Abstract
Many epidemiological studies have shown that the incidence of immune thrombocytopenia (ITP) increases after age 60 years and peaks in patients over age 80 years. Therefore, ITP is a concern for physicians taking care of older patients, especially regarding its diagnosis and management. The diagnostic work-up should exclude other causes of thrombocytopenia and secondary ITP, including myelodysplastic syndrome and drug-induced ITP. The treatment decision is influenced by an increased risk of bleeding, infectious diseases and thrombosis in this population and should take into account comorbidities and concomitant medications such as anticoagulant drugs. First-line treatment is based on short corticosteroids courses and intravenous immunoglobulin, which should be reserved for patients with more severe bleeding complications, with their higher risk of toxic effects as compared with younger patients. Second-line treatment should be tailored to the patient’s history, comorbidities and preferences. Preferred second-line treatments are thrombopoietin receptor agonists for most groups and guidelines given their good efficacy/tolerance ratio, but the thrombotic risk is increased in older people. Other second-line options that can be good alternatives depending on the clinical context include rituximab, dapsone, fostamatinib or immunosuppressive drugs. Splenectomy is less often performed but remains an option for fit patients with chronic refractory disease. Emerging treatments such as Syk or Bruton tyrosine kinase inhibitors and FcRn antagonists are becoming available for ITP and may modify the treatment algorithm in the near future. The aim of this review is to describe the particularities of the diagnosis and treatment of ITP in older people, including the response and tolerance to the currently available drugs. We also discuss some situations related to co-morbidities that can frequently lead to adapt the management strategy in older patients.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by antibody-mediated platelet destruction and impaired platelet production resulting in bleeding symptoms.Citation1 Although it can affect individuals of all age categories, the disease incidence peaks in older patients.Citation2,Citation3 Hence, combined with the worldwide trend in the ageing of the population, ITP is of particular interest for physicians taking care of older patients. ITP management is challenging in older versus younger patients given the frequent comorbidities and increased risk of bleeding, infections and thrombosis of the former group.Citation4,Citation5
A growing number of studies focusing on older patients with ITP are now available, as are new treatments for ITP. In this review, we provide an update on the diagnosis, prognosis and treatment of older patients with ITP in light of these recent data. We also discuss some situations related to co-morbidities that can frequently lead to adapt the management strategy in older patients. To date, no prospective study focusing on this population has been conducted and therefore most recommendations presented here are not evidence-based but rather extrapolated from observational and retrospective studies as well as our own experience.
ITP Diagnosis and Epidemiology
Epidemiology
Several large epidemiological studies have shown that the ITP epidemiology is influenced by sex and age,Citation2,Citation3,Citation6 with peaks in young women and old men. ITP is also a geriatric disease, with incidence rates reaching 23.9/100 000 in men >80 years old in the United KingdomCitation3 and 9/100 000 person-years in men >75 years old in France,Citation2 that is, an approximately 10-fold increase as compared with men aged 30 to 39 years in both studies. In a recent French study including 541 adults with incident ITP included in a prospective national registry, 251 (46%) were ≥65 years and among them, 47% were ≥80 years. In this later group of very old patients, 37.9% were exposed to antiplatelet drugs and 18.4% to anticoagulants.Citation7
Diagnosis
According to international guidelines, primary ITP is defined by isolated thrombocytopenia <100 x 109/L of an autoimmune origin in the absence of any underlying cause or disorder.Citation8 ITP usually presents as isolated thrombocytopenia, and the diagnostic work-up mainly focuses on eliminating other etiologies because of no gold-standard diagnostic test. Secondary ITP refers to immune thrombocytopenia associated with other conditions (eg, hematological malignancies, systemic lupus, primary immunodeficiencies) at diagnosis. The main differential diagnoses of thrombocytopenia and causes of secondary ITP are shown in .
Table 1 Other Main Causes of Thrombocytopenia
Patient history, physical examination, complete blood count and peripheral blood smear examination are the cornerstone of ITP diagnosis. In addition, some exams of interest to identify particular situations or secondary ITP include serologies for HIV, hepatitis C and B virus, antinuclear antibodies and protein serum electrophoresis. Other blood tests should be oriented to the clinical context. Because of lack of sensitivity and specificity, the search for antibodies against platelet antigens is not recommended in routine practice.Citation8,Citation9
However, the diagnostic approach proposed in the international guidelines is a global approach that addresses adults without taking into account age. In older patients, some points deserve consideration in the ITP diagnostic work-up. First, drug-induced ITP should be considered because exposure to treatments increases with age. Several mechanisms can be responsible for drug-induced ITP, but most commonly, platelet destruction is mediated by a drug-dependent antibody.Citation10 Patients usually present profound thrombocytopenia and bleeding symptoms soon (in a few hours to a few days) after drug introduction. Because in vitro confirmation of drug-induced ITP is usually unavailable, the diagnosis relies on a complete history of drug intake, the relative chronology with thrombocytopenia, and spontaneous recovery upon drug discontinuation. Many drugs but also vaccines have been implicated in drug-induced ITP and updated reviews have been published recently.Citation10–12
Interest of Bone-Marrow Evaluation (BME) in Older Patients
Another differential diagnosis of thrombocytopenia in older patients is myelodysplastic syndrome (MDS) affecting megakaryocyte lineage. Although most MDS cases exhibit associated thrombocytopenia (65%),Citation13 isolated thrombocytopenia is less frequent (12% of cases).Citation14 Therefore, the presence of other cytopenia or macrocytosis on complete blood count and signs of dyserythropoiesis and/or dysgranulopoiesis on peripheral blood smear examination should prompt a BME to rule out a myelodysplastic syndrome. Notably, patients with early ITP can present some abnormalities in megakaryocyte morphology as well as some reticulin fiber deposits on bone-marrow biopsy.Citation15,Citation16 The coexistence of MDS and ITP has been described.Citation17
We suggest to use a pragmatic approach and to propose a short course of corticosteroids or intravenous immunoglobulin (IVIg) when suspecting ITP. Although not validated in prospective studies, a response to these treatments suggests an immunological contribution to thrombocytopenia but does not rule out MDS.Citation5
Performing systematic BME in patients >60 years presenting isolated thrombocytopenia has been debated in the past years. However, growing evidence suggests that the rate of abnormality on bone-marrow smear in this context is very low. In the prospective French registry, only one in 197 (0.8%) patients with ITP and older than 60 years had an abnormal bone-marrow smear result.Citation18 In a recent retrospective study of 324 patients with relapsed/refractory ITP, 56% had a BME, which resulted in eight other diagnoses (8% of tested patients). Of note, age was not associated with abnormal findings on BME in this study.Citation19
Current international guidelines recommend BME only in patients with systemic symptoms, abnormal signs, or a suspected different diagnosis independent of age. BME can also be performed before splenectomy or in case of treatment failure.Citation8,Citation20 If performed in older patients to rule out MDS, a cytogenetic study and next-generation sequencing should be performed at the same time; flow cytometry analysis and bone-marrow biopsy should be performed in case of suspected lymphoma.Citation8
ITP Prognosis: What Particularities in Older Patients?
Given the increased frequency of ITP in older patients, interest has been growing in determining whether the disease course, severity and complications are similar to those of younger patients. Infectious and thrombotic risk are also relevant when considering therapeutic options, especially in older patients.
Disease Course
ITP can be classified according to disease duration as newly diagnosed (0–3 months), persistent (3–12 months), or chronic (>12 months).Citation8 In adults, newly diagnosed ITP evolves toward chronic disease in about 70% of cases. Chronic ITP has a very low probability of spontaneous recovery.Citation21 Whether older patients diagnosed with ITP are at increased risk of chronic disease is unknown.
Bleeding Risk
Data from the literature are conflicting about bleeding risk in older patients. Some studies did not find an association between age and bleeding,Citation22,Citation23 and others found more bleeding in older patients with similar platelet counts.Citation2,Citation24–31 In a recent series comparing 311 patients aged <65 years to 154 aged ≥65 years, Palandri et al found no differences in platelet count at diagnosis or in bleeding symptoms but more grade 3/4 bleeding and thrombosis in older patients, with a first-line therapy started for higher platelet counts.Citation32 Several other factors such as comorbidities and drug exposure could influence the bleeding risk, particularly in older patients. Comorbidities such as cardiovascular diseases, kidney or liver failure, or gastrointestinal diseases increase in number with age.Citation4,Citation7,Citation29 In a French cross-sectional study, age was significantly associated with severe bleeding on univariate analysis but not after adjustment for other covariates, including anticoagulant drugs. In this study, platelet count, female sex and exposure to non-steroidal anti-inflammatory drugs were associated with risk of any bleeding, and exposure to anticoagulant drugs was a major risk factor for severe bleeding.Citation33
The definition of “older” has also varied among studies and may explain some discrepancies. In a recent study from our group, the disease course of very old patients (aged ≥80 years) was compared to those of old patients (aged 65–79 years). Although platelet counts at ITP diagnosis were similar, severe bleeding and mortality were more frequent in very old patients although not significantly. Comorbidities were also more frequent, and exposure to anticoagulant drugs was strongly associated with severe bleeding.Citation7
Overall, these data suggest that platelet count and bleeding symptoms at ITP onset are probably independent of age as well as disease course but that older patients probably are at increased risk of severe bleeding, especially when exposed to anticoagulant drugs. In other words, the care of a patient over age 65 years without co-morbidities and not exposed to anticoagulants can probably be modelled on that of younger patients. However, a more aggressive strategy and a higher platelet threshold to decide to treat should probably be considered in very old patients, especially in the presence of a co-morbidity and exposure to anticoagulants because of greater risk of bleeding complications.
Thrombosis and Infection
Multiple factors can contribute to arterial and venous thrombosis observed during the ITP course.Citation34 Paradoxically, ITP could be a prothrombotic disease, and thrombosis can occur even with low platelet counts.Citation35,Citation36 Thrombotic risk also increases with age,Citation37,Citation38 and cardiovascular risk factors are more frequent in older patients. Some ITP treatments that could favor thrombosis include IVIg,Citation39 splenectomyCitation40 or thrombopoietin receptor agonists (TPO-RAs).Citation41–45 In this context, the addition of treatment-related prothrombotic factors to patient- and ITP-related prothrombotic factors should be weighed carefully.
Older patients may be more sensitive to infections than younger patients. Infectious risk is also increased in ITP probably because of immunosuppressive drug use and splenectomy,Citation50 and contribute to mortality.Citation51,Citation52 This is important to consider for ITP management and treatment decisions in older patients with a possibly increased risk of infection observed with some treatments such as corticosteroids, immunosuppressive drugs and rituximab.
ITP Treatments: What Tolerance and What Efficacy in Older Patients?
Treatment Decision
The treatment decision is mainly based on bleeding symptoms and platelet count, although other factors important to consider include disease duration, other medications, comorbidities, expected tolerance, accessibility of care, quality of life and patient expectations. Age but more importantly frailty also influence the treatment choice, but in any case, treatment should always be tailored to the patient, and guidelines highlight that ITP treatment should be personalized. This is especially true for older people because for the same number of platelets, many individual factors can influence the decision. Also, patients’ concerns and fears and disease burden are often misjudged by the physician.Citation53,Citation54 Therefore, the decision to treat must always be shared with the patient, as it has been shown in other hematologic diseases.Citation55 If the patient has high functional impairment, a fair discussion with the caregivers outlining the benefits, limitations and side effects of treatment is essential.
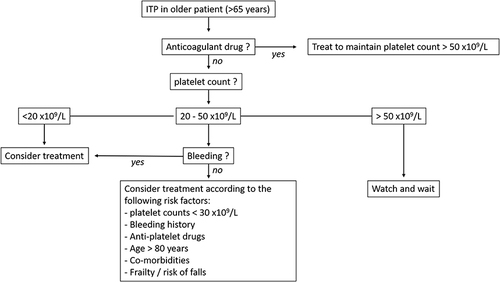
International guidelines have long proposed a threshold of 30 x 109/L platelets to indicate treatment, even in a patient without bleeding.Citation56 We now have considerable evidence to suggest that this threshold can be lowered to 20 x 109/L.Citation33 Because bleeding risk clearly increases with platelet count <20 x 109/L, particularly in older patients (see above), the recommendation is to treat older patients with platelet count below this threshold.Citation8 However, bleeding symptoms secondary to thrombocytopenia are unusual with platelet count >50 x 109/L, and a watch-and-wait approach should be proposed in this case unless a higher platelet count is required for an intervention.Citation8
When platelet counts are 20 to 50 x 109/L, no evidence-based medicine is available and the treatment decision should be based on the presence of additional risk factors of bleeding such as a history of bleeding; co-morbidities (eg, severe hypertension, renal or liver failure, peptic ulcer); age >80 years and frailty, including risk of falls; and drug exposure. In this setting, a watch-and-wait approach is easier when a rapid response to corticosteroids or IVIg is known from previous treatment courses, allowing for safe management of the potential occurrence of a bleeding complication.
When the patient is taking drugs interfering with hemostasis, the referring physician should be contacted to assess the risk/benefit balance. These drugs should not eventually be discontinued when indicated (except transiently when platelet counts are very low [ie, <10 x 109/L] or in case of bleeding) because a low platelet count does not preclude thrombotic risk.Citation35,Citation36 ITP treatment should be considered to maintain a safe platelet count. Anticoagulant drugs have been associated with increased risk of severe bleeding, and some experts and epidemiological studies argue that maintaining the platelet count above 50 x 109/L is safer.Citation7,Citation33,Citation57 Although anti-platelet drugs interfere with primary hemostasis, the bleeding risk seems lower with these agents,Citation7,Citation33 perhaps because platelet turnover is increased in ITP. Therefore, maintaining a higher platelet count is probably safer when the patient takes anti-platelet drugs, although the optimal threshold is unknown, but a threshold of 30 x 109/L could probably be tolerated.
If a watch-and-wait approach is favored, patients should have a close monitoring of platelet count and bleeding symptoms. Our current strategy for ITP treatment decisions in older patients is summarized in .
Figure 1 Proposed algorithm for treatment decision. In patients with immune thrombocytopenia (ITP) without anticoagulant drugs and with platelet counts of 20 to 50 x109/L, treatment can be delayed if there are no bleeding risk factors but should be considered if there are more than 2 bleeding risk factors.
First-Line Treatments
Corticosteroids
ITP first-line therapy relies on a short course of corticosteroids.Citation8 Long-term corticosteroids should be avoided in most cases because they are poorly effective in maintaining remission at a low dose and do not affect the ITP natural history while generating side effects.Citation58,Citation59 Prednisone 1 mg/kg for 3 weeks or dexamethasone 40 mg/day for 4 days (repeated up to three times if necessary) can be used. A number of controlled studies have suggested a faster and greater platelet response with dexamethasone over prednisolone, but the rate of long-term response was similar in both groups.Citation60,Citation61
Age does not affect the response to corticosteroids, but this treatment generates more adverse events in older patients.Citation27,Citation29 Tolerance remains acceptable with short courses of corticosteroids: in a retrospective study of 440 patients aged >60 years who received corticosteroids (prednisone or dexamethasone) as first-line ITP therapy, only 16 (3.7%) discontinued treatment because of toxic effects (mainly diabetes and psychiatric symptoms).Citation62 The risk of infection is also increased with corticosteroids but mainly with prolonged treatment, which should be strongly avoided in ITP.Citation63
Several studies have suggested lower than standard corticosteroids dose regimens as an alternative strategy.Citation26,Citation64 We frequently use a reduced dexamethasone dose of 20 mg/day (instead of 40 mg/day) in frail patients. Although particularly relevant in older patients to limit toxic effects, this dose remains to be validated in this particular population.
Intravenous Immunoglobulin (IVIg)
A combined course of IVIg and corticosteroids has been found more effective than corticosteroids alone for time to response, platelet count and response duration.Citation65 Therefore, IVIg is a treatment of choice for patients with a high bleeding score,Citation66 although its cost, availability, transient response (less than 1 month) and potential adverse events limits its more general use.Citation8 The recommended dose is 1 g/kg/day, which can be repeated on day 2 in case of severe bleeding with a life-threatening situation or on day 3 with less severe bleeding and in case of lack of response after the first infusion.Citation67 Side effects include anaphylaxis, hemolytic reactions, and minor symptoms such as headache, nausea, or fever. Other adverse events such as renal failure, fluid overload and thrombosis seem more frequent in older patients and justify a lower daily dosage (0.5 g/kg days 1–4 or 0.4 g/kg days 1–5), with careful monitoring of hydration and renal function.Citation39,Citation68–70
Anti-Rho(D) Drugs
Intravenous anti-Rho(D) drugs are not licensed in Europe but have been proposed to treat Rho(D)-positive patients who have not undergone splenectomy, with high response rates at the cost of rare but life-threatening side effects.Citation71 Fatal disseminated intravascular coagulation associated with acute hemoglobinemia or hemoglobinuria after Rho(D) intravenous administration has been reported, particularly in older people in whom this treatment should be avoided.Citation72
Management of Life-Threatening Bleeding and Refractory Disease
In case of life-threatening bleeding situations such as visceral hemorrhage with decreased hemoglobin level or intracranial bleeding, the addition of platelet transfusions to IVIg and dexamethasone should be administered with a proven beneficial hemostatic effect.Citation73,Citation74 Drugs interfering with hemostasis should be stopped immediately. In patients with active bleeding and with disease refractory to first-line therapy, vinca alkaloids such as vinblastin (weekly injection of 5 mg/m² with a maximum of 10 mg) rather than vincristine due to better digestive tolerance can provide rapid response but should not be used for long-term treatment because of neurological toxic effects, particularly in older people.Citation8 TPO-RAs can also be used as an off-label salvage therapy in our experience but with a risk of thrombosis.Citation75 Until these encouraging results are confirmed by other groups, the use of high-dose TPO-RAs should be reserved for patients with very severe bleeding resistant to total therapy (IVIg + high-dose corticosteroids + multiple and repeated platelet transfusions).
Second-Line Treatments
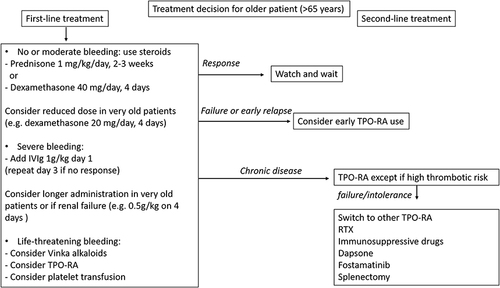
Most adults with ITP have a chronic disease course. When disease is refractory to or relapses after first-line treatment, an increasing number of therapeutic options are available for second-line treatment. Each has an expected pattern of response, but to date, there are no accurate predictors to help select the ITP treatment. The treatment choice also relies on caveats specific to each molecule that are described below and in . Co-morbidities and patient preference are also important in therapeutic decisions. According to the context, treatment should aim to avoid bleeding by maintaining a safe platelet count while limiting short- and long-term toxic effects. An overview of our current treatment strategy is proposed in .
Table 2 Factors influencing second-line treatment choices for ITP
Figure 2 Proposed therapeutic strategy for ITP in older patients.
Thrombopoietin Receptor Agonists (TPO-RAs)
TPO-RAs are drugs increasing platelet production without immunosuppression that have a demonstrated good efficacy/safety profile, which explains why they have become the preferred second-line treatment in older patients with ITP by most groups and guidelines ().Citation4,Citation5,Citation46 Two TPO-RAs, namely eltrombopag and romiplostim, are currently licensed for chronic ITP in Europe, and a third one, avatrombopag, is also available in some countries. Although no head-to-head comparison is available, response rates seem similar between both drugsCitation76 and close to 80% within 2 weeks.Citation41,Citation42 The molecule choice mostly relies on the administration route preference: romiplostim requires a weekly subcutaneous injection, whereas eltrombopag is administered orally but at the cost of food or drug interactions and potential poor compliance in patients with cognitive disorders. In case of non-response or intolerance to a TPO-RA, a switch to the other drug can provide a better clinical result.Citation77 In case of failure to eltrombopag and romiplostim, response can be obtained with a switch to avatrombopag.Citation78 Because they increase platelet production, TPO-RAs have long been considered to very rarely lead to sustained remission off-treatment in ITP, but we now have evidence for sustained remission after their discontinuation in 10% to 30% of patients.Citation79,Citation80 Therefore, after a response is obtained, the treatment should be tapered in order to use the lowest dose to maintain platelet counts.Citation8
Several studies have suggested that TPO-RA efficacy is similar in older and younger patients.Citation47,Citation48,Citation81 Although the overall tolerance of TPO-RA is good, there are some concerns about long- and short-term adverse events. The risk of reticulin deposits in bone marrow has been raised in patients with long-term use of TPO-RAs but does not lead to bone-marrow failure and is reversible after drug discontinuation.Citation82,Citation83
Although not evidenced in pivotal trials, some studies have suggested a two- to three-fold increase in thrombosis risk with TPO-RA use as compared with untreated ITP patients.Citation41–45 This risk is particularly relevant in older patients because age is an independent risk factor for thrombosis,Citation37,Citation38 in addition to the risks with other ITP treatments such as IgIVCitation39 or splenectomyCitation40 and the disease itself.Citation35,Citation36 Despite these multiple confounding factors, a higher rate of thrombotic events has been described in older patients particularly those exposed to TPO-RAs.Citation46–49
Anti-CD20 Monoclonal Antibodies
The efficacy of rituximab is well documented in ITP, with initial response rates of about 60%, decreasing to 40% at 2 years and to almost 30% after 5 years.Citation84–90 Although low, these long-term responses suggest that rituximab can be a curative treatment for ITP, and re-treatment was found effective in most relapsing patients.Citation88,Citation89,Citation91
Rituximab is usually administered as four weekly infusions of 375 mg/m², but a more convenient dosage of 1000 mg on days 1 and 15 also used in other autoimmune diseases is considered equivalent.Citation88 Treatment with lower doses (100 mg weekly for 4 weeks) has comparable short-term response but with slower time to response and shorter response duration.Citation92 Several retrospective studies suggested a lower response rate in older patientsCitation85,Citation86 and in patients with longer disease duration.Citation88 However, a large prospective register including almost 250 patients with 5 years of follow-up did not show a difference in long-term response according to age.Citation89
The tolerance profile of rituximab is good, but some points are important to consider when treating older patients. The infectious risk is only moderately increased,Citation93 although severe infections are predominantly observed in older patients, without excluding that concomitant prolonged corticosteroids and/or immunosuppressive drugs could have been the cause of these severe infectious events.Citation88 Hypogammaglobulinemia is uncommon after one course of rituximab, and the risk of severe hypogammaglobulinemia after repeated courses and/or the addition of immunosuppressive agents is unknown in ITP but seems rare when anti-CD20 antibodies are used to treat other autoimmune diseases such as rheumatoid arthritis.Citation94 Rituximab is associated with a risk of hepatitis B virus reactivation that is preventable.Citation95 Progressive multifocal leukoencephalopathy is exceptional.Citation96 Most importantly, an impaired vaccine response in the months following rituximab treatment has been well documentedCitation97 and is particularly relevant for older patients requiring yearly influenza and/or SARS-CoV-2 vaccination. Anti-pneumococcal vaccination should also be performed before rituximab administration in older patients. During the COVID-19 pandemic, most groups avoided rituximab use in older patients because of the fear that rituximab would increase the risk of fatal infections, even if this risk has not been confirmed in the setting of ITP, for which rituximab is rarely associated with a strong immunosuppressive treatment.
Despite these limitations, rituximab remains a valid option for older patients and should be assessed on an individual basis. Anti-CD20 treatment is also a good option in the particular case of ITP associated with B-cell lymphoid malignancies or with associated systemic autoimmunity. Associating rituximab with other agents such as dexamethasone and/or ciclosporin has also been proposed, with higher response rates but at the cost of greater toxic effects, including infections and decreased gamma globulin levels.Citation98–101 Promising results were obtained by combining belimumab with rituximab in a pilot study.Citation102 The efficacy and tolerance of these combined strategies in older patients are unknown.
Splenectomy
Splenectomy has been used to treat ITP for decades and provides a high rate of long-term response with an overall long-term remission rate of 60% to 70%.Citation103 It is now admitted that splenectomy should be deferred for at least 12 months to allow for spontaneous remission.Citation8 Given the availability of new ITP treatments, the rate of splenectomy is decreasing, particularly in older patients.Citation32,Citation104 Response rates in older patients could be lower with increased surgical risk. Although laparoscopic surgery can reduce the risk of post-operative complications,Citation103,Citation105 some studies reported a higher rate of immediate surgical complications such as severe bleedingCitation105,Citation106 in older patients, which could be explained by the frequency of co-morbidities in this population.Citation107 Some studies have suggested a lower response rateCitation105 and higher relapse rateCitation104,Citation105,Citation108 in older patients. In a recent multicenter retrospective study after TPO-RA introduction in France, lack of sustained response after splenectomy was associated with older age on multivariable analysis (60–75 years: odds ratio 0.39 [95% confidence interval 0.17–0.86], p =.02; >75 years: 0.28 [0.10–0.75], p =.013).Citation104 Splenectomy also exposes to increased risk of thrombosis, which should be prevented by prophylactic use of an anticoagulant immediately after the procedure, but given that this risk persists over time, it should be taken into account in the treatment decision.Citation40,Citation109 Lastly, splenectomy also favors infection, particularly infection to encapsulated germs, and prior immunization against influenza and Streptococcus pneumoniae is recommended, as is patient education to take immediate antibiotics in case of fever.Citation40,Citation109
Nonetheless, our current opinion is that splenectomy can still be considered a third-line treatment in older patients, particularly for those with few comorbidities and low risk of thrombosis, and should not be contra-indicated solely on an age basis.Citation29 Some groups suggest that a platelet isotopic study could be useful to select the best patient candidates for splenectomy because in case of a predominant splenic sequestration pattern, most studies reported an excellent positive predictive value of response to splenectomy.Citation110 However, conflicting results were published in case of a hepatic or mixed sequestration pattern. A large UK study showed that most patients with mixed platelet destruction experienced clinical benefit after splenectomy despite being classified as non-responding.Citation111
Partial splenic embolization was recently reported as a safe and effective treatment by Japanese colleagues.Citation112 If these good results are confirmed, this technique could be an interesting alternative in older patients with disease refractory to medical treatment and in whom splenectomy appears dangerous because of comorbidities.
Immunosuppressive Agents
Immunosuppressive drugs have been used in ITP with various response rates. Mycophenolate mofetilCitation113–116 and azathioprineCitation117,Citation118 are the most widely used, but data on ciclosporin,Citation119–121 rapamycinCitation122–124 or cyclophosphamideCitation125,Citation126 efficacy have also been published. The treatment usually takes several months to obtain a response and requires long-term use with a high risk of relapse in case of stopping and with increased infectious risk that can be problematic in older patients. Despite these limitations, mycophenolate mofetil is widely prescribed as second-line therapy in the United Kingdom and has even been proposed as first-line therapy in a prospective controlled study.Citation116 This attitude is not widely adopted, and in France, our strategy is to reserve immunosuppressive drugs for patients with failure to respond to TPO-RAs and rituximab. We have also observed good responses in refractory patients when combining immunosuppressive drugs with TPO-RAs (unpublished observations).
Dapsone
Dapsone is an effective treatment for ITP that can be used in older patients with moderate response rates (20–60%).Citation127–130 Previous splenectomy is associated with low efficacy, and it should not be used when a rapid response is needed given that the median time to response is close to 1 month. This treatment induces hemolysis, requiring a close monitoring of hemoglobin level especially in the first weeks of treatment, but in many patients, the decrease in hemoglobin level is transient (a few weeks) and limited (1 to 2 g/dL) and does not require discontinuation of the treatment. In contrast, the patient should be aware of the risk of dapsone-induced hypersensitivity syndrome that combines a generalized skin eruption with fever, with lymphadenopathy and hepatitis in the more severe forms. The syndrome always occurs in the first 4 weeks after the beginning of treatment. In our experience, skin eruption is observed in 7% of patients, and its severity is probably diminished when dapsone is associated with a short course of prednisone.Citation131
Danazol
Danazol has been used for years as second-line treatment for ITP and is considered efficient, with 40% to 70% response rates, even in older patients.Citation27,Citation132,Citation133 Its use is limited by its long time to response and its toxic effects (androgenic effects in women, risk of accelerated prostate cancer in men, liver cytotoxicity and increased thrombotic risk) but also by the concurrence of alternative second-line treatments for ITP.
Fostamatinib and Emerging Agents
Fostamatinib, a Syk inhibitor, has been approved recently in the United States and the European Medicines Agency for treating chronic ITP. Both pivotal trials included older adults,Citation134 but to date there are no data regarding its specific use in older people. Common adverse events reported in a pivotal study, such as diarrhea, hypertension, nausea, and increased transaminase levels, should be considered in the therapeutic decision. Other treatments such as anti-FcRn, rizalbrutinib (which inhibits Bruton tyrosine kinase) or daratumumab (an anti-CD38 monoclonal antibody) are currently under development for chronic ITP, but their respective efficacy/tolerance ratio in older patients remains to be determined.Citation135
Management of Particular Situations
B-Cell Malignancies
The frequency of clonal B-cell disorders increases with age, and ITP is a well-known complication of B-cell lymphoid malignancies such as indolent lymphomas or chronic lymphoid leukemias. In this particular setting, lymphoma treatment should prevail over ITP treatment. With no indication for treating the B-cell disorder, rituximabCitation136–138 and TPO-RAsCitation139 can be used, and splenectomy should be avoided.
Myelodysplastic Syndrome (MDS)
In older people, MDS can coexist with ITP or complicate the ITP course and is associated with increased bleeding risk.Citation17 The overall therapeutic strategy is unchanged, but the risk of acute myeloid leukemia transformation favored by TPO-RAs has been a concern. However, the risk seems limited and reassuring data have been produced recently.Citation17,Citation140–143 In a randomized double-blind trial, the risk of leukemic progression in thrombocytopenic patients with low-risk MDS was similar between romiplostim and placebo,Citation143 although there are fewer data available for the use of TPO-RAs in high-risk MDS.
Management of Thrombosis and TPO-RAs
Thrombotic events have been described in older patients receiving TPO-RAs.Citation46–49 In a real-life study of 384 patients >60 years and receiving TPO-RAs with a median follow-up of 2.7 years, Palandri et al observed 43 thromboses in 35 patients (including 22 arterial thromboses), corresponding to a cumulative incidence of 6.2% at 12 months.Citation48 The authors found a significant association between thrombosis history and thrombosis. Median platelet count was 127 x 109/L at the time of thrombosis event, which confirms that thrombosis can occur even with low/moderate platelet counts. Of note, thrombosis recurrence was observed mostly in patients who continued TPO-RAs, particularly those not receiving long-term anti-thrombotic treatment, which suggests that it could be used as a secondary prophylaxis if TPO-RA continuation is needed. These data strongly suggest that TPO-RAs should be used with caution in older patients with a history of thrombosis, especially if they are no longer receiving anticoagulants. In this situation, we suggest considering another therapeutic strategy.
Refractory Patients
Despite the increasing therapeutic options to treat ITP, some patients do not respond to or lose response after the initiation of second-line treatment.Citation144 In these cases, a differential diagnosis should be ruled out, if necessary, by performing BME. When the disease is refractory to several second-line treatments such as both TPO-RAs and rituximab, the treatment choice should be personalized according to the context and the expected benefit/risk ratio. A better knowledge of the pathophysiology of ITP suggested that a combination of treatments could be synergic.Citation135 According to this hypothesis, we propose that the combination of immunosuppressive drugs and TPO-RA could produce a significant response in multirefractory ITP with previous failure to respond to TPO-RAs and immunosuppressive drugs alone.Citation140 Splenectomy can also be discussed for patients with few comorbidities and low risk of thrombosis.
Conclusion
ITP is increasingly frequent in older patients, and the diagnostic work-up is mainly the same as in younger patients, with special attention to ruling out a differential diagnosis. The underlying mechanisms responsible for disease may differ and are probably a hot topic for research because it could help tailor therapy in the future. One of the limits of this review is that evidence-based medicine is lacking in older patients, although there is a growing number of retrospective studies focusing on this particular population. Age is associated with the accumulation of chronic conditions but is probably not a prognostic factor in ITP after controlling for other variables. Thus, the therapeutic decision should be patient-tailored according to the patient’s preferences, co-morbidities and fitness rather than age itself. The efficacy of second-line treatments has relegated splenectomy to patients with refractory disease only, and the good efficacy/safety profile of TPO-RAs has changed the therapeutic landscape of ITP in older people at the cost of increased thrombosis risk. The availability of new treatments will likely modify our therapeutic strategy in the next years.
Disclosure
Mahévas received funds for research from GSK and fees from Amgen and Novartis for lectures. Bertrand Godeau served as an expert for Amgen, Novartis, Grifols and Sobi. Marc Michel received honoraria (advisory boards, speaker fees) from Novartis, Amgen, UCB, Argenx, Alexion and Sanofi, and personal fees from Sobi. Etienne Crickx received honoraria (advisory boards, speaker fees) from Novartis, UCB, and Sanofi. The authors report no other potential conflicts of interest in this work.
Acknowledgment
We thank Laura Smales for English editing.
Additional information
Funding
References
- Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–632. doi:10.1016/j.autrev.2017.04.012
- Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. 2014;124(22):3308–3315. doi:10.1182/blood-2014-05-578336
- Doobaree IU, Conway K, Miah H, et al. Incidence of adult primary immune thrombocytopenia in England-an update. Eur J Haematol. 2022;109(3):238–249. doi:10.1111/ejh.13803
- Lucchini E, Fanin R, Cooper N, Zaja F. Management of immune thrombocytopenia in elderly patients. Eur J Intern Med. 2018;58:70–76. doi:10.1016/j.ejim.2018.09.005
- Mahévas M, Michel M, Godeau B. How we manage immune thrombocytopenia in the elderly. Br J Haematol. 2016;173(6):844–856. doi:10.1111/bjh.14067
- Terrell DR, Beebe LA, Neas BR, Vesely SK, Segal JB, George JN. Prevalence of primary immune thrombocytopenia in Oklahoma. Am J Hematol. 2012;87(9):848–852. doi:10.1002/ajh.23262
- Sokal A, de Nadaï T, Maquet J, et al. Primary immune thrombocytopenia in very elderly patients: particularities in presentation and management: results from the prospective CARMEN-France registry. Br J Haematol. 2022;196(5):1262–1270. doi:10.1111/bjh.17935
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi:10.1182/bloodadvances.2019000812
- McMillan R. Antiplatelet antibodies in chronic immune thrombocytopenia and their role in platelet destruction and defective platelet production. Hematol Oncol Clin North Am. 2009;23(6):1163–1175. doi:10.1016/j.hoc.2009.08.008
- Vayne C, Guéry EA, Rollin J, Baglo T, Petermann R, Gruel Y. Pathophysiology and diagnosis of drug-induced immune thrombocytopenia. J Clin Med. 2020;9(7):E2212. doi:10.3390/jcm9072212
- Crickx E, Moulis G, Ebbo M, et al. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br J Haematol. 2021. doi:10.1111/bjh.17813
- Fuentes S, Chrétien B, Dolladille C, et al. An updated list of drugs suspected to be associated with immune thrombocytopenia based on the WHO pharmacovigilance database. Blood. 2022;140(8):922–927. doi:10.1182/blood.2022015936
- Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer. 2007;109(9):1705–1714. doi:10.1002/cncr.22602
- Waisbren J, Dinner S, Altman J, et al. Disease characteristics and prognosis of myelodysplastic syndrome presenting with isolated thrombocytopenia. Int J Hematol. 2017;105(1):44–51. doi:10.1007/s12185-016-2081-4
- Brynes RK, Orazi A, Theodore D, et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: data from the EXTEND study. Am J Hematol. 2015;90(7):598–601. doi:10.1002/ajh.24011
- Rivière É, Viallard JF, Guy A, et al. Intrinsically impaired platelet production in some patients with persistent or chronic immune thrombocytopenia. Br J Haematol. 2015;170(3):408–415. doi:10.1111/bjh.13444
- Jachiet V, Moulis G, Hadjadj J, et al. Clinical spectrum, outcome and management of immune thrombocytopenia associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Haematologica. 2021;106(5):1414–1422. doi:10.3324/haematol.2020.272559
- Comont T, Germain J, Beyne-Rauzy O, Adoue D, Moulis G; Group and the C investigators. Positivity rate of systematic bone marrow smear in patients over 60 years old with newly diagnosed immune thrombocytopenia. Blood Adv. 2020;4(10):2136–2138. doi:10.1182/bloodadvances.2020001654
- Taparia K, Wall E, Arnold DM, Sun HL. Frequency and utility of bone marrow examination in relapsed/refractory immune thrombocytopenia. J Thromb Haemost. 2022;20(9):2119–2126. doi:10.1111/jth.15802
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi:10.1182/bloodadvances.2019000966
- Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98(5):436–442. doi:10.1016/s0002-9343(99)80342-8
- Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94(3):909–913. doi:10.1182/blood.V94.3.909.415k02_909_913
- Vianelli N, Valdrè L, Fiacchini M, et al. Long-term follow-up of idiopathic thrombocytopenic purpura in 310 patients. Haematologica. 2001;86(5):504–509.
- Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991;77(1):31–33. doi:10.1182/blood.V77.1.31.31
- Bourgeois E, Caulier MT, Delarozee C, Brouillard M, Bauters F, Fenaux P. Long-term follow-up of chronic autoimmune thrombocytopenic purpura refractory to splenectomy: a prospective analysis. Br J Haematol. 2003;120(6):1079–1088. doi:10.1046/j.1365-2141.2003.04211.x
- Bizzoni L, Mazzucconi MG, Gentile M, et al. Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 2006;76(3):210–216. doi:10.1111/j.1600-0609.2005.00602.x
- Daou S, Federici L, Zimmer J, Maloisel F, Serraj K, Andrès E. Idiopathic thrombocytopenic purpura in elderly patients: a study of 47 cases from a single reference center. Eur J Intern Med. 2008;19(6):447–451. doi:10.1016/j.ejim.2007.07.006
- Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–1638. doi:10.1001/archinte.160.11.1630
- Michel M, Rauzy OB, Thoraval FR, et al. Characteristics and outcome of immune thrombocytopenia in elderly: results from a single center case-controlled study. Am J Hematol. 2011;86(12):980–984. doi:10.1002/ajh.22170
- Mithoowani S, Cervi A, Shah N, et al. Management of major bleeds in patients with immune thrombocytopenia. J Thromb Haemost. 2020;18(7):1783–1790. doi:10.1111/jth.14809
- Zhou H, Fu R, Wang H, et al. Immune thrombocytopenia in the elderly: clinical course in 525 patients from a single center in China. Ann Hematol. 2013;92(1):79–87. doi:10.1007/s00277-012-1567-2
- Palandri F, Catani L, Auteri G, et al. Understanding how older age drives decision-making and outcome in immune thrombocytopenia. A single centre study on 465 adult patients. Br J Haematol. 2019;184(3):424–430. doi:10.1111/bjh.15668
- Piel-Julian ML, Mahévas M, Germain J, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16(9):1830–1842. doi:10.1111/jth.14227
- Swan D, Newland A, Rodeghiero F, Thachil J. Thrombosis in immune thrombocytopenia - current status and future perspectives. Br J Haematol. 2021;194(5):822–834. doi:10.1111/bjh.17390
- Sarpatwari A, Bennett D, Logie JW, et al. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom general practice research database. Haematologica. 2010;95(7):1167–1175. doi:10.3324/haematol.2009.018390
- Doobaree IU, Nandigam R, Bennett D, Newland A, Provan D. Thromboembolism in adults with primary immune thrombocytopenia: a systematic literature review and meta-analysis. Eur J Haematol. 2016;97(4):321–330. doi:10.1111/ejh.12777
- Ekstrand C, Linder M, Baricault B, et al. Impact of risk factors on the occurrence of arterial thrombosis and venous thromboembolism in adults with primary immune thrombocytopenia – results from two nationwide cohorts. Thromb Res. 2019;178:124–131. doi:10.1016/j.thromres.2019.04.016
- Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–699. doi:10.1111/j.1538-7836.2007.02450.x
- Paran D, Herishanu Y, Elkayam O, Shopin L, Ben-Ami R. Venous and arterial thrombosis following administration of intravenous immunoglobulins. Blood Coagul Fibrinolysis. 2005;16(5):313–318. doi:10.1097/01.mbc.0000172694.85233.a8
- Thai LH, Mahévas M, Roudot-Thoraval F, et al. Long-term complications of splenectomy in adult immune thrombocytopenia. Medicine. 2016;95(48):e5098. doi:10.1097/MD.0000000000005098
- Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. doi:10.1182/blood-2017-04-748707
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–423. doi:10.1111/bjh.12260
- Catalá-López F, Corrales I, de la Fuente-Honrubia C, et al. Risk of thromboembolism with thrombopoietin receptor agonists in adult patients with thrombocytopenia: systematic review and meta-analysis of randomized controlled trials. Med Clin. 2015;145(12):511–519. doi:10.1016/j.medcli.2015.03.014
- Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112–1123. doi:10.3324/haematol.2018.212845
- Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–2171. doi:10.1182/blood-2008-04-150078
- Lozano ML, Mingot-Castellano ME, Perera MM, et al. A decade of changes in management of immune thrombocytopenia, with special focus on elderly patients. Blood Cells Mol Dis. 2021;86:102505. doi:10.1016/j.bcmd.2020.102505
- Michel M, Wasser J, Godeau B, et al. Efficacy and safety of the thrombopoietin receptor agonist romiplostim in patients aged ≥ 65 years with immune thrombocytopenia. Ann Hematol. 2015;94(12):1973–1980. doi:10.1007/s00277-015-2485-x
- Palandri F, Rossi E, Bartoletti D, et al. Real-world use of thrombopoietin receptor agonists in older patients with primary immune thrombocytopenia. Blood. 2021;138(7):571–583. doi:10.1182/blood.2021010735
- Ruggeri M, Tosetto A, Palandri F, et al. Thrombotic risk in patients with primary immune thrombocytopenia is only mildly increased and explained by personal and treatment-related risk factors. J Thromb Haemost. 2014;12(8):1266–1273. doi:10.1111/jth.12636
- Nørgaard M, Jensen AØ, Engebjerg MC, et al. Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Blood. 2011;117(13):3514–3520. doi:10.1182/blood-2010-10-312819
- Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97(9):2549–2554. doi:10.1182/blood.v97.9.2549
- Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the general practice research database. Br J Haematol. 2009;145(2):235–244. doi:10.1111/j.1365-2141.2009.07615.x
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. 2021;96(2):188–198. doi:10.1002/ajh.26045
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. 2021;96(2):199–207. doi:10.1002/ajh.26036
- Giordano P, Lassandro G, Valente M, Molinari AC, Ieranò P, Coppola A. Current management of the hemophilic child: a demanding interlocutor. Quality of life and adequate cost-efficacy analysis. Pediatr Hematol Oncol. 2014;31(8):687–702. doi:10.3109/08880018.2014.930768
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi:10.1182/blood-2009-06-225565
- Matzdorff A, Beer JH. Immune thrombocytopenia patients requiring anticoagulation--maneuvering between scylla and charybdis. Semin Hematol. 2013;50(Suppl 1):S83–S88. doi:10.1053/j.seminhematol.2013.03.020
- Godeau B, Bierling P. High-dose dexamethasone as initial treatment for immune thrombocytopenic purpura. N Engl J Med. 2003;349(23):2267–2268;author reply 2267–2268. doi:10.1056/NEJM200312043492318
- Pirunsarn A, Kijrattanakul P, Chamnanchanunt S, Polprasert C, Rojnuckarin P. A randomized multicenter trial comparing low-dose prednisolone versus observation for prevention of recurrences in adult immune thrombocytopenia. Clin Appl Thromb Hemost. 2018;24(6):867–873. doi:10.1177/1076029618764843
- Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302;quiz 370. doi:10.1182/blood-2015-07-659656
- Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3(10):e489–e496. doi:10.1016/S2352-3026(16)30109-0
- Palandri F, Santoro C, Carpenedo M, et al. Management of elderly patients with immune thrombocytopenia: real-world evidence from 451 patients older than 60 years. Thromb Res. 2020;185:88–95. doi:10.1016/j.thromres.2019.11.026
- Moulis G, Palmaro A, Sailler L, Lapeyre-Mestre M, Latus J. Corticosteroid risk function of severe infection in primary immune thrombocytopenia adults. A nationwide nested case-control study. PLoS One. 2015;10(11):e0142217. doi:10.1371/journal.pone.0142217
- Bellucci S, Charpak Y, Chastang C, Tobelem G. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71(4):1165–1169. doi:10.1182/blood.V71.4.1165.1165
- Godeau B, Chevret S, Varet B, et al. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359(9300):23–29. doi:10.1016/S0140-6736(02)07275-6
- Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90(6):829–832.
- Godeau B, Caulier MT, Decuypere L, et al. Intravenous immunoglobulin for adults with autoimmune thrombocytopenic purpura: results of a randomized trial comparing 0.5 and 1 g/kg b.w. Br J Haematol. 1999;107(4). doi:10.1046/j.1365-2141.1999.01766.x
- Caress JB, Kennedy BL, Eickman KD. Safety of intravenous immunoglobulin treatment. Expert Opin Drug Saf. 2010;9(6):971–979. doi:10.1517/14740338.2010.484419
- Marie I, Maurey G, Hervé F, Hellot MF, Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br J Dermatol. 2006;155(4):714–721. doi:10.1111/j.1365-2133.2006.07390.x
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171–178. doi:10.1016/j.tmrv.2013.05.004
- Gaines AR. Acute onset hemoglobinemia and/or hemoglobinuria and sequelae following Rh(o)(D) immune globulin intravenous administration in immune thrombocytopenic purpura patients. Blood. 2000;95(8):2523–2529. doi:10.1182/blood.V95.8.2523
- Ar G. Disseminated intravascular coagulation associated with acute hemoglobinemia or hemoglobinuria following Rh(0)(D) immune globulin intravenous administration for immune thrombocytopenic purpura. Blood. 2005;106:5. doi:10.1182/blood-2004-11-4303
- Salama A, Kiesewetter H, Kalus U, Movassaghi K, Meyer O. Massive platelet transfusion is a rapidly effective emergency treatment in patients with refractory autoimmune thrombocytopenia. Thromb Haemost. 2008;100(5):762–765. doi:10.1160/TH08-06-0418
- Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol. 2008;83(2):122–125. doi:10.1002/ajh.21060
- Roumier M, Le Burel S, Audia S, et al. High dose romiplostim as a rescue therapy for adults with severe bleeding and refractory immune thrombocytopenia. Am J Hematol. 2021;96(2):E43–E46. doi:10.1002/ajh.26040
- Zhang J, Liang Y, Ai Y, et al. Eltrombopag versus romiplostim in treatment of children with persistent or chronic immune thrombocytopenia: a systematic review incorporating an indirect-comparison meta-analysis. Sci Rep. 2018;8(1):576. doi:10.1038/s41598-017-19099-8
- Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol. 2015;101(3):255–263. doi:10.1007/s12185-014-1731-7
- Al-Samkari H, Jiang D, Gernsheimer T, et al. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: a multicentre US study. Br J Haematol. 2022;197(3):359–366. doi:10.1111/bjh.18081
- Bussel JB, Wang X, Lopez A, Eisen M. Case study of remission in adults with immune thrombocytopenia following cessation of treatment with the thrombopoietin mimetic romiplostim. Hematology. 2016;21(4):257–262. doi:10.1179/1607845415Y.0000000041
- Mahévas M, Fain O, Ebbo M, et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br J Haematol. 2014;165(6):865–869. doi:10.1111/bjh.12888
- González-López TJ, Sánchez-González B, Jarque I, et al. Use of eltrombopag for patients 65 years old or older with immune thrombocytopenia. Eur J Haematol. 2020;104(3):259–270. doi:10.1111/ejh.13370
- Ghanima W, Geyer JT, Lee CS, et al. Bone marrow fibrosis in 66 patients with immune thrombocytopenia treated with thrombopoietin-receptor agonists: a single-center, long-term follow-up. Haematologica. 2014;99(5):937–944. doi:10.3324/haematol.2013.098921
- Rizvi H, Butler T, Calaminici M, et al. United Kingdom immune thrombocytopenia registry: retrospective evaluation of bone marrow fibrosis in adult patients with primary immune thrombocytopenia and correlation with clinical findings. Br J Haematol. 2015;169(4):590–594. doi:10.1111/bjh.13330
- Godeau B, Porcher R, Fain O, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter Phase 2 study. Blood. 2008;112(4):999–1004. doi:10.1182/blood-2008-01-131029
- Auger S, Duny Y, Rossi JF, Quittet P. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol. 2012;158(3):386–398. doi:10.1111/j.1365-2141.2012.09169.x
- Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol. 2017;98(4):371–377. doi:10.1111/ejh.12839
- Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25–33. doi:10.7326/0003-4819-146-1-200701020-00006
- Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124(22):3228–3236. doi:10.1182/blood-2014-06-582346
- Deshayes S, Khellaf M, Zarour A, et al. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: results at 5 years from the French prospective registry ITP-ritux. Am J Hematol. 2019;94(12):1314–1324. doi:10.1002/ajh.25632
- Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. doi:10.1182/blood-2011-11-393975
- Hasan A, Michel M, Patel V, et al. Repeated courses of rituximab in chronic ITP: three different regimens. Am J Hematol. 2009;84(10):661–665. doi:10.1002/ajh.21512
- Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. doi:10.1111/j.1600-0609.2010.01486.x
- Chugh S, Darvish-Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2015;2(2):e75–e81. doi:10.1016/S2352-3026(15)00003-4
- van Vollenhoven RF, Emery P, Bingham CO, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72(9):1496–1502. doi:10.1136/annrheumdis-2012-201956
- Cho CH, Hwang WL, Cheng SB, Lee TY, Teng CL. Hepatitis B reactivation induced by Rituximab maintenance therapy for lymphoma. Ann Hematol. 2011;90(1):111–112. doi:10.1007/s00277-010-0962-9
- Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the research on adverse drug events and reports project. Blood. 2009;113(20):4834–4840. doi:10.1182/blood-2008-10-186999
- Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946–1953. doi:10.1182/blood-2013-04-494096
- Bussel JB, Lee CS, Seery C, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99(7):1264–1271. doi:10.3324/haematol.2013.103291
- Choi PYI, Roncolato F, Badoux X, Ramanathan S, Ho SJ, Chong BH. A novel triple therapy for ITP using high-dose dexamethasone, low-dose rituximab, and cyclosporine (TT4). Blood. 2015;126(4):500–503. doi:10.1182/blood-2015-03-631937
- Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755–2762. doi:10.1182/blood-2009-07-229815
- Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11):1976–1981. doi:10.1182/blood-2012-09-455691
- Mahévas M, Azzaoui I, Crickx E, et al. Efficacy, safety and immunological profile of combining rituximab with belimumab for adults with persistent or chronic immune thrombocytopenia: results from a prospective phase 2b trial. Haematologica. 2021;106(9):2449–2457. doi:10.3324/haematol.2020.259481
- Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104(9):2623–2634. doi:10.1182/blood-2004-03-1168
- Mageau A, Terriou L, Ebbo M, et al. Splenectomy for primary immune thrombocytopenia revisited in the era of thrombopoietin receptor agonists: new insights for an old treatment. Am J Hematol. 2022;97(1):10–17. doi:10.1002/ajh.26378
- Gonzalez-Porras JR, Escalante F, Pardal E, et al. Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 2013;91(3):236–241. doi:10.1111/ejh.12146
- Andrès E, Zimmer J, Noel E, Kaltenbach G, Koumarianou A, Maloisel F. Idiopathic thrombocytopenic purpura: a retrospective analysis in 139 patients of the influence of age on the response to corticosteroids, splenectomy and danazol. Drugs Aging. 2003;20(11):841–846. doi:10.2165/00002512-200320110-00005
- Kavic SM, Segan RD, Park AE. Laparoscopic splenectomy in the elderly: a morbid procedure? Surg Endosc. 2005;19(12):1561–1564. doi:10.1007/s00464-005-0125-6
- Park YH, Yi HG, Kim CS, et al. Clinical outcome and predictive factors in the response to splenectomy in elderly patients with primary immune thrombocytopenia: a multicenter retrospective study. Acta Haematol. 2016;135(3):162–171. doi:10.1159/000442703
- Vianelli N, Palandri F, Polverelli N, et al. Splenectomy as a curative treatment for immune thrombocytopenia: a retrospective analysis of 233 patients with a minimum follow up of 10 years. Haematologica. 2013;98(6):875–880. doi:10.3324/haematol.2012.075648
- Cuker A, Cines DB. Evidence-based mini-review: is indium-labeled autologous platelet scanning predictive of response to splenectomy in patients with chronic immune thrombocytopenia? Hematol Am Soc Hematol Educ Program. 2010;2010(1):385–386. doi:10.1182/asheducation-2010.1.385
- Sarpatwari A, Provan D, Erqou S, Sobnack R, David Tai FW, Newland AC. Autologous 111 in-labelled platelet sequestration studies in patients with primary immune thrombocytopenia (ITP) prior to splenectomy: a report from the United Kingdom ITP registry. Br J Haematol. 2010;151(5):477–487. doi:10.1111/j.1365-2141.2010.08377.x
- Togasaki E, Shimizu N, Nagao Y, et al. Long-term efficacy of partial splenic embolization for the treatment of steroid-resistant chronic immune thrombocytopenia. Ann Hematol. 2018;97(4):655–662. doi:10.1007/s00277-018-3232-x
- Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70(6):353–357. doi:10.1034/j.1600-0609.2003.00076.x
- Taylor A, Neave L, Solanki S, et al. Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol. 2015;171(4):625–630. doi:10.1111/bjh.13622
- Provan D, Moss AJ, Newland AC, Bussel JB. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol. 2006;81(1):19–25. doi:10.1002/ajh.20515
- Bradbury CA, Pell J, Hill Q, et al. Mycophenolate mofetil for first-line treatment of immune thrombocytopenia. N Engl J Med. 2021;385(10):885–895. doi:10.1056/NEJMoa2100596
- Vesely SK, Perdue JJ, Rizvi MA, Terrell DR, George JN. Management of adult patients with persistent idiopathic thrombocytopenic purpura following splenectomy: a systematic review. Ann Intern Med. 2004;140(2):112–120. doi:10.7326/0003-4819-140-3-200402030-00012
- Mishra K, Pramanik S, Sandal R, et al. Safety and efficacy of azathioprine in immune thrombocytopenia. Am J Blood Res. 2021;11(3):217–226.
- Choudhary DR, Naithani R, Mahapatra M, Kumar R, Mishra P, Saxena R. Efficacy of cyclosporine as a single agent therapy in chronic idiopathic thrombocytopenic purpura. Haematologica. 2008;93(10):e61–e62;discussion e63. doi:10.3324/haematol.13481
- Kappers-Klunne MC, Van’t Veer MB. Cyclosporin A for the treatment of patients with chronic idiopathic thrombocytopenic purpura refractory to corticosteroids or splenectomy. Br J Haematol. 2001;114(1):121–125. doi:10.1046/j.1365-2141.2001.02893.x
- Emilia G, Morselli M, Luppi M, et al. Long-term salvage therapy with cyclosporin A in refractory idiopathic thrombocytopenic purpura. Blood. 2002;99(4):1482–1485. doi:10.1182/blood.V99.4.1482
- Li H, Ji J, Du Y, et al. Sirolimus is effective for primary relapsed/refractory autoimmune cytopenia: a multicenter study. Exp Hematol. 2020;89:87–95. doi:10.1016/j.exphem.2020.08.001
- Feng Y, Xiao Y, Yan H, et al. Sirolimus as rescue therapy for refractory/relapsed immune thrombocytopenia: results of a single-center, prospective, single-arm study. Front Med. 2020;7:110. doi:10.3389/fmed.2020.00110
- Li J, Wang Z, Dai L, et al. Effects of rapamycin combined with low dose prednisone in patients with chronic immune thrombocytopenia. Clin Dev Immunol. 2013;2013:548085. doi:10.1155/2013/548085
- Verlin M, Laros RK, Penner JA. Treatment of refractory thrombocytopenic purpura with cyclophosphamine. Am J Hematol. 1976;1(1):97–104. doi:10.1002/ajh.2830010111
- Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: multicentric trial of the cooperative Latin American group on hemostasis and thrombosis. Blood. 1984;64(6):1179–1183. doi:10.1182/blood.V64.6.1179.1179
- Godeau B, Oksenhendler E, Bierling P. Dapsone for autoimmune thrombocytopenic purpura. Am J Hematol. 1993;44(1):70–72. doi:10.1002/ajh.2830440117
- Godeau B, Durand JM, Roudot-Thoraval F, et al. Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 1997;97(2):336–339. doi:10.1046/j.1365-2141.1997.412687.x
- Zaja F, Marin L, Chiozzotto M, Puglisi S, Volpetti S, Fanin R. Dapsone salvage therapy for adult patients with immune thrombocytopenia relapsed or refractory to steroid and rituximab. Am J Hematol. 2012;87(3):321–323. doi:10.1002/ajh.22266
- Durand JM, Lefèvre P, Hovette P, Mongin M, Soubeyrand J. Dapsone for idiopathic autoimmune thrombocytopenic purpura in elderly patients. Br J Haematol. 1991;78(3):459–460. doi:10.1111/j.1365-2141.1991.tb04467.x
- Sauvetre G, MahÉvas M, Limal N, et al. Cutaneous rash and dapsone-induced hypersensitivity syndrome a common manifestation in adult immune thrombocytopenia. Presentation and outcome in 16 cases. Am J Hematol. 2015;90(10):E201–E202. doi:10.1002/ajh.24068
- Maloisel F, Andrès E, Zimmer J, et al. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: long-term results. Am J Med. 2004;116(9):590–594. doi:10.1016/j.amjmed.2003.12.024
- Liu W, Gu X, Fu R, et al. The effect of danazol in primary immune thrombocytopenia: an analysis of a large cohort from a single center in China. Clin Appl Thromb Hemost. 2016;22(8):727–733. doi:10.1177/1076029615622002
- Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two Phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921–930. doi:10.1002/ajh.25125
- Audia S, Mahévas M, Nivet M, Ouandji S, Ciudad M, Bonnotte B. Immune thrombocytopenia: recent advances in pathogenesis and treatments. Hemasphere. 2021;5(6):e574. doi:10.1097/HS9.0000000000000574
- Zent CS, Ding W, Reinalda MS, et al. Autoimmune cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma: changes in clinical presentation and prognosis. Leuk Lymphoma. 2009;50(8):1261–1268. doi:10.1080/10428190903026492
- Rogers KA, Woyach JA. Secondary autoimmune cytopenias in chronic lymphocytic leukemia. Seminars in Oncology. 2016;43(2):300–310. doi:10.1053/j.seminoncol.2016.02.011
- Hauswirth AW, Skrabs C, Schützinger C, et al. Autoimmune thrombocytopenia in non-Hodgkin’s lymphomas. Haematologica. 2008;93(3):447–450. doi:10.3324/haematol.11934
- Ferretti A, Baldacci E, Miulli E, et al. Thrombopoietin receptor agonists to control immune thrombocytopenia in patients with active lymphoma. Br J Haematol. 2019;186(6):e217–e219. doi:10.1111/bjh.16114
- Kantarjian HM, Giles FJ, Greenberg PL, et al. Phase 2 study of romiplostim in patients with low- or intermediate-risk myelodysplastic syndrome receiving azacitidine therapy. Blood. 2010;116(17):3163–3170. doi:10.1182/blood-2010-03-274753
- Giagounidis A, Mufti GJ, Fenaux P, et al. Results of a randomized, double-blind study of romiplostim versus placebo in patients with low/intermediate-1-risk myelodysplastic syndrome and thrombocytopenia. Cancer. 2014;120(12):1838–1846. doi:10.1002/cncr.28663
- Fenaux P, Muus P, Kantarjian H, et al. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: long-term safety and efficacy. Br J Haematol. 2017;178(6):906–913. doi:10.1111/bjh.14792
- Kantarjian HM, Fenaux P, Sekeres MA, et al. Long-term follow-up for up to 5 years on the risk of leukaemic progression in thrombocytopenic patients with lower-risk myelodysplastic syndromes treated with romiplostim or placebo in a randomised double-blind trial. Lancet Haematol. 2018;5(3):e117–e126. doi:10.1016/S2352-3026(18)30016-4
- Mahévas M, Gerfaud-Valentin M, Moulis G, et al. Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood. 2016;128(12):1625–1630. doi:10.1182/blood-2016-03-704734
