Abstract
Purpose
Our study aimed to identify new-onset atrial fibrillation (NOAF) risk factors in acute myocardial infarction (AMI) patients after treatment with percutaneous coronary intervention (PCI) and investigate whether their nutritional status can be a predicting factor of NOAF.
Patients and Methods
We analyzed 662 AMI patients after PCI for NOAF occurrence during follow-up hospitalization and divided them into an NOAF and non-NOAF group. The patients’ nutritional status was assessed using the controlling nutritional status (CONUT) score and geriatric nutritional risk index (GNRI). The Kaplan‒Meier analysis was used to assess NOAF-free survival in varying degrees of malnutrition. Cox proportional hazards models were used to identify the risk factors for NOAF.
Results
Eighty-four (12.7%) patients developed NOAF during hospitalization. There was a statistically significant difference in the occurrence of NOAF among different categories of nutritional status. The CONUT score and GNRI classifications were independent predictors of NOAF. NOAF occurrence was associated with older age, higher uric acid levels, higher N-terminal pro-B-type natriuretic peptide levels, greater left atrial size, and worse Killip class upon admission.
Conclusion
The nutritional status can affect NOAF occurrence in AMI patients after PCI. The CONUT score and GNRI are ideal tools for evaluating the nutritional status of AMI patients, with an excellent predictive effect on NOAF.
Introduction
Atrial fibrillation (AF) is the most prevalent type of cardiac arrhythmia worldwide. It is also a common complication of acute myocardial infarction (AMI), occurring at a rate of 3% to 22%Citation1‒3 and is related to negative outcomes.Citation4,Citation5 Many studiesCitation6–9 have associated new-onset atrial fibrillation (NOAF) in AMI patients with in-hospital mortality and poor prognosis. Therefore, it is important to identify AMI patients who are at a higher risk of developing NOAF.
The nutritional status has been consistently linked to cardiovascular disorders such as heart failure, myocardial infarction and arrhythmias. However, malnutrition is a widespread condition among hospitalized patients. Some studies have shown that it is also associated with poorer outcomes in AMI, such as prolonged hospital stays, the risk of adverse complications and increased mortality.Citation10,Citation11 While timely nutritional intervention can improve patient outcomes, only a few studies have investigated the relationship between nutritional status and NOAF development in AMI patients.
Our study aimed to investigate whether nutritional status can be a predicting factor of NOAF and identify NOAF risk factors in AMI patients after treatment with percutaneous coronary intervention (PCI).
Materials and Methods
Study Population
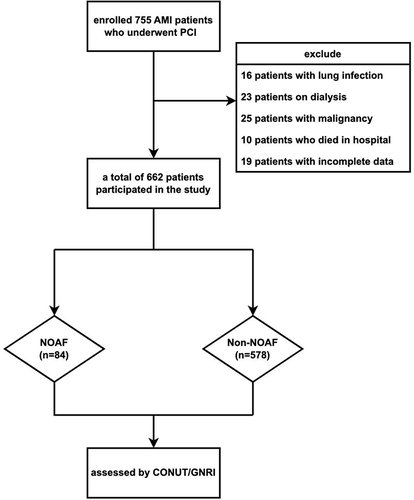
This retrospective study was conducted between January 2019 and January 2021 at a single center in China. We recruited 755 AMI patients without a history of AF who were undergoing PCI in the ZheJiang Provincial People’s Hospital. AMI included ST-segment elevation myocardial infarction (STEMI) and non- STEMI. These diagnostic standards met the European Society of Cardiology/American College of Cardiology diagnostic criteria for AMI.Citation12,Citation13 NOAF was defined as AF occurring during hospitalization after surgery based on documentation of AF episodes (≥ 30s in duration) using continuous telemetry throughout hospitalization, 12-lead electrocardiogram (ECG), or Holter monitoring. Exclusion criteria included (1) age < 18 years; (2) severe cardiac valve diseases or congenital heart disease; (3) end-stage renal disease (estimated glomerular filtration rate [eGFR] < 15 mL/min/1.73m2) or severe liver malfunction, cirrhosis, a history of hepatic malignancy, blood system diseases, or severe infections and other malignancies; (4) death during hospitalization; and (5) insufficient data. First, we enrolled 755 AMI patients who underwent PCI. Subsequently, we excluded 16 patients with lung infection, 23 on dialysis, 25 with malignancy, 10 who died in the hospital, and 19 with incomplete data. Ultimately, 662 patients participated in the study ().
Data Collection
The following general data were obtained from the patients’ medical records: age, sex, height, weight, body mass index assessed at the clinic, heart rate, systolic blood pressure, diastolic blood pressure, medication use, current smoking and drinking status, laboratory test results on the day of admission (white blood cell, lymphocyte, monocyte, neutrophil, platelet, and red blood cell count, hemoglobin, high-sensitivity C-reactive protein, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum creatinine, eGFR, N-terminal pro-B-type natriuretic peptide [NT-proBNP], B-type natriuretic peptide [BNP], and cardiac troponin I levels), presence of comorbidities such as hypertension, diabetes mellitus, history of stroke, history of coronary heart disease, chronic obstructive pulmonary disease, type of AMI, type of coronary artery stenosis, Killip class, and echocardiography results such as left atrial diameter (LAD) and left ventricular ejection fraction (LVEF). The body mass index was calculated as weight (kg) divided by height (m) squared. Coronary artery stenosis is defined as stenosis of ≥ 50% in any coronary artery (including the left main artery, left anterior descending artery, left circumflex artery, and right coronary artery) by coronary angiography. During physical examination, the patients were divided into four classes according to the Killip‒Kimball classification:Citation14 class I: no signs of heart failure; class II: left heart failure with lung rales occupying < 50% of the lung field; class III: acute pulmonary edema; and class IV: cardiogenic shock with varying degrees of hemodynamic changes. A well-trained, experienced sonographer performed transthoracic echocardiography.
Nutritional Status Evaluation
The controlling nutritional status (CONUT)Citation15 score was determined using serum albumin and total cholesterol levels and lymphocyte count (). According to the CONUT score, the patients were divided into three groups: normal (0–1), mild malnutriton (2-4), and moderate-severe malnutrition (≥ 5).Citation16 The geriatric nutritional risk index (GNRI) score was calculated using the serum albumin level and body weight using the following formula: GNRI = [1.489 × albumin (g/dL)] + [41.7 × body weight (kg)/ideal body weight (kg)]. The ideal body weight (kg) in men was calculated as height (cm) − 100 − [(height (cm) − 150)/4], and in women, it was calculated as height (cm) − 100 − [(height (cm) − 150)/2.5].Citation17 Similarly, according to the GNRI scores, the patients were divided into four groups: normal (> 98), mild malnutrition (92–98), moderate malnutrition (82–91), and severe malnutrition (< 82) groups.Citation18
Table 1 CONUT Scoring System
Follow-Up
All patients underwent continuous telemetry throughout hospitalization, using a 12-lead ECG at least once a day. They also completed a 24-hour Holter monitoring during hospitalization. The follow-up endpoint was the patient’s discharge. The follow-up period for each ECG was recorded daily. Analysis of ECG, continuous telemetry, and Holter monitoring showed that AF was considered to indicate NOAF occurrence.
Statistical Analysis
Normally distributed continuous variables are presented as mean and standard deviation, whereas non-normally distributed data are presented as median with interquartile range (IQR). The Kolmogorov–Smirnov test was used to analyze normally distributed data. The Mann‒Whitney U-test was used to analyze non-normally distributed data, while the Student’s t-test was used to analyze normally distributed data. Categorical data were described as frequencies (percentages) and analyzed with Pearson’s Chi-squared test. NOAF occurrence probability was calculated using a Kaplan–Meier analysis with the Log rank test over time. The multivariate Cox stepwise regression risk model was used to investigate the independent factors associated with NOAF after undergoing PCI for AMI. We used time-dependent covariate analysis to meet the proportional hazards assumption. The time-dependent covariates were examined, but none were found to be statistically significant. The potential risk factors of NOAF were initially identified by univariate Cox regression analysis. To validate that these factors had independent effects, significant univariate correlates were then added to the multivariate Cox regression. The P-value < 0.001 threshold value was set for variables included in the regression equation. All statistical analyses were performed using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA) and R version 4.1.2 (The R Project for Statistical Computing; http://www.R-project.org/). Statistical significance was defined as a two-sided P-value < 0.05.
Results
Baseline Data of the Two Groups of Patients
summarizes the patients’ baseline characteristics. The study’s 662 AMI patients enrolled comprised 325 (49.1%) STEMI and 337 (50.9%) non-STEMI patients. The patients’ average age was 63.71 ± 13.71 years, with approximately 80% of them being males. Eighty-four (12.7%) patients developed NOAF during hospitalization. The median time from admission to NOAF onset was 2 days, while the median duration of hospital stay was 7 days. There was no statistically significant difference between the two groups in terms of sex distribution, height, current smoking or drinking status, white blood cell, monocyte, and platelet counts, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, cardiac troponin I, medications, history of hypertension, stroke, chronic obstructive pulmonary disease, coronary heart disease, type of AMI, and coronary artery stenosis type. Compared with the patients in the non-NOAF group, those in the NOAF group were older and more likely to have higher heart rate, neutrophil count, high-sensitivity C-reactive protein, serum creatinine, uric acid, and NT-proBNP level, BNP, LAD, and Killip class (P < 0.05). Patients in the NOAF group had lower weight, body mass index, systolic blood pressure, diastolic blood pressure, lymphocyte and red blood cell counts, hemoglobin, serum albumin, and triglyceride levels, eGFR, and LVEF than those in the non-NOAF group (P < 0.05).
Table 2 Patients Characteristics
Nutritional Status Based on CONUT Score and GNRI
NOAF patients had a substantially higher CONUT score than non-NOAF patients (4.00 [2.00-6.00, IQR] vs 1.00 [0.50-3.50, IQR], P < 0.001; ). According to the CONUT score, 344 (52.0%), 210 (31.7%), and 108 (16.3%) patients were classified into the normal (0–1), mild malnutrition (2-4), and moderate-severe malnutrition (≥ 5) groups, respectively. Compared with the non-NOAF group, the NOAF group had significantly lower GNRI scores (92.50 [86.25-98.00, IQR] vs 101.00 [95.50-107.00, IQR], P < 0.001; ). Based on the GNRI scores, 263 (39.7%)patients were classified as normal (score > 98), 291 (44.0%)patients had mild malnutrition (score 92–98), 83 (12.5%) patients had moderate malnutrition (score 82–91), and 25 (3.8%) patients had severe malnutrition (score < 82). Compared with the non-NOAF group, the NOAF group had a higher prevalence of moderate and severe malnutrition according to the CONUT and GNRI scores.
Nutritional Status and NOAF Correlation
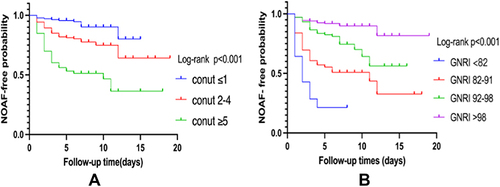
In total, 84 AMI patients developed NOAF during hospitalization: 11 (13.1%) patients in the normal group, 35 (41.7%) patients in the mild malnutrition group, and 38 (45.2%) patients in the moderate-severe malnutrition group based on the CONUT scoring system. In addition, 11 (13.1%), 25 (29.8%), 29 (34.5%), and 19 (22.6%) patients were categorized into the normal, mild malnutrition, moderate malnutrition, and severe malnutrition groups, respectively, based on the GNRI scoring system. The stratification of nutritional status, the occurrence of NOAF, and the time from admission to the occurrence of NOAF were included in the Kaplan–Meier analysis. shows that there was a statistically significant difference in NOAF occurrence among different nutritional status categories based on the CONUT (log-rank P<0.001; ) and GNRI (log-rank P<0.001; ) scores. The survival analysis plot shows that the curve of the group with the worst nutritional status changed dramatically during the first 5 days, indicating that patients with the worst nutritional status were those who are most susceptible to NOAF development.
Figure 2 Kaplan–Meier analysis of NOAF. (A) Kaplan–Meier analysis of AMI patients in different CONUT scores; (B) Kaplan– Meier analysis of AMI patients in different GNRI scores. p < 0.05 was deemed statistically significant.
Cox Regression Analysis of NOAF
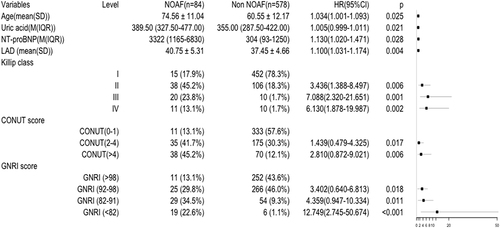
We used univariate Cox regression analysis based on variables with a P < 0.05 in the baseline data for both groups. The potential risk factors of NOAF were initially identified by univariate Cox regression analysis. As presented in , age, high-sensitivity C-reactive protein, uric acid, NT-proBNP levels, LAD, LVEF, Killip class, CONUT score and GNRI classification were significantly associated with NOAF (P < 0.05 for all variables). The subsequent multivariate Cox regression analysis demonstrated that age (adjusted hazard ratio [HR], 1.034; 95% confidence interval [CI]: 1.001–1.093; P = 0.025), uric acid level (adjusted HR, 1.005; 95% CI: 0.999-1.011; P = 0.021), NT-proBNP level (adjusted HR, 1.130; 95% CI: 1.020–1.471; P = 0.028), LAD (adjusted HR, 1.100; 95% CI: 1.031–1.174; P = 0.004), Killip class (II adjusted HR, 3.346; 95% CI: 1.388–8.497; P = 0.006; III adjusted HR, 7.088; 95% CI: 2.320–21.651; P = 0.001; IV adjusted HR, 6.130; 95% CI: 1.878–19.987; P = 0.002, vs I), CONUT score classification (mild malnutrition adjusted HR, 1.439; 95% CI: 0.479–4.325; P = 0.017; moderate-severe malnutrition adjusted HR, 2.810; 95% CI: 0.872–9.021; P = 0.006, vs normal) and GNRI classification (mild malnutrition adjusted HR, 3.402; 95% CI: 0.640–6.813; P = 0.018; moderate malnutrition adjusted HR, 4.359; 95% CI: 0.947–10.334; P = 0.011; severe malnutrition adjusted HR, 12.749; 95% CI: 2.745–50.674; P < 0.001, vs normal) were independent predictors of NOAF ().
Table 3 Cox Regression Analysis of Risk Factors Associated with NOAF
Figure 3 Forest plot of multivariable regression analyses.
Discussion
In this study, we assessed the relationship between risk factors and the nutritional status level and NOAF in patients with AMI after PCI. Our results highlight the fact that the nutritional status of patients with AMI should not be ignored. Patients with worse nutritional status, assessed using the CONUT and GNRI scoring systems, were more susceptible to developing NOAF within 5 days of admission. Additional findings associated with an increased risk of developing NOAF include older age, higher uric acid level, higher NT-proBNP level, increased left atrial size, and worse Killip class upon admission.
Patients with AMI and AF often have poorer prognoses with increased risks of all-cause mortality and associated comorbidities such as ischemic stroke and heart failure.Citation19–21 The incidence of NOAF as an AMI complication is 3%–22%.Citation25 In our study, NOAF occurred in 12.6% of patients, which is consistent with findings from previous studies.
Many studies have shown that NOAF development in AMI patients is associated with older age and higher uric acid and NT-proBNP levels, LAD, and Killip class. Advanced age has been recognized as a risk factor for AF.Citation22,Citation23 A study by Fu et alCitation24 suggested that older people are more likely to develop NOAF after AMI occurrence. Other studiesCitation25–27 have reported similar conclusions.
There is accumulating evidence that hyperuricemia is a risk factor for cardiovascular disease.Citation28,Citation29 As these previous studies did, we also discovered that higher uric acid level is an independent risk factor for NOAF in AMI patients. Kawasoe et alCitation30 revealed that men and women with uric acid levels higher than 6.5 mg/dL and 4.9 mg/dL, respectively, are more likely to experience AF in the future. Wang et alCitation31 revealed that uric acid was linked to NOAF in older patients with non-STEMI who had high uric acid level. It may be beneficial to propose a mechanism for observing the relationship between high uric acid levels and AF.
Both BNP and NT-proBNP are frequently utilized as heart failure diagnostic biomarkers.Citation32,Citation33 A number of studiesCitation34–36 have demonstrated that increased BNP or NT-proBNP levels are predictive biomarkers for the future development of AF. Our study found that NT-proBNP level is a risk factor for NOAF, while BNP level is not. This finding suggested that NT-proBNP is a more sensitive indicator of early and subtle hemodynamic alterations than BNP, as NT-proBNP is associated with other factors such as chamber enlargement, valvular disease, and left ventricular hypertrophy.Citation37 Asanin et alCitation38 also reported that a high level of BNP 24 hours after AMI symptom onset independently predicts NOAF occurrence in STEMI patients treated with primary PCI.
The Killip class is a simple and easy clinical tool for cardiac function assessment and risk stratification in AMI. Poorer cardiac function and outcomes are associated with a worse Killip classification. We noted that Killip class and enlarged left atrial size were independent predictors of NOAF, suggesting that elevated left atrial filling pressures and/or acute atrial dilatation plays an important role in AF development.Citation39 Experimental studies have demonstrated that increased atrial pressure results in a significant increase in AF vulnerability.Citation40
The assessment of nutritional status in patients with AF has largely focused on the relationship between obesity and AF, while the role of malnutrition has been largely ignored, particularly in AMI patients. However, malnutrition is very common in hospitalized patients. Recent data from the United States of America and Europe show that nearly a third of inpatients have malnutrition or are at risk of malnutrition at the time of admission.Citation41–43 Malnutrition is not only a result of illness, but also hastens its progression. Prior studiesCitation44 have also established a link between malnutrition and poor clinical outcomes, such as length of stay, mortality rate, readmission rate, and hospitalization costs, all of which makes early detection significant in preventing morbidity and the increase in healthcare costs.
The CONUT and GNRI scoring systems are frequently used to screen malnutrition in clinical settings.Citation15,Citation17,Citation45–47 Therefore, we used these scoring systems in our study. The predictive effect of malnutrition on the prognosis and recurrence of AF following radiofrequency ablation have been reported;Citation18,Citation48,Citation49 however, to our knowledge, this is the first study to illustrate the predictive role of varying degrees of malnutrition in the development of NOAF in patients treated with PCI after AMI. Furthermore, we observed that most patients developed NOAF within 5 days after admission and those with worsening nutritional status were more susceptible to NOAF. Therefore, timely nutritional risk assessment and early nutritional intervention are necessary.
Serum albumin is a well-known protein related to nutritional assessment. Hypoalbuminemia is deemed as a manifestation of middle and late stages of the disease and its existence is linked to a worse prognosis.Citation50–53 In a recent meta-analysis by Wang et al of nine studies with a total of 32,130 participants,Citation54 a significant negative linear relationship between serum albumin levels and the chance of developing AF was documented. This observation was consistent with previous findings and our results.Citation55 The anti-inflammatory properties of serum albumin have been well established. Oxidative stress has now emerged as an essential pathway in the pathogenesis of AF.Citation56 Moreover, low serum albumin levels promote pulmonary edema and fluid retention,Citation57 highlighting another possible mechanism contributing to the onset of AF, in which fat and muscle mass are reduced, including cholesterol and lipoproteins with bacterial endotoxin-neutralizing properties.Citation58,Citation59 Myostatin is a myokine produced and released by myocytes. This molecule plays an important role in muscle mass loss. Markedly lower expression of myostatin has been found in the atrial appendages of patients with persistent AF and in the hearts of decorin knockout mice.Citation60 In addition, others believe malnutrition leads to or is at least associated with deficiencies in trace elements, such as zinc, copper, magnesium, and vitamin D. Deficiencies in these trace elements and vitamins are associated with an increased risk of AF.Citation61,Citation62
In our study, we observed that AMI patients, especially those with poor nutritional status, were the most likely to develop NOAF within 5 days after PCI. It is unclear whether their poor nutritional status accelerates AF occurrence or if AF is caused by manipulation during cardiac interventional procedures. The incidence of postoperative AF has remained up to 30%.Citation63 By under-recognizing, and more importantly, under-treating poor nutritional status, the incidence rate of postoperative AF is not likely to decrease.Citation64 Therefore, future quality improvement strategies should consider patients’ nutritional status during the periprocedural periods to reduce the risk of AF.
Limitations
First, this was a single-center retrospective study with a small sample size, which may not be representative. Therefore, a multicenter study with a large sample size is required for further validation. Second, some patients could not calculate their CONUT and GNRI scores due to insufficient data at admission; hence, these patients were excluded. Third, as mitral E peak, left atrial volume index and E/Em were not demonstrated in our hospital’s cardiac ultrasound reports, we unfortunately could not include these indices in the study. Finally, we only investigated the relationship between the nutritional status and NOAF and did not further investigate the outcome after nutritional improvement. In future studies, we may investigate this relationship after the nutritional status has improved with a larger sample from multiple centers.
Conclusions
Among the various contributing factors of NOAF occurrence after PCI in AMI patients, one factor that cannot be ignored is the patient’s nutritional status. For early identification of NOAF, the GNRI and CONUT scoring systems used to assess nutritional status showed an excellent predictive effect in AMI patients after PCI.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study protocol was reviewed and approved by Ethics Committee of Zhejiang Provincial People’s Hospital, approval number: QT2022101. Informed consent was waived due to the retrospective nature of the study. At the same time, patient data was anonymized or maintained with confidentiality and this study was in line with the Declaration of Helsinki.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
First, I (Liuyang Wu) would like to express my gratitude to my teacher, Dr. Lihong Wang, who provided me with invaluable guidance in every stage of the writing. I also like to thank my classmates, Wei Wang, Yang Gui, and Qiqi Yan, who participated in this study with great cooperation.
Additional information
Funding
References
- Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30(9):1038–1045. doi:10.1093/eurheartj/ehn579
- Angeli F, Reboldi G, Garofoli M, et al. Atrial fibrillation and mortality in patients with acute myocardial infarction: a systematic overview and meta-analysis. Curr Cardiol Rep. 2012;14(5):601–610. doi:10.1007/s11886-012-0289-3
- Rathore SS, Berger AK, Weinfurt KP, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101(9):969–974. doi:10.1161/01.CIR.101.9.969
- Komici K, Vitale DF, Mancini A, et al. Impact of malnutrition on long-term mortality in elderly patients with acute myocardial infarction. Nutrients. 2019;11(2):224. doi:10.3390/nu11020224
- Nakamura T, Haraguchi Y, Matsumoto M, Ishida T, Momomura SI. Prognostic impact of malnutrition in elderly patients with acute myocardial infarction. Heart Vessels. 2022;37(3):385–391. doi:10.1007/s00380-021-01922-y
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi:10.1093/eurheartj/ehx393
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–1250. doi:10.1016/j.jacc.2015.10.005
- DeGeare VS, Boura JA, Grines LL, O’Neill WW, Grines CL. Predictive value of the killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2001;87:1035–1038. doi:10.1016/S0002-9149(01)01457-6
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45.
- Luo J, Liu B, Li H, et al. Prognostic impact of the symptom of new-onset atrial fibrillation in acute myocardial infarction: insights from the NOAFCAMI-SH registry. Front Cardiovasc Med. 2021;8:677695. doi:10.3389/fcvm.2021.677695
- Kim D, Shim J, Kim YG, et al. Malnutrition and risk of procedural complications in patients with atrial fibrillation undergoing catheter ablation. Front Cardiovasc Med. 2021;8:736042. doi:10.3389/fcvm.2021.736042
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
- Bishara R, Telman G, Bahouth F, Lessick J, Aronson D. Transient atrial fibrillation and risk of stroke after acute myocardial infarction. Thromb Haemost. 2011;106(5):877–884. doi:10.1160/TH11-05-0343
- Zhu S, Zhao H, Zheng M, Peng J. The impact of malnutrition on atrial fibrillation recurrence post ablation. Nutr Metab Cardiovasc Dis. 2021;31(3):834–840. doi:10.1016/j.numecd.2020.12.003
- Lee JH, Kim SH, Lee W, et al. New-onset paroxysmal atrial fibrillation in acute myocardial infarction: increased risk of stroke. BMJ Open. 2020;10(9):e039600. doi:10.1136/bmjopen-2020-039600
- Shiyovich A, Axelrod M, Gilutz H, Plakht Y. Early versus late new-onset atrial fibrillation in acute myocardial infarction: differences in clinical characteristics and predictors. Angiology. 2019;70(10):921–928. doi:10.1177/0003319719867542
- Wi J, Shin DH, Kim JS, et al. Transient new-onset atrial fibrillation is associated with poor clinical outcomes in patients with acute myocardial infarction. Circ J. 2016;80(7):1615–1623. doi:10.1253/circj.CJ-15-1250
- Kinjo K, Sato H, Sato H, et al. Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol. 2003;92(10):1150–1154. doi:10.1016/j.amjcard.2003.07.021
- Luo J, Xu S, Li H, et al. Long-term impact of new-onset atrial fibrillation complicating acute myocardial infarction on heart failure. ESC Heart Fail. 2020;7(5):2762–2772. doi:10.1002/ehf2.12872
- Fauchier L, Bisson A, Bodin A, et al. Outcomes in patients with acute myocardial infarction and new atrial fibrillation: a nationwide analysis. Clin Res Cardiol. 2021;110(9):1431–1438. doi:10.1007/s00392-021-01805-2
- Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120(9):1501–1517. doi:10.1161/CIRCRESAHA.117.309732
- Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4–20. doi:10.1161/CIRCRESAHA.120.316340
- Fu Y, Pan Y, Gao Y, Yang X, Chen M. Predictive value of CHA2DS2-VASc score combined with hs-CRP for new-onset atrial fibrillation in elderly patients with acute myocardial infarction. BMC Cardiovasc Disord. 2021;21(1):175. doi:10.1186/s12872-021-01978-8
- Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123(15):1587–1593. doi:10.1161/CIRCULATIONAHA.110.986661
- He J, Yang Y, Zhang G, Lu XH. Clinical risk factors for new-onset atrial fibrillation in acute myocardial infarction: a systematic review and meta-analysis. Medicine. 2019;98(26):e15960. doi:10.1097/MD.0000000000015960
- Yi JE, Seo SM, Lim S, et al. Gender differences in the impact of new-onset atrial fibrillation on long-term risk of ischemic stroke after acute myocardial infarction. J Clin Med. 2021;10(21):5141. doi:10.3390/jcm10215141
- Wang J, Yang YM, Zhu J. Mechanisms of new-onset atrial fibrillation complicating acute coronary syndrome. Herz. 2015;40:18–26. doi:10.1007/s00059-014-4149-3
- Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–57. doi:10.1016/j.jjcc.2020.12.013
- Hisatome I, Li P, Miake J, et al. Uric acid as a risk factor for chronic kidney disease and cardiovascular disease – Japanese guideline on the management of asymptomatic hyperuricemia. Circ J. 2021;85(2):130–138. doi:10.1253/circj.CJ-20-0406
- Kawasoe S, Kubozono T, Yoshifuku S, et al. Uric acid level and new-onset atrial fibrillation in the Japanese general population – longitudinal study. Circ J. 2018;83(1):156–163. doi:10.1253/circj.CJ-18-0508
- Wang Y, Wang XD, Yao JW, et al. The impact of the duration of cardiac troponin I elevation on the clinical prognosis as well as incidence of new-onset atrial fibrillation respectively in elderly non-ST-elevation acute myocardial infarction patients without PCI. J Inflam Res. 2021;14:6907–6916. doi:10.2147/JIR.S345576
- Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American heart association. Circulation. 2017;135(22):e1054–e1091. doi:10.1161/CIR.0000000000000490
- Tsutsui H, Isobe M, Ito H, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure – digest version. Circ J. 2019;83(10):2084–2184. doi:10.1253/circj.CJ-19-0342
- Nagata T, Hata J, Sakata S, et al. Serum N-terminal pro-B-type natriuretic peptide as a predictor for future development of atrial fibrillation in a general population: the hisayama study. Int J Cardiol. 2020;320:90–96. doi:10.1016/j.ijcard.2020.06.018
- Asselbergs FW, van den Berg MP, Bakker SJ, et al. N-terminal pro B-type natriuretic peptide levels predict newly detected atrial fibrillation in a population-based cohort. Neth Heart J. 2008;16(3):73–78. doi:10.1007/BF03086122
- Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121(2):200–207. doi:10.1161/CIRCULATIONAHA.109.882241
- Dorje T, Wang X, Shao M, et al. Plasma N-terminal pro-brain natriuretic peptide levels predict new-onset atrial fibrillation in patients with acute myocardial infarction. Int J Cardiol. 2013;168(3):3135–3137. doi:10.1016/j.ijcard.2013.04.032
- Asanin M, Stankovic S, Mrdovic I, et al. B-type natriuretic peptide predicts new-onset atrial fibrillation in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Peptides. 2012;35(1):74–77. doi:10.1016/j.peptides.2012.02.022
- Bahouth F, Mutlak D, Furman M, et al. Relationship of functional mitral regurgitation to new-onset atrial fibrillation in acute myocardial infarction. Heart. 2010;96(9):683–688. doi:10.1136/hrt.2009.183822
- Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi:10.1161/01.CIR.96.5.1686
- Gomes F, Schuetz P, Bounoure L, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–353. doi:10.1016/j.clnu.2017.06.025
- Felder S, Braun N, Stanga Z, et al. Unraveling the link between malnutrition and adverse clinical outcomes: association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann Nutr Metab. 2016;68(3):164–172. doi:10.1159/000444096
- Schuetz P, Seres D, Lobo DN, Gomes F, Kaegi-Braun N, Stanga Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet. 2021;398(10314):1927–1938. doi:10.1016/S0140-6736(21)01451-3
- Adejumo AC, Adejumo KL, Adegbala OM, et al. Inferior outcomes of patients with acute myocardial infarction and comorbid protein-energy malnutrition. JPEN J Parenter Enter Nutr. 2020;44(3):454–462. doi:10.1002/jpen.1680
- Takahashi T, Watanabe T, Otaki Y, et al. Prognostic significance of the controlling nutritional (CONUT) score in patients with acute coronary syndrome. Heart Vessels. 2021;36(8):1109–1116. doi:10.1007/s00380-021-01792-4
- Kato T, Yaku H, Morimoto T, et al. Association with Controlling Nutritional Status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep. 2020;10(1):3320. doi:10.1038/s41598-020-60404-9
- Ruan GT, Zhang Q, Zhang X, et al. Geriatric nutrition risk index: prognostic factor related to inflammation in elderly patients with cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12(6):1969–1982. doi:10.1002/jcsm.12800
- Díez-Manglano J, Clemente-Sarasa C. The nutritional risk and short-, medium- and long-term mortality of hospitalized patients with atrial fibrillation. Aging Clin Exp Res. 2019;31(12):1775–1781. doi:10.1007/s40520-019-01152-3
- Furui K, Morishima I, Morita Y, et al. Impact of preoperative nutritional status on the outcome of catheter ablation for atrial fibrillation. Circ J. 2022;86(2):268–276. doi:10.1253/circj.CJ-21-0218
- Çinier G, Hayıroğlu Mİ, Kolak Z, et al. The value of C-reactive protein-to-albumin ratio in predicting long-term mortality among HFrEF patients with implantable cardiac defibrillators. Eur J Clin Invest. 2021;51(8):e13550. doi:10.1111/eci.13550
- Çınar T, Hayıroğlu Mİ, Çiçek V, et al. Is prognostic nutritional index a predictive marker for estimating all-cause in-hospital mortality in COVID-19 patients with cardiovascular risk factors? Heart Lung. 2021;50(2):307–312. doi:10.1016/j.hrtlng.2021.01.006
- Hayıroğlu Mİ, Çınar T, Çinier G, et al. Prognostic value of serum albumin for long-term mortality in patients with dual-chamber permanent pacemakers. Biomark Med. 2022;16(5):341–348. doi:10.2217/bmm-2021-0991
- Hayıroğlu Mİ, Çınar T, Çinier G, et al. Cardiac variables associated with atrial fibrillation occurrence and mortality in octogenarians implanted with dual chamber permanent pacemakers. Aging Clin Exp Res. 2022;34(10):2533–2539. doi:10.1007/s40520-022-02194-w
- Wang Y, Du P, Xiao Q, et al. Relationship between serum albumin and risk of atrial fibrillation: a dose-response meta-analysis. Front Nutr. 2021;8:728353. doi:10.3389/fnut.2021.728353
- Liao LZ, Zhang SZ, Li WD, et al. Serum albumin and atrial fibrillation: insights from epidemiological and mendelian randomization studies. Eur J Epidemiol. 2020;35(2):113–122. doi:10.1007/s10654-019-00583-6
- Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115(2):135–143. doi:10.1016/j.ijcard.2006.04.026
- Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail. 2011;17(6):451–458. doi:10.1016/j.cardfail.2011.02.010
- Anaszewicz M, Budzyński J. Clinical significance of nutritional status in patients with atrial fibrillation: an overview of current evidence. J Cardiol. 2017;69(5):719–730. doi:10.1016/j.jjcc.2016.06.014
- Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356(9233):930–933. doi:10.1016/S0140-6736(00)02690-8
- Barallobre-Barreiro J, Gupta SK, Zoccarato A, et al. Glycoproteomics reveals decorin peptides with anti-myostatin activity in human atrial fibrillation. Circulation. 2016;134(11):817–832. doi:10.1161/CIRCULATIONAHA.115.016423
- Yan YQ, Zou LJ. Relation between zinc, copper, and magnesium concentrations following cardiopulmonary bypass and postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Biol Trace Elem Res. 2012;148(2):148–153. doi:10.1007/s12011-012-9356-2
- Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost. 2014;20(1):98–103. doi:10.1177/1076029612453762
- Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52(4):665–672. doi:10.1093/ejcts/ezx039
- Goldman SM. Commentary: postoperative atrial fibrillation: “no magic bullet”. J Thorac Cardiovasc Surg. 2021;161(5):1812–1813. doi:10.1016/j.jtcvs.2019.11.067