 90% of older survivors of critical illness report one or more geriatric syndromes, which represents a 2.6-fold increase from baseline.Citation7 Presence of these syndromes may contribute to ongoing higher care or nursing care needs, decreased independence, and reduced quality of life after critical illness.Citation8
Aging is associated with an increase in comorbidities and a higher risk of “multimorbidity” or the co-occurrence of two or more chronic conditions.Citation9,Citation10 Common comorbidities in aging include hypertension, diabetes, chronic obstructive pulmonary disease, heart failure, cancer, and cognitive impairment.Citation10 Multimorbidity is associated with increased short- and long-term mortality among all ICU patientsCitation11 and poses a significant risk for older populations.Citation12 The aging process and comorbidities in older adults increase the risk of developing frailty, a syndrome resulting from decline across multiple physiologic systems that decreases the body’s reserve for managing stressful events and increases vulnerability to adverse outcomes.Citation13 Frail older adults experience higher hospital and long-term mortality than their non-frail counterparts.Citation14
Geriatric syndromes lead to unique preoperative evaluation requirements in older surgical patients, with interventions aimed at improving perioperative outcomes in these high-risk patients. Geriatric specific risk factors that contribute to perioperative morbidity, unplanned postoperative ICU admission, and mortality include baseline cognitive impairment, cardiopulmonary disease, frailty, and poor functional status.Citation15,Citation16 Identifying modifiable risk factors prior to surgery and intervention with multidisciplinary collaboration has been found to decrease postoperative complications.Citation17 Discussing these risks with patients, in addition to patient’s goals, is an important component of informed surgical consent.Citation18
Geriatric syndromes and multimorbidity add to the complexity of ICU care in older adults. As more older adults are admitted with and survive critical illness, the health care system will require increased resources and long-term support for this population. Further, intensivists and ICU staff must be trained and equipped to manage geriatric-specific considerations.
System-Specific Considerations in Older Adults
A summary of aging-specific considerations by organ system for older adults admitted to the ICU is shown in .
Figure 1 System-specific considerations for the critically Ill older adult patient. Age-related changes to the central nervous, cardiovascular, respiratory, musculoskeletal, renal, and immune systems are highlighted. Together these changes can impact overall patient health at baseline, and may be of particular concern during acute illness when physiologic homeostasis is particularly altered. Consideration of these changes should be made by all clinicians caring for older adults to help guide clinical decision making, family discussions, and overall goals of care. Image is powered by Piktochart.
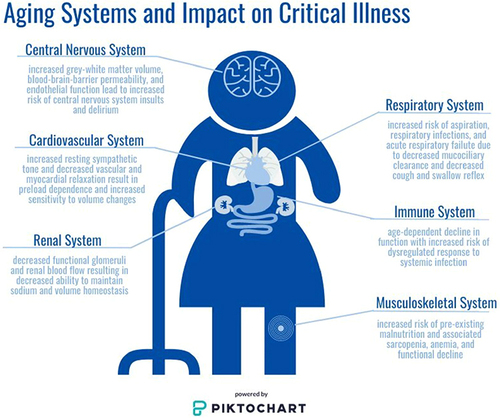
Neurologic
The aging brain undergoes changes that make older adults more vulnerable to developing neurocognitive dysfunction. Imaging studies have shown notable losses in grey and white matter volume and integrity with aging.Citation19 Cerebral blood flow also declines, leading to impaired oxygen delivery, slowed metabolism, and altered activity and production of neurotransmitters.Citation20,Citation21 Further, endothelial cells lose function, and the blood–brain barrier becomes more permeable, exposing older adults to increased risk to the central nervous system from systemic insults.Citation22,Citation23 Increasing age is also a risk factor for the development of cerebral microbleeds detected on MRI.Citation24 In a large population-based study, participants aged 60–69, 70–79, and 80–97 years old were found to have an 18.4%, 32.4%, and 38.1% incidence of microbleeds, respectively.Citation24 Higher microbleed burden is associated with increased physical frailty in a community dwelling adult population, and occurrence of microbleeds is associated with decline in cognitive function.Citation25,Citation26 Loss of ability to perform activities of daily living and impaired cognition are not considered part of “normal aging” and warrant structured evaluation to identify and alleviate all modifiable factors.Citation27
Acute brain dysfunction, or delirium, and long-term cognitive impairment are highly prevalent among ICU patients and survivors. Age is a well-established risk factor for both delirium and cognitive impairment. Routine screening with a validated assessment tool (ie, Confusion Assessment Method for the ICU [CAM-ICU] or Intensive Care Delirium Screening Checklist [ICDSC]) is essential as the diagnosis is often missed or delayed when relying on clinician identification alone. Application of routine screening has shown that a remarkable 60–80% of patients across medical, surgical, and trauma ICUs will develop delirium during their critical illness.Citation28,Citation29 Early diagnosis is imperative as delirium is an independent predictor of increased duration of mechanical ventilation, duration of ICU and hospital stay, increased health care costs, long-term cognitive impairment, and mortality.Citation30–34
Older adults admitted to the ICU with neurologic pathology such as traumatic brain injury, stroke, or postoperative neurosurgical intervention, may require specific neurologic care. These interventions include specialized monitoring electroencephalogram, intracranial pressure monitors, and serial neurologic exams. Serial hourly neurologic exams early in hospitalization may be beneficial for early detection of expanding intracranial hematoma or increasing cerebral edema requiring emergent intervention. Serial neurologic monitoring, however, requires frequent sleep interruptions and should be discontinued as soon as possible to mitigate sleep disruption.Citation35 Sleep disruption impacts several organ systems and has been found to be independently associated with delirium in the ICU.Citation36,Citation37 Continuation of sleep disruption with hourly exams when no longer indicated may contribute to ICU delirium. Older age alone has been identified as a factor affecting sleep quality in the ICU.Citation38 One retrospective review of traumatic brain injury patients monitored with hourly neurologic exams found 20.2% of these patients were assessed hourly for greater than four days and had greater length of stay.Citation39 Given older age is associated with disrupted sleep in the ICU and sleep disruption associated increased risk delirium, the need for hourly neurologic exams should be assessed continually and interval increased as soon as possible.
Patients undergoing cardiac surgery are a unique population as they are often older and are placed at risk for delirium both from the surgery and from the often requisite ICU stay after surgery. Delirium develops in 20–50% of postoperative cardiac patients.Citation40,Citation41 Risk factors for post-cardiac surgery delirium include advancing age, low ejection fraction, diabetes mellitus, postoperative atrial fibrillation, chronic kidney disease, and prolonged hospitalization.Citation40 Patients older than 65 years old undergoing cardiac surgery who had postoperative delirium were found to have prolonged hospitalization, prolonged ICU stays, and longer time on mechanical ventilation.Citation40 Delirium has also been shown to be an independent predictor of functional decline after cardiac surgery.Citation42
There is currently no FDA-approved treatment for delirium. The best evidence for both prevention and management of delirium involves multi-component bundles of care, such as The Society of Critical Care Medicine’s Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption (PADIS) Guidelines.Citation43 The PADIS Guidelines were designed to optimize care and minimize iatrogenic risk factors that contribute to poor outcomes after critical illness and are summarized into the ABCDEF implementation bundle. Each letter addresses a component of care associated with attempts to maintain brain health in the ICU: A, Assess, prevent and treat pain; B, perform Both spontaneous awakening and spontaneous breathing trials; C, Choose analgesic and sedative medications thoughtfully; D, perform frequent Delirium assessments and provide appropriate prevention and management; E, engage in Early mobility and exercise; F, encourage Family engagement.Citation44 Large-scale implementation studies of the ABCDEF bundle have demonstrated decreasing mortality, delirium, and coma with increased compliance with all components of the bundle.Citation45,Citation46
ABCDEF bundle implementation practices face hurdles and barriers for initiation, and summarizes the bundle components, age-related barriers to implementation, and strategies for overcoming these barriers. Factors related to the structure of the ICU, implementation planning with training and staff support, and educational prompts may lead to success of the ABCDEF bundle implementation.Citation47 High staff turnover, lack of continued education, and a culture that does not encourage multidisciplinary cooperation in quality improvement efforts may be barriers to bundle implementation.Citation47
Table 1 ABCDEF Bundle Components and Age-Specific Barrier Guidance for Clinicians
Cardiovascular
Physiologic changes of the aging cardiovascular system can make hemodynamic management challenging in older ICU patients. The autonomic nervous system becomes more imbalanced over time, with attenuated parasympathetic responses and increased resting sympathetic tone.Citation48 These imbalances predispose older adults to cardiovascular disease. The sinus node intrinsic rate and AV conduction slow, resulting in lower heart rates. Anatomic changes include valvular calcification and increased arterial and ventricular stiffness. In sum, these changes create higher preload dependence in older adults.Citation49 Intra-arterial and venous wall changes cause vessel stiffness and fragility, most notably in peripheral and coronary arteries. Thus, older adults are more vulnerable to the shifts in intravascular volume status that are common when critically ill.
The 2020 scientific statement from the American Heart Association reported increased incidence of myocardial infarction (MI) with age, both with type 1 MI due to plaque erosion and type 2 MI if blood supply and demand mismatch occur.Citation50 In older adults, less reserve and more demand resulting in type 2 MI were found to have five times increased risk of in-hospital mortality.Citation51 As such, demand ischemia from the acute critical illness insult is more likely to occur and complicate further management. Vascular changes contribute to the peak acute aortic dissection occurrences in the sixth and seventh decade of life;Citation52 however, management of Type A aortic dissection with open surgical repair remains high risk in the elderly. One meta-analysis found higher short-term mortality postoperatively in patients undergoing open aortic repair aged 70 years and older.Citation53 Postoperative in-hospital or 30-day mortality pooled incidence in older adults was 19.9% compared to 14.9% for younger adults, with a relative risk of 2.25 for the older adults.
The overall incidence of heart failure increases with age, and etiology is multifactorial with underlying causes including coronary artery disease (CAD), hypertension, and valvular disease.Citation54 In addition to increased incidence, mortality associated with heart failure also increases with age.Citation55 Development of congestive heart failure may result from increased left ventricle wall stiffness and decreased relaxation due to increased interstitial connective tissue within the left ventricle myocardium, myocyte hypertrophy, and changes in calcium channels in the sarcoplasmic reticulum.Citation54 This leads to reduced left ventricle early diastolic filling and decreased left ventricle compliance. The resultant decrease in cardiovascular reserve further contributes to older patients’ sensitivity to changes in volume status, with hypovolemia resulting in decreased cardiac output. Conversely, hypervolemia in the setting of decreased cardiac compliance causes an increase in the left atrial stretch and pulmonary edema.Citation54
Respiratory disorders, sepsis, or acute coronary syndrome may precipitate acute heart failure in older adults with limited cardiac reserve.Citation56 While intravascular depletion is detrimental in critically ill patients, resulting in hypotension and acute kidney injury, intravascular volume overload from intravenous fluid can also result in cardiac dysfunction and pulmonary edema with evidence for increased mortality in mechanically ventilated older patients with volume overload.Citation57
Aging is also associated with an increased incidence of cardiac arrhythmias.Citation58,Citation59 Cardiovascular changes with aging that can contribute to the development of arrhythmias include increased fibrous content resulting in dysfunction of the atrioventricular node and even atrioventricular block. The most common cardiac arrhythmia is atrial fibrillation, with an increased incidence in older adults and the most common arrhythmia in ICU patients.Citation60,Citation61 In atrial fibrillation, the late diastolic filling of the left ventricle is lost. Along with impaired early filling as described earlier, this further increases the risk of hemodynamic compromise. Older adults may have chronic atrial fibrillation or may develop acute atrial fibrillation in the ICU, and atrial fibrillation is a predictor of mortality in critically ill patients regardless of chronicity.Citation62 For management of atrial fibrillation, the initial step is to recognize hemodynamic instability and if present proceed with emergent cardioversion. In the hemodynamically stable patient, treatment to control the ventricular response rate with beta blockade, calcium channel blockers, or amiodarone is administered.Citation63 Decreasing the heart rate allows for more left ventricle filling and improved stroke volume. Managing reversible causes of atrial fibrillation, such as volume overload and acute infection, must also be addressed. Associated morbidity with atrial fibrillation is cardioembolic stroke risk. Anticoagulation for atrial fibrillation has been associated with increased risk of bleeding complications in critically ill patients.Citation64 In the ICU or even after discharge, bleeding risk may preclude therapeutic anticoagulation in older patients due to other comorbidity and/or fall risk.
An early conversation for older patients in the ICU should involve code status in the event of cardiac arrest. The most common documented dysrhythmias in geriatric cardiac arrest are ventricular fibrillation and pulseless electrical activity.Citation65 Survival rates from in-hospital arrest and cardiopulmonary resuscitation (CPR) decrease with age. In one systematic review of in-hospital cardiac arrest for patients over age 90 years, the overall return of spontaneous circulation (ROSC) rate was 38.6%; the immediate survival rates of CPR for patients age 90 years and older was 11.6%, 15.4% for age 80–89 years, and 18.7% for age 70–79 years.Citation66 For elderly patients who survive in-hospital cardiac arrest and survive until hospital discharge, the risk-adjusted rate of 1-year survival was 63.7% for age 65–74 years, 58.6% for age 75–84 years, and 49.7% for age 85 years and older.Citation67 Out-of-hospital arrest survival rates after cardiac arrest and CPR are even lower. One-month survival for persons aged 85–94 years old was 0.59%, and 0.27% for ages >95 years old.Citation68 Careful attention is needed in administration of post-arrest management, as age is an independent predictor of underutilization of targeted temperature management. It is unclear if this is due to clinician bias or post-ROSC goals of care discussions.Citation50 One recent meta-analysis comparing hypothermic to normothermic management did not find a difference in 6-month mortality or functional outcomes using the Rankin score for both older and younger age groups.Citation69 While older age is associated with decreased survival after cardiac arrest, there is limited evidence on functional and neurologic outcomes specific to geriatric patients. This lack of data may impact code status discussions with patients and their family members and should be a goal of future studies.
Respiratory
Respiratory system changes with aging increase vulnerability to pulmonary infections and respiratory failure. Over time, mucociliary transport becomes dysfunctional which can contribute to difficulty with mucus and secretion clearance.Citation70 Connective tissue changes in the lung parenchyma lead to decreased elasticity, along with an overall reduced number of alveoli and increase in alveolar duct size. These changes cause an increase in the alveolar-arterial (A-a) gradient.Citation71 With kyphosis of the spine in addition to parenchymal changes, there is a decrease in forced expiratory volume (FEV) and a decrease in vital capacity.Citation72,Citation73 In addition, age is associated with reduced muscular strength, reduced cough strength, and decreased ability to clear secretions.Citation74
Physiologic changes of aging may also lead to increased risk of aspiration prior to, during, and after critical illness. Due to decrease in the cough and swallow reflex, chronic tracheal aspiration may go undetected until older patients present with aspiration pneumonia.Citation75,Citation76 Cough and swallow reflexes have a sensory and motor component. The sensory limb seems to be more affected by aging and is the primary target for ongoing investigation to preserve cough and swallow reflexes.Citation77
Advanced age is associated with an increased risk of acute respiratory failure.Citation78 The incidence of acute respiratory failure has been found to increase almost exponentially with each decade of life up to 85 years old.Citation78 With increased risk of respiratory failure and the growing aging population, more older adults with respiratory failure are being admitted to the ICU.Citation79 The incidence of mechanical ventilation requirement increases 10-fold from age 55 to 85 years old.Citation78 As the population ages and more geriatric patients require ICU care, there has been shown to be an increase in acute respiratory infection diagnoses and increase in number of hospitalizations for respiratory illness over the decade from 2006 to 2015.Citation79 The number of hospitalizations for patients age 75 years and older increased 1.6-fold, for age 85–90 by 2.5-fold, and for age 90 years and older by 2.1-fold. Older adults admitted to the ICU with respiratory failure are also at risk for complications including ventilator-induced lung injury and acute respiratory distress syndrome (ARDS).Citation80,Citation81
In respiratory failure requiring mechanical ventilation, increasing age is associated with increased mortality. In one study of patients aged 80 or older admitted to the ICU with acute respiratory illness, survival to hospital discharge was 75%.Citation82 When compared to a propensity score case-matched controls, the hospitalized cohort had a 10-fold increased risk of death 6 months post hospitalization. In mechanically ventilated older adults with acute lung injury and ARDS, adults aged 70 years and older had longer duration of mechanical ventilation, had longer stay in the ICU, and had higher mortality.Citation83 In this cohort, survival rate decreased for each increasing decade in age. Another observational study of patients age 65 and greater found decreasing survival with increasing age and also significant decrease in functional outcomes with increasing age.Citation84 For patients who survived one year, some functional recovery was regained. Increased mortality with age and potential functional recovery are both important considerations when caring for older adults with respiratory failure.
Respiratory changes with aging in addition to multiple comorbidities predisposed geriatric patients to develop severe infection and high mortality rates during the COVID pandemic.Citation85 In older adults, COVID may have atypical presentation of non-respiratory symptoms which can lead to delay in diagnosis and seeking supportive care; these symptoms can include fatigue, headache, diarrhea, and loss of sense and smell. A retrospective review of COVID hospitalizations in France during March 2020 found that age and history of cardiovascular disease were predictive of in-hospital mortality for patients hospitalized with severe SARS-CoV-2 infection.Citation86 The COVID international database to identify risk factors for mortality revealed that age >75 years, in addition to dementia, hypoxia, lymphopenia, and quick sequential organ failure assessment (qSOFA) score >1, was an independent predictor for mortality.Citation87
Nutrition
Aging patients are at risk for preexisting malnutrition and inadequate dietary intake.Citation88 Malnutrition is associated with functional decline, sarcopenia, anemia, and poor wound healing.Citation89 Nutritional assessment may be useful in predicting complications; there is great variation in available assessment tools, however, and currently no consensus is available on the best tool for older adults in the ICU. The Geriatric Nutritional Risk Index was created to predict nutrition-related risk of morbidity and mortality in hospitalized older patients.Citation90 In a geriatric trauma patient population, the Geriatric Nutritional Risk Index was found to predict the length of stay and development of postoperative delirium in elderly patients.Citation91 According to the European Society for Parenteral and Enteral Nutrition (ESPEN), the Mini Nutrition Assessment (MNA) is the most commonly used and highly validated screening tool in older adults, though the requirement for anthropometric measurements and clinical history make implementation challenging in the ICU.Citation89,Citation92 The Nutrition Risk in the Critically Ill (NUTRIC) score was designed specifically for use in the ICU, relies on clinical data not history or physical examination, and has been associated with increased duration of mechanical ventilation and 28-day mortality.Citation93
While preexisting malnutrition may predispose patients to worse outcomes, nutrition requirements during critical illness also influence outcomes, and all critically ill patients are at risk for developing malnutrition. Critical illness is thought to have an acute phase with hemodynamic instability and early metabolic instability with a substantial increase in catabolism followed by later muscle wasting and stabilization of metabolic changes.Citation94 Current ESPEN guidelines recommend early implementation of oral or enteral feeding that slowly ramps up to full nutritional requirements within 3 to 7 days of admission and consideration of parenteral nutrition by days 3 to 7 if oral or enteral nutrition is contraindicated.Citation94 Enteral nutrition in older ICU patients preserves intestinal function.Citation95 While total parenteral nutrition (TPN) provides nutrition to patients with intestinal failure, it is associated with morbidity and mortality, and ESPEN guidelines should be followed with regard to initiation. In older adults, TPN use during hospitalization was associated with increased mortality when compared to younger adults.Citation96 All decisions on temporary and permanent procedures to supply nutrition to patients should be performed under the overall goals of patient care, as discussed within the Ethics section below.
Given the increased risk of baseline malnutrition, screening for refeeding syndrome and associated electrolyte abnormalities is also critical in the older population. Early screening for malnutrition and rapid steps to begin nutritional supplementation may improve outcomes in the particularly vulnerable population of older adults.
Renal
The kidneys undergo many physiologic changes with age. There is a decrease in renal mass due to loss of renal cortex.Citation97 This is in conjunction with a decrease in the number of functioning glomeruli and an increase in size of the remaining glomeruli.Citation98 The effective renal blood flow decreases up to 10% per decade of life.Citation99 When trending lab data, there is a variable decrease in glomerular filtration rate (GFR) with age, and many GFR calculations do not account for the physiologic changes of aging.Citation100 With increasing age, the physiologic changes to maintaining sodium homeostasis lead to decreased ability to concentrate urine and potential increased risk for volume depletion.Citation101
Acute kidney injury (AKI) in critical illness is common.Citation102 Geriatric patients are at an increased risk of AKI due to the decrease in effective renal blood flow causing increased susceptibility to episodes of hypotension or reduced cardiac output. In combination with dehydration and disturbances in autoregulation, these episodes can lead to renal ischemia and AKI.Citation103 Using GFR and serum creatinine to diagnose AKI in older adults has limitations. Serum creatinine levels are less reliable, and an increase in serum creatinine can lag days behind the initial AKI insult.Citation104
Acute renal failure in the setting of critical illness raises questions if elderly patients can tolerate the hemodynamic effects of renal replacement therapy (hemodialysis or hemofiltration). Acute illness with sequela of acute renal failure, along with renal replacement therapy, puts patients at risk of hemodynamic instability, decreased cardiac reserve, autonomic dysfunction, bleeding, and neurologic complications.Citation105 In one study, requirement of dialysis in older patients in the ICU was associated with higher risk of chronic dialysis than in younger patients.Citation106 Review of current evidence, however, does not show increase in mortality for older patients with acute renal failure requiring dialysis in the ICU when compared to younger patients.Citation107
Geriatric patients are more likely to have chronic kidney disease (CKD) due to renal physiologic changes with aging. Geriatric patients on chronic dialysis have high annual mortality risk.Citation108,Citation109 In the outpatient setting, for geriatric patients with chronic renal disease that progresses to renal failure, goals of care discussion should take place prior to initiating dialysis therapy.Citation107 Patient and family discussions and education about what chronic hemodialysis entails may help inform decision-making for unplanned hospitalizations or continued decline in renal function. In the acute setting, these goals should be readdressed if a patient declines to renal failure requiring dialysis as part of the holistic approach and prioritizing quality of life.
Infection and Immune System
Aging leads to an age-dependent decline in immune system effectiveness. Known as immunosenescence, these changes include higher level of proinflammatory cytokine secretion at baseline and a decrease in the ability to stimulate the immune response to antigens.Citation49 This results in chronic hyperstimulation of the immune system and likely higher risk of dysregulated systemic response to infection.
In older patients, respiratory infection is the most common source of sepsis. Patient’s age, need for mechanical ventilation, need for renal replacement therapy, and need for inotropic support are all predictive of mortality in older adults with sepsis.Citation110 Sepsis from a urinary source is the second most common source of infection in septic older patients.Citation111 Presenting symptoms of urinary infection in geriatric patients can be atypical, leading to delays in diagnosis and often progression of infection prior to initiating appropriate treatments.Citation112 Finally, decline in immune system function in older patients increases their risk for secondary infections or hospital-acquired infections.
With increasing age, there is evidence that the gut microbiome also undergoes changes, with an increase in pro-inflammatory bacteria associated with inflammatory dysregulation and immunosenescence.Citation113 Changes in microbiota have been linked to increased intestinal permeability and age-associated inflammation, resulting in greater susceptibility to infections.Citation114 The microbiome is also affected by diet, lifestyle, medications, and overall health status, and there are many ongoing studies evaluating potential therapeutic targets. These changes in microbiome may also result in a reduction of essential amino acid production that may contribute to poor nutritional status and even sarcopenia in older adults.Citation113
Musculoskeletal
Sarcopenia is an aging-related loss of muscle mass and function, which commonly impacts older adults presenting with critical illness.Citation115,Citation116 In addition, structural and functional alterations in both muscles and nerves may occur throughout the course of critical illness and leave survivors with a new condition termed ICU-acquired weakness (ICUAW).Citation117,Citation118 Nervous system changes include axonal degeneration, microvascular changes in the setting of sepsis, and channelopathies that all contribute to neuropathy.Citation119–122 Muscular changes in critical illness can begin early during hospitalization, including early atrophy from increased catabolism and decreased synthesis.Citation123 Many components of critical illness contribute to loss of muscle mass including inflammation, immobilization, endocrine stress responses, nutritional deficit, and impaired microcirculation.Citation124 Pro-inflammatory mediators such as TNF alpha, interleukin-1, interleukin-6, GDF-15, illness-induced sodium channel dysfunction, and altered intracellular calcium homeostasis have all been connected to muscle breakdown in critically ill patients.Citation125
A retrospective observational study of outcomes in critically ill patients found increased age and sarcopenia were associated with increased hospital length of stay.Citation126 In addition, studies examining skeletal muscle mass measured on computed tomography imaging found that patients with lower muscle mass on admission to the ICU were more likely to have higher severity of illness during the ICU stay, longer duration of mechanical ventilation, increased length of ICU stay, and higher mortality.Citation127–130 Similarly, in older adults requiring cardiac surgery, sarcopenia diagnosed by psoas muscle area and handgrip strength predicted longer length of hospital stay.Citation131 The precise mechanism of developing ICUAW remains unknown; however, studies have shown that patients with increased severity of illness, including sepsis and shock, and increasing degree of multiorgan failure are more likely to develop ICUAW.Citation132,Citation133
Proposed methods to prevent muscle loss, sarcopenia, and ICUAW include maintaining normal glycemic levels during hospitalization, reducing duration of immobilization, using electrical muscle stimulation, and employing early enteral nutrition.Citation134–139 The early catabolic phase during critical illness, however, is not prevented by early parenteral nutrition.Citation138,Citation139 Early mobilization of ICU patients has been a key area of interest and strategy for reducing the burden of physical decline after critical illness and potentially reducing muscle loss and dysfunction.Citation140,Citation141 In mechanically ventilated patients, daily physical and occupational therapy sessions that were coordinated with interruption of sedation and initiated within 72 hours of mechanical ventilation improved the likelihood of being discharged with functional independence and reduced days of ICU delirium, days on the ventilator, and length of ICU and hospital stay.Citation142 Similar findings were reported in a study of early goal-directed mobility in surgical ICU patients.Citation143 A recent meta-analysis confirmed that physical rehabilitation in the ICU reduced ICU and hospital length of stay and improved physical function at discharge; however, there was no difference in physical function at 6-month follow-up.Citation144 Another recent meta-analysis comparing efficacy and safety of early mobilization and long-term outcomes found no effect on days alive and out of the hospital up to 6 months, but early mobilization was associated with improved physical function in survivors at 6-month follow-up.Citation145 In this meta-analysis, early mobilization, however, was also associated with an increase in adverse events potentially due to the mobilization, such as arrhythmia, blood pressure changes, and oxygen desaturation. Neither of these recent meta-analyses focused on outcomes specific to older adults. Future studies are needed to examine interventions that will improve long-term recovery, particularly in older and frailer adults and those with sarcopenia.
Oncologic
Cancer risk increases with age, and ICU admissions related to cancer, cancer treatments (ie, chemotherapy, radiation, surgery), and comorbid conditions are also increasing with the aging of the population. Data suggest that that approximately 20% of all ICU patients have underlying cancer diagnoses.Citation146 Breast, hematological, lung, colorectal, prostate, pancreatic and biliary, urinary tract, and sarcoma make up the majority of ICU admissions for cancer. The specific care associated with different cancer types, along with toxicities related to specific cancer therapies, is out of the scope of this manuscript. Although increasing age is a risk factor for many cancers, management is not different based on age but is rather centered around the oncologic pathology. Cancer and its therapies are additionally associated with immunosuppression, malnutrition, anemia, and frailty, which have all been previously discussed. A cancer diagnosis carries a slightly higher odds of death after accounting for additional risk factors, and cancer patients have a higher likelihood of death years after discharge following ICU admission.Citation147 As such, early goals of care and palliative discussions for oncology patients may be warranted, especially in the older patient population with limited neurologic or functional capacity or significant comorbid disease.
Post-Intensive Care Syndrome and Long-Term Outcomes
Post-intensive care syndrome includes the physical, neurocognitive, and psychological symptoms that frequently occur in survivors of critical illness.Citation148 Together, these symptoms can impact physical function, mental health, and overall quality of life for months to years after hospital discharge.Citation149–151 In older populations, it is often difficult to qualify deficits found following acute illness as secondary to the illness or attributed to premorbid status or aging. Surrogate markers of pre-illness disability and cognitive function are employed but limited by recall bias of both patients and family members.Citation152,Citation153 Larger cohort studies have been constructed to understand the impact of critical illness on a population level, understanding that individual patients’ experience can be variable.
Physical Disability
Baseline physical function status has been shown in several studies to be either associated with no change in post-discharge physical functional statusCitation154–157 or associated with significant decline.Citation158–161 These results are limited in interpretation by the retrospective collection of preadmission functional status. Larger population-based studies investigating overall change in functional status have demonstrated that older mechanically ventilated patients have worsened functional status when compared to similar patients who did not require mechanical ventilation—indicating baseline functional impairment is likely associated with worse disability among older ICU survivors.Citation162 However, the overall link between pre-admission disability and post-survival physical dysfunction remains ill-defined.Citation163 Regardless of pre-admission functional status, survivors of critical illness experience physical deficits that impair their overall physical function and daily independence.Citation164 Identifying and optimizing modifiable factors associated with disability including optimizing nutritional status, early mobility, and incorporating care bundles have shown the best success in helping to mitigate the risk of physical disability among these patients.Citation140,Citation142,Citation165
Neurocognitive Deficits
Cognitive impairments are prevalent, persistent, and can be severe among adult ICU survivors across all age groups, affecting up to 60% of survivors.Citation166–169 Baseline cognitive impairment, as well as advanced age, has been shown to be a strong predictor of long-term cognitive impairment among survivors.Citation169–172 While these strong predictors are non-modifiable, important modifiable factors have also been identified, introducing important targets for clinicians to potentially mitigate the downstream risk of cognitive impairment among older adults.
Delirium is the most prominent independent risk factor for cognitive impairment,Citation167,Citation173–175 with up to 40% of cases during hospitalization deemed preventable.Citation176 The most effective strategies to date include multicomponent nonpharmacologic treatment bundles such as the ABCDEF bundle.Citation44,Citation177–179 There are often barriers or perceived barriers that prevent incorporation of these bundles within the care of older adults. outlines the ABCDEF bundle, age-related barriers, and strategies for mitigating these barriers for patients.
Sleep may play an important role in recovery, as sleep disruption has been shown to be associated with worse cognition in older ICU survivorsCitation180 as well as worsened functional decline.Citation181 Further studies are necessary to define and understand these associations. While other modifiable risk factors have presented within the literature, evidence surrounding their correlation and causation remains mixed.
Older patients are vulnerable to medication side effects due to increased comorbid disease, decreased physiologic reserve, and impaired drug metabolism. Many common medications in the ICU used to treat anxiety, pain, and insomnia are centrally acting, including sedatives, analgesics, antipsychotics, and anticholinergics specifically identified by The American Geriatric Society as potentially inappropriate medications in older adults.Citation182 After leaving the ICU, these medications are frequently continued in up to a third of all patients, with an unclear understanding of how they may impact long-term health and recovery after the ICU.Citation183–185 Nonetheless, potentially inappropriate medications should be discontinued as soon as possible as their use is associated with lower quality of life, higher incidence of hospital readmission, worsening cognitive impairment, adverse drug events, and increased mortality in non-critically ill adults.Citation186–188
Psychological Symptoms
Up to 30% of ICU survivors experience long-term psychological symptoms from the acute stress, trauma, and anxiety that accompanied their ICU stay.Citation149,Citation189 These symptoms often include post-traumatic stress symptoms, anxiety, and depression that carry significant implications for patients,Citation151,Citation190 ranging from impacting daily quality of life, employment, relationships, and worsening cognitive impairment to associated increases in patient mortality.Citation149,Citation191–193 It can also lead to avoidance behaviors which may potentially impact willingness to continue necessary medical care after discharge.
ICU delirium is a risk factor for the development of neuropsychiatric disorders, perhaps in part due to the distorted memories and experiences that patients can experience while delirious.Citation151,Citation194 Additional risk factors include socioeconomic status and isolation, both of which in older adults are associated with worsened mental health outcomes following ICU discharge.Citation195–197 Patient-centered approaches, including palliative aspects, to care such as ICU diaries, reorientation, cognitive and physical stimulation, and family involvement may reduce these psychological symptoms in older ICU survivors by emphasizing patient needs and quality of life goals.Citation198
Overall Long-Term Outcomes
Prognosticating outcomes, including survival, is difficult across all patient groups, including older patients. Similar to other age groups, older adults (≥65 years) and very old (>85 years) adults with less comorbidities, lower severity of illness, and lower frailty index scores have shown better survival and function up to 1 year following hospital discharge.Citation199–203 Age in and of itself should not be a contraindication for ICU admission. The highest proportion of death occurs 6–12 months after hospital discharge, suggesting potential windows for optimum intervention and highlighting the impact of post-discharge impairments.Citation204 Future research should focus on identifying targets for improvement including post-ICU recovery clinics, rehabilitation, and therapeutic interventions to help identify modifiable factors and guide interventions to continue to improve outcomes among older ICU survivors.
Ethics and End-of-Life Discussions
Age alone is not a reliable predictor of mortality in the ICU, and other systems for assessing severity of illness and risk quantification should be validated and used to guide care-based decisions.Citation205–208 Acute illness, baseline comorbidities, and frailty should be interpreted alongside specific patient conditions and concerns to guide medical care, as illustrated in . Along with defining goals of care, code status and end-of-life discussions are valuable to ensure care teams and families are communicating and informed on risks associated with acute illnesses as well as life-sustaining interventions, regardless of patient status on admission.Citation209–211 Care teams including social workers, case managers, interpreters, religious members, and palliative care teams can be helpful in aiding and facilitating these conversations within hospital settings with these ancillary teams readily available. Life-prolonging interventions that may not improve quality of life and should be discussed to delineate patient’s goals of care may include mechanical ventilation, enteral feeding tube placement, TPN, and tracheostomy.
Figure 2 Conditions and concerns for older adults admitted to the intensive care unit. Older adults admitted to the intensive care unit present with a unique constellation of physiologic effects of aging that should be considered. In addition to their acute illness, baseline frailty/functional status, and geriatric syndromes/multimorbidities greatly impact outcomes. Additional influences that must be considered by the clinician includes presenting or iatrogenic polypharmacy and malnutrition as pose as potentially modifiable clinical factors. Physiologic conditions including cognitive impairment, alterations in metabolism, decreased muscle mass, immunosenescence, cardiac reserve, and an increased risk of lung injury are all common among older adults. Image is powered by Piktochart.
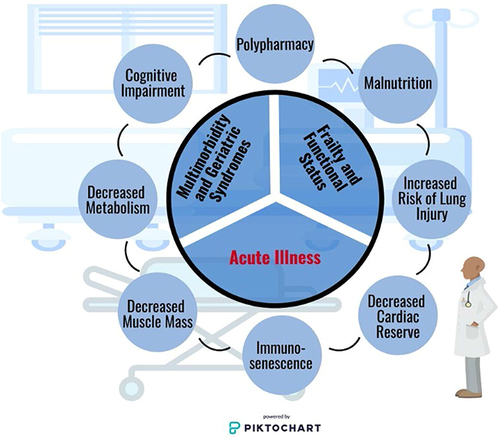
The ethical conflicts of withholding versus withdrawing care may be avoided by these early discussions.Citation212,Citation213 Training in goals of care discussions is not ubiquitous in the many pathways to becoming an intensivist, and specific education in leading these discussions is imperative as the ICU patient population ages. The 5M approach is a proposed holistic framework for non-geriatricians to use when approaching care of an older adult. The 5M’s: Mind, Mobility, Medications, Multicomplexity, and Matters Most incorporate unique characteristics of older patients in the ICU designed to remind clinicians to frame care goals around patient-specific values.Citation214 While this is good practice regardless of patient age, these conversations are particularly important in older critically ill adults who represent a higher proportion of mortalities attributed to withdrawal of care compared to younger cohorts.Citation215,Citation216 This may in part be due to findings that the majority of older adults reported valuing quality of life over life extension—frequently preferring a shorter life expectancy over dying within an ICU.Citation217–219 Conversations surrounding prolonged mechanical ventilation with the patient, if able to participate, and their family should include tracheostomy procedure details, likely need for prolonged hospitalization, and potential need for long-term acute care facility. Additionally, most older adults reported viewing immobility and ventilator-dependence as equal to or worse than death.Citation220
The American Thoracic Society has established five goals of clinician-family communication with the understanding that multiple strategies and organizations go into each and should be individualized to the situation and parties involved. These goals include (1) establishing a trusting relationship, (2) providing emotional support to families, (3) helping families understand diagnosis, prognosis, and treatment options, (4) allowing clinicians to understand the patient as a person, and (5) creating conditions for careful deliberation about difficult conditions.Citation221 Strategies used to achieve these goals are often individualized and learned from either experience, colleagues, coursework, or other training as it is generally not yet integrated into medical studies. Patient goals remaining a focus of care is an overarching theme in these efforts. While not unique to older adults, these discussions are imperative within older populations as they experience high rates of both in-hospital and long-term mortality, along with the constellation of syndromes included within post-intensive care syndrome (PICS) that frequently require long-term caregiver support.Citation222 Together, these add social complexities to already medically complex patients.
Summary and Conclusion
Increasing age of the population means there will be increased demand for specialized geriatric care across medical and surgical specialties, including the ICU. Aging affects multiple organ systems, and these changes must be considered when caring for these patients. The multiorgan physiologic effects of aging, in addition to other complexities associated with geriatric patients, such as frailty and pre-existing comorbidities, all must be considered and incorporated into a greater holistic approach to caring for these patients in the ICU.
Many ICU trials identify mortality as primary outcome; in geriatric patients, however, morbidity and quality of life are often more important than survival. More studies are needed in older ICU patients with morbidity outcomes that affect quality of life. Finally, there is a well-described need for geriatric specific education and further implementation of geriatric knowledge via multidisciplinary ICU teams as the population ages and ICU admissions increase.
Disclosure
CSB and CGH are consultants for and received fees from Sedana Medical. CGH has received research grant funding from Kohler Chemie. The authors declare no other competing interests for this work.
Additional information
Funding
References
- Mather MJ, Pollard KM. Aging in the United States. Popul Bull. 2015;70(2):1–18.
- Laake JH, Dybwik K, Flaatten HK, Fonneland IL, Kvale R, Strand K. Impact of the post-World War II generation on intensive care needs in Norway. Acta Anaesthesiol Scand. 2010;54(4):479–484. doi:10.1111/j.1399-6576.2009.02170.x
- Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in Hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49–55. doi:10.1097/BOT.0b013e3182a5a045
- Halvachizadeh S, Grobli L, Berk T, et al. The effect of geriatric comanagement (GC) in geriatric trauma patients treated in a level 1 trauma setting: a comparison of data before and after the implementation of a certified geriatric trauma Center. PLoS One. 2021;16(1):e0244554. doi:10.1371/journal.pone.0244554
- Heydari A, Sharifi M, Moghaddam AB. Challenges and Barriers to Providing Care to Older Adult Patients in the Intensive Care Unit: a Qualitative Research. Open Access Maced J Med Sci. 2019;7(21):3682–3690. doi:10.3889/oamjms.2019.846
- Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi:10.1111/j.1532-5415.2007.01156.x
- Tang HJ, Tang HJ, Hu FW, Chen CH. Changes of geriatric syndromes in older adults survived from Intensive Care Unit. Geriatr Nurs. 2017;38(3):219–224. doi:10.1016/j.gerinurse.2016.10.011
- Dolezalova J, Tothova V, Neugebauer J, Sadilek P. Impact of selected geriatric syndromes on the quality of life in the population aged 60 and older. Healthcare. 2021;9(6):657. doi:10.3390/healthcare9060657
- Guidet B, Vallet H, Boddaert J, et al. Caring for the critically ill patients over 80: a narrative review. Ann Intensive Care. 2018;8(1):114. doi:10.1186/s13613-018-0458-7
- Beil M, Flaatten H, Guidet B, et al. The management of multi-morbidity in elderly patients: ready yet for precision medicine in intensive care? Crit Care. 2021;25(1):330. doi:10.1186/s13054-021-03750-y
- Miller PE, Thomas A, Breen TJ, et al. Prevalence of Noncardiac multimorbidity in patients admitted to two cardiac intensive care units and their association with mortality. Am J Med. 2021;134(5):653–661 e5. doi:10.1016/j.amjmed.2020.09.035
- Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intensive Care Med. 2017;43(12):1820–1828. doi:10.1007/s00134-017-4940-8
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Sci Med Sci. 2001;56(3):M146–56. doi:10.1093/gerona/56.3.m146
- Haas LEM, Boumendil A, Flaatten H, et al. Frailty is associated with long-term outcome in patients with sepsis who are over 80 years old: results from an observational study in 241 European ICUs. Age Ageing. 2021;50(5):1719–1727. doi:10.1093/ageing/afab036
- Tang B, Green C, Yeoh AC, Husain F, Subramaniam A. Post-operative outcomes in older patients: a single-centre observational study. ANZ J Surg. 2018;88(5):421–427. doi:10.1111/ans.14433
- Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215(4):453–466. doi:10.1016/j.jamcollsurg.2012.06.017
- McDonald SR, Heflin MT, Whitson HE, et al. Association of Integrated Care Coordination With Postsurgical Outcomes in High-Risk Older Adults: the Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018;153(5):454–462. doi:10.1001/jamasurg.2017.5513
- Steffens NM, Tucholka JL, Nabozny MJ, Schmick AE, Brasel KJ, Schwarze ML. Engaging Patients, Health Care Professionals, and Community Members to Improve Preoperative Decision Making for Older Adults Facing High-Risk Surgery. JAMA Surg. 2016;151(10):938–945. doi:10.1001/jamasurg.2016.1308
- Kochunov P, Ramage AE, Lancaster JL, et al. Loss of cerebral white matter structural integrity tracks the gray matter metabolic decline in normal aging. Neuroimage. 2009;45(1):17–28. doi:10.1016/j.neuroimage.2008.11.010
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11(4):684–689. doi:10.1038/jcbfm.1991.121
- Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. doi:10.1016/j.jagp.2013.09.005
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol. 2009;94(3):317–321. doi:10.1113/expphysiol.2008.043356
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–352. doi:10.1016/j.neurobiolaging.2007.07.015
- Poels MM, Ikram MA, van der Lugt A, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42(3):656–661. doi:10.1161/STROKEAHA.110.607184
- Chung CP, Chou KH, Chen WT, et al. Cerebral microbleeds are associated with physical frailty: a community-based study. Neurobiol Aging. 2016;44:143–150. doi:10.1016/j.neurobiolaging.2016.04.025
- Takashima Y, Mori T, Hashimoto M, et al. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. J Stroke Cerebrovasc Dis. 2011;20(2):105–110. doi:10.1016/j.jstrokecerebrovasdis.2009.11.007
- Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–752. doi:10.1016/j.cger.2013.07.002
- Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. doi:10.1001/jama.286.21.2703
- Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi:10.1097/TA.0b013e31814b2c4d
- Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi:10.1007/s00134-001-1132-2
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi:10.1001/jama.291.14.1753
- Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi:10.1097/01.ccm.0000119429.16055.92
- Vasilevskis EE, Chandrasekhar R, Holtze CH, et al. The Cost of ICU Delirium and Coma in the Intensive Care Unit Patient. Med Care. 2018;56(10):890–897. doi:10.1097/MLR.0000000000000975
- Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi:10.1007/s00134-006-0399-8
- McLaughlin DC, Hartjes TM, Freeman WD. Sleep Deprivation in Neurointensive Care Unit Patients From Serial Neurological Checks: how Much Is Too Much? J Neurosci Nurs. 2018;50(4):205–210. doi:10.1097/JNN.0000000000000378
- Trompeo AC, Vidi Y, Locane MD, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77(6):604–612.
- Chang VA, Owens RL, LaBuzetta JN. Impact of sleep deprivation in the neurological intensive care unit: a narrative review. Neurocrit Care. 2020;32(2):596–608. doi:10.1007/s12028-019-00795-4
- Bihari S, Doug McEvoy R, Matheson E, Kim S, Woodman RJ, Bersten AD. Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307. doi:10.5664/jcsm.1920
- Stone JJ, Childs S, Smith LE, Battin M, Papadakos PJ, Huang JH. Hourly neurologic assessments for traumatic brain injury in the ICU. Neurol Res. 2014;36(2):164–169. doi:10.1179/1743132813Y.0000000285
- Kotfis K, Szylinska A, Listewnik M, et al. Early delirium after cardiac surgery: an analysis of incidence and risk factors in elderly (>/=65 years) and very elderly (>/=80 years) patients. Clin Interv Aging. 2018;13:1061–1070. doi:10.2147/CIA.S166909
- Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236. doi:10.1161/CIRCULATIONAHA.108.795260
- Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58(4):643–649. doi:10.1111/j.1532-5415.2010.02762.x
- Devlin JW, Skrobik Y, Gelinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/ Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi:10.1097/CCM.0000000000003299
- Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF Bundle in Critical Care. Crit Care Clin. 2017;33(2):225–243. doi:10.1016/j.ccc.2016.12.005
- Barnes-Daly MA, Phillips G, Ely EW. Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: implementing PAD Guidelines Via the ABCDEF Bundle in 6064 Patients. Crit Care Med. 2017;45(2):171–178. doi:10.1097/CCM.0000000000002149
- Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2019;47(1):3–14. doi:10.1097/CCM.0000000000003482
- Carrothers KM, Barr J, Spurlock B, Ridgely MS, Damberg CL, Ely EW. Contextual issues influencing implementation and outcomes associated with an integrated approach to managing pain, agitation, and delirium in adult ICUs. Crit Care Med. 2013;41(9 Suppl 1):S128–35. doi:10.1097/CCM.0b013e3182a2c2b1
- Almeida-Santos MA, Barreto-Filho JA, Oliveira JL, Reis FP, Da cunha oliveira CC, Sousa AC. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr. 2016;63:1–8. doi:10.1016/j.archger.2015.11.011
- Flaatten H, Beil M, Guidet B. Elderly Patients in the Intensive Care Unit. Semin Respir Crit Care Med. 2021;42(1):10–19. doi:10.1055/s-0040-1710571
- Damluji AA, Forman DE, van Diepen S, et al. Older Adults in the Cardiac Intensive Care Unit: factoring Geriatric Syndromes in the Management, Prognosis, and Process of Care: a Scientific Statement From the American Heart Association. Circulation. 2020;141(2):e6–e32. doi:10.1161/CIR.0000000000000741
- Lichtman JH, Spertus JA, Reid KJ, et al. Acute noncardiac conditions and in-hospital mortality in patients with acute myocardial infarction. Circulation. 2007;116(17):1925–1930. doi:10.1161/CIRCULATIONAHA.107.722090
- Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33(1):26–35b. doi:10.1093/eurheartj/ehr186
- Bruno VD, Chivasso P, Guida G, Vohra HA. Surgical repair of Stanford type A aortic dissection in elderly patients: a contemporary systematic review and meta-analysis. Ann Cardiothorac Surg. 2016;5(4):257–264. doi:10.21037/acs.2016.06.03
- Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997;45(8):968–974. doi:10.1111/j.1532-5415.1997.tb02968.x
- Gillum RF. Epidemiology of heart failure in the United States. Am Heart J. 1993;126(4):1042–1047. doi:10.1016/0002-8703(93)90738-u
- Arrigo M, Gayat E, Parenica J, et al. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail. 2017;19(2):201–208. doi:10.1002/ejhf.682
- Herbert JA, Valentine MS, Saravanan N, et al. Conservative fluid management prevents age-associated ventilator induced mortality. Exp Gerontol. 2016;81:101–109. doi:10.1016/j.exger.2016.05.005
- Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi:10.1161/CIRCOUTCOMES.111.962688
- Mirza M, Strunets A, Shen WK, Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28(4):555–573. doi:10.1016/j.cger.2012.08.005
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi:10.1161/01.CIR.0000140263.20897.42
- Artucio H, Pereira M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit Care Med. 1990;18(12):1383–1388. doi:10.1097/00003246-199012000-00015
- Shaver CM, Chen W, Janz DR, et al. Atrial Fibrillation Is an Independent Predictor of Mortality in Critically Ill Patients. Crit Care Med. 2015;43(10):2104–2111. doi:10.1097/CCM.0000000000001166
- Michelena HI, Ezekowitz MD. Approach to management of the patient over age 75 with atrial fibrillation. Cardiol Rev. 2000;8(1):9–16. doi:10.1097/00045415-200008010-00004
- Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother. 2013;47(10):1266–1271. doi:10.1177/1060028013500938
- Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi:10.1001/jama.295.1.50
- Zanders R, Druwe P, Van Den Noortgate N, Piers R. The outcome of in- and out-hospital cardiopulmonary arrest in the older population: a scoping review. Eur Geriatr Med. 2021;12(4):695–723. doi:10.1007/s41999-021-00454-y
- Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med. 2013;368(11):1019–1026. doi:10.1056/NEJMoa1200657
- Funada A, Goto Y, Maeda T, Teramoto R, Hayashi K, Yamagishi M. Improved Survival With Favorable Neurological Outcome in Elderly Individuals With Out-of-Hospital Cardiac Arrest in Japan- A Nationwide Observational Cohort Study. Circ J. 2016;80(5):1153–1162. doi:10.1253/circj.CJ-15-1285
- Holgersson J, Meyer MAS, Dankiewicz J, et al. Hypothermic versus Normothermic Temperature Control after Cardiac Arrest. NEJM Evid. 2022;1(11):EVIDoa2200137. doi:10.1056/EVIDoa2200137
- Wanner A. Clinical aspects of mucociliary transport. Am Rev Respir Dis. 1977;116(1):73–125. doi:10.1164/arrd.1977.116.1.73
- Chebotarev DF, Korkushko OV, Ivanov LA. Mechanisms of hypoxemia in the elderly. J Gerontol. 1974;29(4):393–400. doi:10.1093/geronj/29.4.393
- Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013;8:1489–1496. doi:10.2147/CIA.S51152
- Burrows B, Lebowitz MD, Camilli AE, Knudson RJ. Longitudinal changes in forced expiratory volume in one second in adults. Methodologic considerations and findings in healthy nonsmokers. Am Rev Respir Dis. 1986;133(6):974–980. doi:10.1164/arrd.1986.133.6.974
- Freitas FS, Ibiapina CC, Alvim CG, Britto RR, Parreira VF. Relationship between cough strength and functional level in elderly. Rev Bras Fisioter. 2010;14(6):470–476. doi:10.1590/S1413-35552010000600004
- Kobayashi H, Sekizawa K, Sasaki H. Aging effects on swallowing reflex. Chest. 1997;111(5):1466. doi:10.1378/chest.111.5.1466
- Katsumata U, Sekizawa K, Ebihara T, Sasaki H. Aging effects on cough reflex. Chest. 1995;107(1):290–291. doi:10.1378/chest.107.1.290-b
- Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29–33. doi:10.1007/s00408-011-9334-z
- Behrendt CE. Acute respiratory failure in the United States: incidence and 31-day survival. Chest. 2000;118(4):1100–1105. doi:10.1378/chest.118.4.1100
- Laporte L, Hermetet C, Jouan Y, et al. Ten-year trends in intensive care admissions for respiratory infections in the elderly. Ann Intensive Care. 2018;8(1):84. doi:10.1186/s13613-018-0430-6
- Nin N, Lorente JA, De paula M, et al. Aging increases the susceptibility to injurious mechanical ventilation. Intensive Care Med. 2008;34(5):923–931. doi:10.1007/s00134-007-0960-0
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi:10.1056/NEJMoa050333
- Guillon A, Hermetet C, Barker KA, et al. Long-term survival of elderly patients after intensive care unit admission for acute respiratory infection: a population-based, propensity score-matched cohort study. Crit Care. 2020;24(1):384. doi:10.1186/s13054-020-03100-4
- Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136(1):25–36. doi:10.7326/0003-4819-136-1-200201010-00007
- Lieberman D, Nachshon L, Miloslavsky O, Dvorkin V, Shimoni A, Lieberman D. How do older ventilated patients fare? A survival/functional analysis of 641 ventilations. J Crit Care. 2009;24(3):340–346. doi:10.1016/j.jcrc.2009.01.015
- Bansod S, Ahirwar AK, Sakarde A, et al. COVID-19 and geriatric population: from pathophysiology to clinical perspectives. Horm Mol Biol Clin Investig. 2021;42(1):87–98. doi:10.1515/hmbci-2020-0053
- Le Borgne P, Dellenbach Q, Alame K, et al. The Impact of Age on In-Hospital Mortality in Critically Ill COVID-19 Patients: a Retrospective and Multicenter Study. Diagnostics. 2022;12(3):666. doi:10.3390/diagnostics12030666
- Becerra-Munoz VM, Nunez-Gil IJ, Eid CM, et al. Clinical profile and predictors of in-hospital mortality among older patients hospitalised for COVID-19. Age Ageing. 2021;50(2):326–334. doi:10.1093/ageing/afaa258
- Bollwein J, Volkert D, Diekmann R, et al. Nutritional status according to the mini nutritional assessment (MNA(R)) and frailty in community dwelling older persons: a close relationship. J Nutr Health Aging. 2013;17(4):351–356. doi:10.1007/s12603-013-0009-8
- Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. doi:10.1016/j.clnu.2018.05.024
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
- Liu HT, Wu SC, Tsai CH, et al. Association between Geriatric Nutritional Risk Index and Mortality in Older Trauma Patients in the Intensive Care Unit. Nutrients. 2020;12(12):3861. doi:10.3390/nu12123861
- Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–122. doi:10.1016/s0899-9007(98)00171-3
- Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268. doi:10.1186/cc10546
- Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi:10.1016/j.clnu.2018.08.037
- McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: the gut in stress. Clin Nutr. 2018;37(1):19–36. doi:10.1016/j.clnu.2017.07.015
- Oterdoom LH, Ten Dam SM, de Groot SD, Arjaans W, van Bodegraven AA. Limited long-term survival after in-hospital intestinal failure requiring total parenteral nutrition. Am J Clin Nutr. 2014;100(4):1102–1107. doi:10.3945/ajcn.114.087015
- Tauchi H, Tsuboi K, Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17(2):87–97. doi:10.1159/000211811
- Goyal VK. Changes with age in the human kidney. Exp Gerontol. 1982;17(5):321–331. doi:10.1016/0531-5565(82)90032-8
- Brown WW, Davis BB, Spry LA, Wongsurawat N, Malone JD, Domoto DT. Aging and the kidney. Arch Intern Med. 1986;146(9):1790–1796. doi:10.1001/archinte.1986.00360210178026
- Gill J, Malyuk R, Djurdjev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group--a cautionary tale. Nephrol Dial Transplant. 2007;22(10):2894–2899. doi:10.1093/ndt/gfm289
- Epstein M, Hollenberg NK. Age as a determinant of renal sodium conservation in normal man. J Lab Clin Med. 1976;87(3):411–417.
- Meersch M, Schmidt C, Zarbock A. Perioperative Acute Kidney Injury: an Under-Recognized Problem. Anesth Analg. 2017;125(4):1223–1232. doi:10.1213/ANE.0000000000002369
- Chronopoulos A, Rosner MH, Cruz DN, Ronco C. Acute kidney injury in elderly intensive care patients: a review. Intensive Care Med. 2010;36(9):1454–1464. doi:10.1007/s00134-010-1957-7
- Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163(3):356–360. doi:10.1001/archinte.163.3.356
- Karakose F, Akkoyunlu ME, Erkoc R, et al. Geriatric patients with known acute kidney injury and normal renal function at the time of admittance to the intensive care unit/assessment of RRT requirement and mortality: retrospective case-control study. Wien Klin Wochenschr. 2015;127(7–8):290–296. doi:10.1007/s00508-014-0684-4
- Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi:10.1001/jama.2009.1322
- Mandel EI, Bernacki RE, Block SD. Serious Illness Conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854–863. doi:10.2215/CJN.05760516
- Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3Suppl 1):Svii,S1–305. doi:10.1053/j.ajkd.2015.12.014
- Foote C, Kotwal S, Gallagher M, Cass A, Brown M, Jardine M. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: a systematic review and meta-analysis. Nephrology. 2016;21(3):241–253. doi:10.1111/nep.12586
- Nasa P, Juneja D, Singh O, Dang R, Arora V. Severe sepsis and its impact on outcome in elderly and very elderly patients admitted in intensive care unit. J Intensive Care Med. 2012;27(3):179–183. doi:10.1177/0885066610397116
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi:10.1097/00003246-200107000-00002
- Heppner HJ, Yapan F, Wiedemann A. [Urosepsis in Geriatric Patients]. Urosepsis beim geriatrischen Patienten. Aktuelle Urol. 2016;47(1):54–59. doi:10.1055/s-0041-106184
- Rampelli S, Candela M, Turroni S, et al. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging. 2013;5(12):902–912. doi:10.18632/aging.100623
- Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5):e10667. doi:10.1371/journal.pone.0010667
- Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12(3):388–396. doi:10.1111/j.1447-0594.2012.00851.x
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
- Bolton CF, Gilbert JJ, Hahn AF, Sibbald WJ. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–1231. doi:10.1136/jnnp.47.11.1223
- Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29(9):1505–1514. doi:10.1007/s00134-003-1858-0
- Latronico N, Fenzi F, Recupero D, et al. Critical illness myopathy and neuropathy. Lancet. 1996;347(9015):1579–1582. doi:10.1016/s0140-6736(96)91074-0
- Fenzi F, Latronico N, Refatti N, Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol. 2003;106(1):75–82. doi:10.1007/s00401-003-0704-3
- Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32(2):140–163. doi:10.1002/mus.20304
- Novak KR, Nardelli P, Cope TC, et al. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest. 2009;119(5):1150–1158. doi:10.1172/jci36570
- Casaer MP, Langouche L, Coudyzer W, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41(10):2298–2309. doi:10.1097/CCM.0b013e31828cef02
- Kizilarslanoglu MC, Kuyumcu ME, Yesil Y, Halil M. Sarcopenia in critically ill patients. J Anesth. 2016;30(5):884–890. doi:10.1007/s00540-016-2211-4
- Paul JA, Whittington RA, Baldwin MR. Critical Illness and the Frailty Syndrome: mechanisms and Potential Therapeutic Targets. Anesth Analg. 2020;130(6):1545–1555. doi:10.1213/ane.0000000000004792
- Joyce PR, O’Dempsey R, Kirby G, Anstey C. A retrospective observational study of sarcopenia and outcomes in critically ill patients. Anaesth Intensive Care. 2020;48(3):229–235. doi:10.1177/0310057X20922234
- Moon SW, Kim SY, Choi JS, et al. Thoracic skeletal muscle quantification using computed tomography and prognosis of elderly ICU patients. Sci Rep. 2021;11(1):23461. doi:10.1038/s41598-021-02853-4
- Kaplan SJ, Pham TN, Arbabi S, et al. Association of Radiologic Indicators of Frailty With 1-Year Mortality in Older Trauma Patients: opportunistic Screening for Sarcopenia and Osteopenia. JAMA Surg. 2017;152(2):e164604. doi:10.1001/jamasurg.2016.4604
- Moisey LL, Mourtzakis M, Cotton BA, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. doi:10.1186/cc12901
- Weijs PJ, Looijaard WG, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12. doi:10.1186/cc13189
- Zuckerman J, Ades M, Mullie L, et al. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann Thorac Surg. 2017;103(5):1498–1504. doi:10.1016/j.athoracsur.2016.09.005
- De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi:10.1001/jama.288.22.2859
- de Letter MA, Schmitz PI, Visser LH, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–2286. doi:10.1097/00003246-200112000-00008
- Hermans G, Wilmer A, Meersseman W, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–489. doi:10.1164/rccm.200605-665OC
- Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi:10.1212/01.WNL.0000158442.08857.FC
- Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi:10.1097/CCM.0b013e318180b90e
- Maffiuletti NA, Roig M, Karatzanos E, Nanas S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med. 2013;11:137. doi:10.1186/1741-7015-11-137
- Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi:10.1001/jama.2013.278481
- Hermans G, Casaer MP, Clerckx B, et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med. 2013;1(8):621–629. doi:10.1016/S2213-2600(13)70183-8
- Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377. doi:10.1097/MAJ.0b013e31820ab4f6
- Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505. doi:10.1097/CCM.0b013e3181a38937
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi:10.1016/S0140-6736(09)60658-9
- Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–1388. doi:10.1016/S0140-6736(16)31637-3
- Wang YT, Lang JK, Haines KJ, Skinner EH, Haines TP. Physical Rehabilitation in the ICU: a Systematic Review and Meta-Analysis. Crit Care Med. 2022;50(3):375–388. doi:10.1097/CCM.0000000000005285
- Paton M, Chan S, Tipping CJ, et al. The Effect of Mobilization at 6 Months after Critical Illness — meta-Analysis. NEJM Evid;2022:EVIDoa2200234. doi:10.1056/EVIDoa2200234
- Puxty K, McLoone P, Quasim T, Sloan B, Kinsella J, Morrison DS. Characteristics and Outcomes of Surgical Patients With Solid Cancers Admitted to the Intensive Care Unit. JAMA Surg. 2018;153(9):834–840. doi:10.1001/jamasurg.2018.1571
- Niskanen M, Kari A, Halonen P. Five-year survival after intensive care--comparison of 12,180 patients with the general population. Finnish ICU Study Group. Crit Care Med. 1996;24(12):1962–1967. doi:10.1097/00003246-199612000-00006
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Marra A, Pandharipande PP, Girard TD, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit Care Med. 2018;46(9):1393–1401. doi:10.1097/CCM.0000000000003218
- Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018;22(1):310. doi:10.1186/s13054-018-2223-6
- Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi:10.1016/s2213-2600(14)70051-7
- Lum TY, Lin WC, Kane RL. Use of proxy respondents and accuracy of minimum data set assessments of activities of daily living. J Gerontol Series A. 2005;60(5):654–659. doi:10.1093/gerona/60.5.654
- Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med. 2006;32(11):1826–1831. doi:10.1007/s00134-006-0333-0
- Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA. 1993;269(24):3119–3123. doi:10.1001/jama.1993.03500240063027
- Kass JE, Castriotta RJ, Malakoff F. Intensive care unit outcome in the very elderly. Crit Care Med. 1992;20(12):1666–1671. doi:10.1097/00003246-199212000-00011
- Rockwood K, Noseworthy TW, Gibney RT, et al. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med. 1993;21(5):687–691. doi:10.1097/00003246-199305000-00011
- McHugh GJ, Havill JH, Armistead SH, Ullal RR, Fayers TM. Follow up of elderly patients after cardiac surgery and intensive care unit admission, 1991 to 1995. N Z Med J. 1997;110(1056):432–435.
- Montuclard L, Garrouste-Orgeas M, Timsit JF, Misset B, De Jonghe B, Carlet J. Outcome, functional autonomy, and quality of life of elderly patients with a long-term intensive care unit stay. Crit Care Med. 2000;28(10):3389–3395. doi:10.1097/00003246-200010000-00002
- Udekwu P, Gurkin B, Oller D, Lapio L, Bourbina J. Quality of life and functional level in elderly patients surviving surgical intensive care. J Am Coll Surg. 2001;193(3):245–249. doi:10.1016/s1072-7515(01)00994-2
- Vazquez Mata G, Rivera Fernandez R, Gonzalez Carmona A, et al. Factors related to quality of life 12 months after discharge from an intensive care unit. Crit Care Med. 1992;20(9):1257–1262. doi:10.1097/00003246-199209000-00012
- Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–529. doi:10.1001/jamainternmed.2014.7889
- Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183(8):1037–1042. doi:10.1164/rccm.201002-0301OC
- Hennessy D, Juzwishin K, Yergens D, Noseworthy T, Doig C. Outcomes of elderly survivors of intensive care: a review of the literature. Chest. 2005;127(5):1764–1774. doi:10.1378/chest.127.5.1764
- Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. 2015;43(6):1265–1275. doi:10.1097/CCM.0000000000000924
- Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi:10.1097/01.Ccm.0000251130.69568.87
- Honarmand K, Lalli RS, Priestap F, et al. Natural History of Cognitive Impairment in Critical Illness Survivors. A Systematic Review. Am J Respir Crit Care Med. 2020;202(2):193–201. doi:10.1164/rccm.201904-0816CI
- Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Estrup S, Kjer CKW, Vilhelmsen F, Poulsen LM, Gøgenur I, Mathiesen O. Cognitive Function 3 and 12 Months After ICU Discharge-A Prospective Cohort Study. Crit Care Med. 2018;46(12):e1121–e1127. doi:10.1097/ccm.0000000000003391
- Wolters AE, Slooter AJ, van der Kooi AW, van Dijk D. Cognitive impairment after intensive care unit admission: a systematic review. Intensive Care Med. 2013;39(3):376–386. doi:10.1007/s00134-012-2784-9
- Wilson ME, Barwise A, Heise KJ, et al. Long-Term Return to Functional Baseline After Mechanical Ventilation in the ICU. Crit Care Med. 2018;46(4):562–569. doi:10.1097/CCM.0000000000002927
- Rengel KF, Hayhurst CJ, Pandharipande PP, Hughes CG. Long-term Cognitive and Functional Impairments After Critical Illness. Anesth Analg. 2019;128(4):772–780. doi:10.1213/ane.0000000000004066
- Hajeb M, Singh TD, Sakusic A, Graff-Radford J, Gajic O, Rabinstein AA. Functional outcome after critical illness in older patients: a population-based study. Neurol Res. 2021;43(2):103–109. doi:10.1080/01616412.2020.1831302
- Wolters AE, van Dijk D, Pasma W, et al. Long-term outcome of delirium during intensive care unit stay in survivors of critical illness: a prospective cohort study. Crit Care. 2014;18(3):R125. doi:10.1186/cc13929
- Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. doi:10.1097/CCM.0b013e318250acc0
- Hope AA, Morrison RS, Du Q, Wallenstein S, Nelson JE. Risk factors for long-term brain dysfunction after chronic critical illness. Ann Am Thorac Soc. 2013;10(4):315–323. doi:10.1513/AnnalsATS.201211-099OC
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. doi:10.1093/ageing/afl005
- Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev. 2007;(2):Cd005563. doi:10.1002/14651858.CD005563.pub2
- Abraha I, Trotta F, Rimland JM, et al. Efficacy of Non-Pharmacological Interventions to Prevent and Treat Delirium in Older Patients: a Systematic Overview. The SENATOR project ONTOP Series. PLoS One. 2015;10(6):e0123090. doi:10.1371/journal.pone.0123090
- Zhang H, Lu Y, Liu M, et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17(2):R47. doi:10.1186/cc12566
- Elías MN, Munro CL, Liang Z. Daytime-to-Nighttime Sleep Ratios and Cognitive Impairment in Older Intensive Care Unit Survivors. Am J Crit Care. 2021;30(2):e40–e47. doi:10.4037/ajcc2021221
- Elías MN, Munro CL, Liang Z. Sleep Quality Associated With Motor Function Among Older Adult Survivors of Critical Illness. Nurs Res. 2020;69(4):322–328. doi:10.1097/nnr.0000000000000418
- By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi:10.1111/jgs.13702
- Tomichek JE, Stollings JL, Pandharipande PP, Chandrasekhar R, Ely EW, Girard TD. Antipsychotic prescribing patterns during and after critical illness: a prospective cohort study. Crit Care. 2016;20(1):378. doi:10.1186/s13054-016-1557-1
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134. doi:10.1111/jgs.12329
- Marshall J, Herzig SJ, Howell MD, et al. Antipsychotic utilization in the intensive care unit and in transitions of care. J Crit Care. 2016;33:119–124. doi:10.1016/j.jcrc.2015.12.017
- Kouladjian L, Gnjidic D, Chen TF, Mangoni AA, Hilmer SN. Drug Burden Index in older adults: theoretical and practical issues. Clin Interv Aging. 2014;9:1503–1515. doi:10.2147/CIA.S66660
- Harrison SL, Kouladjian O’Donnell L, Bradley CE, et al. Associations between the Drug Burden Index, Potentially Inappropriate Medications and Quality of Life in Residential Aged Care. Drugs Aging. 2018;35(1):83–91. doi:10.1007/s40266-017-0513-3
- Wright RM, Roumani YF, Boudreau R, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57(2):243–250. doi:10.1111/j.1532-5415.2008.02127.x
- Kowalczyk M, Nestorowicz A, Fijałkowska A, Kwiatosz-Muc M. Emotional sequelae among survivors of critical illness: a long-term retrospective study. Eur J Anaesthesiol. 2013;30(3):111–118. doi:10.1097/EJA.0b013e32835dcc45
- Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–1234. doi:10.1097/01.ccm.0000059996.30263.94
- Wewalka M, Warszawska J, Strunz V, et al. Depression as an independent risk factor for mortality in critically ill patients. Psychosom Med. 2015;77(2):106–113. doi:10.1097/psy.0000000000000137
- Vest MT, Murphy TE, Araujo KL, Pisani MA. Disability in activities of daily living, depression, and quality of life among older medical ICU survivors: a prospective cohort study. Health Qual Life Outcomes. 2011;9:9. doi:10.1186/1477-7525-9-9
- Nordness MF, Bipin Patel M, Erickson CR, et al. Depression predicts long-term cognitive impairment in survivors of critical illness. J Trauma Acute Care Surg. 2021;90(1):79–86. doi:10.1097/ta.0000000000002955
- Brown KN, Soo A, Faris P, Patten SB, Fiest KM, Stelfox HT. Association between delirium in the intensive care unit and subsequent neuropsychiatric disorders. Crit Care. 2020;24(1):476. doi:10.1186/s13054-020-03193-x
- Jain S, Murphy TE, O’Leary JR, Leo-Summers L, Ferrante LE. Association Between Socioeconomic Disadvantage and Decline in Function, Cognition, and Mental Health After Critical Illness Among Older Adults : a Cohort Study. Ann Intern Med. 2022;175(5):644–655. doi:10.7326/m21-3086
- Falvey JR, Cohen AB, O’Leary JR, Leo-Summers L, Murphy TE, Ferrante LE. Association of Social Isolation With Disability Burden and 1-Year Mortality Among Older Adults With Critical Illness. JAMA Intern Med. 2021;181(11):1433–1439. doi:10.1001/jamainternmed.2021.5022
- Falvey JR, Murphy TE, Leo-Summers L, Gill TM, Ferrante LE. Neighborhood Socioeconomic Disadvantage and Disability After Critical Illness. Crit Care Med. 2021;50:733–741. doi:10.1097/ccm.0000000000005364
- Andersen SK, Vincent G, Butler RA, et al. ProPACC: protocol for a Trial of Integrated Specialty Palliative Care for Critically Ill Older Adults. J Pain Symptom Manage. 2022;63(6):e601–e610. doi:10.1016/j.jpainsymman.2022.02.344
- Heyland DK, Garland A, Bagshaw SM, et al. Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med. 2015;41(11):1911–1920. doi:10.1007/s00134-015-4028-2
- Michel P, Fadel F, Ehrmann S, Plantefève G, Gelée B. Prognosis of Very Elderly Patients after Intensive Care. J Clin Med. 2022;11(4):897. doi:10.3390/jcm11040897
- Pietiläinen L, Bäcklund M, Hästbacka J, Reinikainen M. Premorbid functional status as an outcome predictor in intensive care patients aged over 85 years. BMC Geriatr. 2022;22(1):38. doi:10.1186/s12877-021-02746-1
- McPeake J, Quasim T, Henderson P, et al. Multimorbidity and Its Relationship With Long-Term Outcomes After Critical Care Discharge: a Prospective Cohort Study. Chest. 2021;160(5):1681–1692. doi:10.1016/j.chest.2021.05.069
- Kaushik R, Ferrante LE. Long-term recovery after critical illness in older adults. Curr Opin Crit Care. 2022;28(5):572–580. doi:10.1097/mcc.0000000000000981
- Baldwin MR. Measuring and predicting long-term outcomes in older survivors of critical illness. Minerva Anestesiol. 2015;81(6):650–661.
- Boumendil A, Angus DC, Guitonneau AL, et al. Variability of intensive care admission decisions for the very elderly. PLoS One. 2012;7(4):e34387. doi:10.1371/journal.pone.0034387
- Guidet B, Leblanc G, Simon T, et al. Effect of Systematic Intensive Care Unit Triage on Long-term Mortality Among Critically Ill Elderly Patients in France: a Randomized Clinical Trial. JAMA. 2017;318(15):1450–1459. doi:10.1001/jama.2017.13889
- Vallet H, Schwarz GL, Flaatten H, De lange DW, Guidet B, Dechartres A. Mortality of Older Patients Admitted to an ICU: a Systematic Review. Crit Care Med. 2021;49(2):324–334. doi:10.1097/CCM.0000000000004772
- Minne L, Ludikhuize J, de Jonge E, de Rooij S, Abu-Hanna A. Prognostic models for predicting mortality in elderly ICU patients: a systematic review. Intensive Care Med. 2011;37(8):1258–1268. doi:10.1007/s00134-011-2265-6
- Brummel NE, Ferrante LE. Integrating Geriatric Principles into Critical Care Medicine: the Time Is Now. Ann Am Thorac Soc. 2018;15(5):518–522. doi:10.1513/AnnalsATS.201710-793IP
- Scheunemann LP, Ernecoff NC, Buddadhumaruk P, et al. Clinician-Family Communication About Patients’ Values and Preferences in Intensive Care Units. JAMA Intern Med. 2019;179(5):676–684. doi:10.1001/jamainternmed.2019.0027
- Shah RD, Rasinski KA, Alexander GC. The Influence of Surrogate Decision Makers on Clinical Decision Making for Critically Ill Adults. J Intensive Care Med. 2015;30(5):278–285. doi:10.1177/0885066613516597
- Vincent JL, Creteur J. Appropriate care for the elderly in the ICU. J Intern Med. 2022;291(4):458–468. doi:10.1111/joim.13371
- Ariyo K, Canestrini S, David AS, Ruck Keene A, Wolfrum S, Owen G. Quality of life in elderly ICU survivors before the COVID-19 pandemic: a systematic review and meta-analysis of cohort studies. BMJ Open. 2021;11(10):e045086. doi:10.1136/bmjopen-2020-045086
- Geen O, Perrella A, Rochwerg B, Wang XM. Applying the geriatric 5Ms in critical care: the ICU-5Ms. Can J Anaesth. 2022;69(9):1080–1085. doi:10.1007/s12630-022-02270-9
- Leblanc G, Boumendil A, Guidet B. Ten things to know about critically ill elderly patients. Intensive Care Med. 2017;43(2):217–219. doi:10.1007/s00134-016-4477-2
- Jacelon CS, Henneman EA. Dignity in the older critically ill adult: the family member’s perspective. Heart Lung. 2014;43(5):432–436. doi:10.1016/j.hrtlng.2014.06.001
- Flaatten H, Beil M, Guidet B. Prognostication in older ICU patients: mission impossible? Br J Anaesth. 2020;125(5):655–657. doi:10.1016/j.bja.2020.08.005
- Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284(19):2476–2482. doi:10.1001/jama.284.19.2476
- Rubin EB, Buehler A, Halpern SD. Seriously Ill Patients’ Willingness to Trade Survival Time to Avoid High Treatment Intensity at the End of Life. JAMA Intern Med. 2020;180(6):907–909. doi:10.1001/jamainternmed.2020.0681
- Rubin EB, Buehler AE, Halpern SD. States Worse Than Death Among Hospitalized Patients With Serious Illnesses. JAMA Intern Med. 2016;176(10):1557–1559. doi:10.1001/jamainternmed.2016.4362
- Seaman JB, Arnold RM, Scheunemann LP, White DB. An Integrated Framework for Effective and Efficient Communication with Families in the Adult Intensive Care Unit. Ann Am Thorac Soc. 2017;14(6):1015–1020. doi:10.1513/AnnalsATS.201612-965OI
- Vallet H, Moïsi L, Thomas C, Guidet B, Boumendil A. Acute critically ill elderly patients: what about long term caregiver burden? J Crit Care. 2019;54:180–184. doi:10.1016/j.jcrc.2019.08.028
