Abstract
Objective
This study aimed to explore possible biomarkers of postoperative delirium (POD) of Parkinson’s disease (PD) patients received deep brain stimulation (DBS) of the subthalamic nuclei.
Materials and methods
This nested case control study analyzed perioperative plasma and cerebral spinal fluid (CSF) of patients (n = 40) who developed POD undergone DBS surgery (n = 10) and those who did not (n = 30). Blood sample was collected before surgery and on the first day postoperative, CSF sample was collected at the beginning of the operation. POD was assessed by the Confusion Assessment Method (CAM) twice a day between 7:00 am and 7:00 pm after the surgery until discharge. Plasma and CSF sample from the two groups were analyzed to investigate possible biomarkers for POD in PD patients.
Results
There was no difference between POD and Non-POD groups on the concentration of Interleukin 6 and Tumor Necrosis Factor-α in CSF, preoperative plasma and postoperative plasma. There was no difference between POD and Non-POD groups on the concentration of S100 calcium-binding protein β protein (S100β) and Neurofilament light chain (NFL) in preoperative plasma and postoperative plasma. The concentration of C-reactive protein (CRP), NFL and S100β were significant higher in POD group than non-POD group in CSF. The concentration of CRP was significantly higher in POD group than non-POD group in preoperative plasma and postoperative plasma. CSF concentration of S100β might be a potential biomarker for POD via the receiver operating characteristic curve analysis and the area under the curve value of 0.973.
Conclusion
For PD patients received DBS surgery, CSF S100β might be a marker for aiding detection of high-risk patients with delirium. This requires further confirmation in clinical trials.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease,Citation1 and the advanced PD patients need the Deep brain stimulation of the subthalamic nuclei(STN-DBS) which improves motor and non-motor symptoms,Citation2–4 and enhance the quality of life.Citation3–5
Unfortunately, as one of the most common neuropsychiatric complications following DBS surgery,Citation6 the incidence of delirium was 22% to 42.6% of patients after DBS surgery.Citation7,Citation8 Postoperative delirium (POD) is common, serious, costly, under-recognized, and often fatal.Citation9 For PD patients, POD is an increased risk factor for developing dementia and death.Citation9
However, the pathogenesis of POD is unclear.Citation10 Detecting PD patients at high risk of POD would promote recovery after DBS surgery. Since the pathogenesis of POD is unclear, and clinical symptoms are diverse and fluctuate, the specific biomarkers to predict POD have important implications.Citation11 The etiology of delirium was multifactorial.Citation12 Currently, there is no convincing evidence showed that pharmacologic prevention is effective.Citation9 Therefore, it is particularly important to screen high-risk patients and intervene in advance. The hypothesized mechanisms of delirium include neurotransmitters,Citation13 inflammation,Citation14 physiologic stressors,Citation15 metabolic derangements,Citation16 electrolyte disorders,Citation17 and genetic factors.Citation18
This present study was conducted to explore possible biomarkers of POD in PD patients after DBS surgery. The findings might provide valuable scientific clues for the early detection and treatment of POD in PD patients after DBS surgery.
Methods
Study Design
This study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Yuquan Hospital of Tsinghua University (20,190,014). All patients enrolled have signed informed consent. The clinical trial registration number was ChiCTR1900027210.
Study Population
The study was conducted between November 2019 and October 2020 in Yuquan Hospital of Tsinghua University (Beijing, China). The PD patients were diagnosed according to the UK Brain Bank criteriaCitation19 and planned to receive bilateral STN-DBS treatment. Bilateral STN-DBS treatment followed the Movement Disorders Society guidelines.Citation20 The inclusion and contraindications for DBS surgery were according to the Chinese deep brain stimulation therapy for Parkinson’s disease Expert Consensus (Second Edition).Citation21
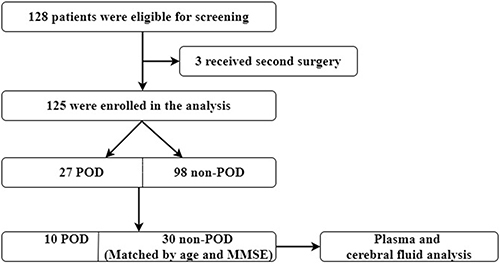
The PD patients (1) were unable to read or had severe visual or auditory deficits, or (2) had a history of alcohol abuse and drug dependence or (3) were unwilling to comply with the study protocol or procedures were excluded.Citation22,128 patients participated in this study, 125 participants (27 POD vs 98 Non-POD) completed the study (, flow diagram). It was a nested case control study. Considering the cost of testing, we randomly chose 10 cases from the 27 POD patients using the random number table in order to make sure the two groups are comparable, 10 POD patients and the matched 30 non-POD patients by age (±2 years) and MMSE score (± 2 points) were chosen to analyze.
Neuropsychological Testing
Preoperatively, Clinical Dementia Rating score, Instrumental Activity of Daily Living score, MMSE score, Montreal Cognitive Assessment score, Hamilton Anxiety Rating Scale, and Hamilton depression Rating Scale were performed in order to estimate if these patients were suitable for surgery. The Confusion assessment method (CAM)Citation9 was used to screen POD twice a day between 7:00 am and 7:00 pm after the surgery until discharge.
Anesthesia and Surgery
A specific team is in charge of the general anesthesia and surgery to avoid interfering factors. The electrocardiograph, non-invasive blood pressure, heart rate (HR), saturation of pulse oximetry, and Bispectral Index (BIS) were monitored during anesthesia and were recorded at fixed intervals of 3 min.
The systemic blood pressure was adjusted to be higher than 90 mm Hg. The anesthesia depth was monitored by the BIS (BIS 40–60).Citation23
In this study, the surgery was divided into two parts. Firstly, under the local anesthesia with minimal sedation, the stereotactic DBS electrodes were implanted in the STN. And the place of the electrodes was confirmed by imaging examination. Secondly, the DBS batteries and leads were placed in the sub-clavicular region under general anesthesia.
The same analgesic strategy was used to relieve postoperative pain as following: sufentanil 2μg/kg + dexmedetomidine 2.3µg/kg diluted to 100 mL, the background rate was 2 mL/h, the dosage of PCA was 0.5 mL, and the locking time was 15 min.
Sample Collection
2 mL venous blood was collected through the peripheral vein before the anesthesia. During the DBS surgery, bilateral electrode implantation sites were determined under stereo positioning, and then a U-shaped incision about 5cm in diameter was made around the implantation site to peel the scalp and expose the skull. A hole is drilled into the skull centered on the site of implantation and the cerebral dura was cut open. At this point, about 2mL of CSF was taken with a 5mL syringe by the surgeon.
The Visual Analog Scale (VAS) scores following surgery were recorded. The intensity of postoperative pain was evaluated twice daily at 7:00 am and 7:00 pm with the VAS. The VAS pain scale ranged from 0 to 10 with 0 corresponding to “no pain” and 10 to the “worst possible pain”, the number patients pointed out to indicate the pain intensity.
On the first postoperative day, 2mL venous blood was taken when routine blood was drawn in the ward for other examinations.
All the samples were centrifuged immediately at 3000 rpm for 10min at 4°C, and the supernatant was gathered and stored at −80°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of Interleukin 6 (IL-6), Tumor Necrosis Factor-α (TNF-α), Neurofilament light chain (NFL), S100 calcium-binding protein β protein (S100β) and C-reactive protein (CRP) in the supernatant of CSF and plasma were determined using commercial ELISA kits according to the manufacturer’s instructions. The CRP and IL-6 kits were from Abcam, ab260058 and ab178013, the TNF-α and NFL kits were from International GmbH, BE58351 and 30,112,458, the S100β kits were from R&D Systems, DY1820-5. The optical density (OD) of each well was determined using an ELISA reader at 450 nm.
Statistical Analysis
The data are described as the mean ± standard deviation (SD), the median, or the number (%). The normality of the variables was test by the Kolmogorov–Smirnov method. Categorical and continuous variables were analyzed using χ2 test and independent-samples t-test respectively. The non-normal variables were analyzed by Mann–Whitney U-test. Statistical significance was considered at p < 0.05. SPSS software (version 21.0; IBM Corp., Armonk, NY, USA) was used for data analysis. In order to evaluate the potential biomarkers for POD, the receiver operating characteristic (ROC) curves and the area under the curve (AUC) were calculated.
Results
Participant Characteristics
In the present study, 10 CSF samples were collected from POD patients, and 30 matched CSF samples were collected from non-POD patients. There were no differences in the basic characteristics between the two groups including age, MMSE score, gender, body mass index, American Society of Anesthesiologists (ASA) physical class, length of anesthesia and surgery, postoperative VAS scores, intraoperative infusion volume, estimated blood loss and intraoperative urinary volume. The length of stay in the hospital was significantly longer in the POD group than in the non-POD group. There were no deaths in either group ().
Table 1 Subject Characteristics
Comparison of CSF Levels of Cytokines Between POD and Non-POD Patients
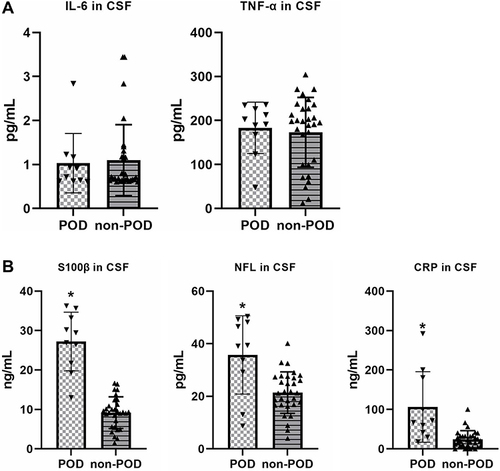
There was no difference between POD and Non-POD groups on the concentration of IL-6 and TNF-α in CSF at the beginning of surgery (). However, the concentration of S100β, NFL and CRP were significantly higher in POD group than non-POD group at the beginning of surgery ().
Figure 2 (A) There was no difference between POD and Non-POD groups on the concentration of IL-6 and TNF-α in cerebral spinal fluid at the beginning of surgery; (B) the concentration of CRP, NFL and S100β were significant higher in POD group than non-POD group at the beginning of surgery. *p < 0.05 compared with non-POD group.
Comparison of Preoperative Plasma Levels of Cytokines Between POD and Non-POD Patients
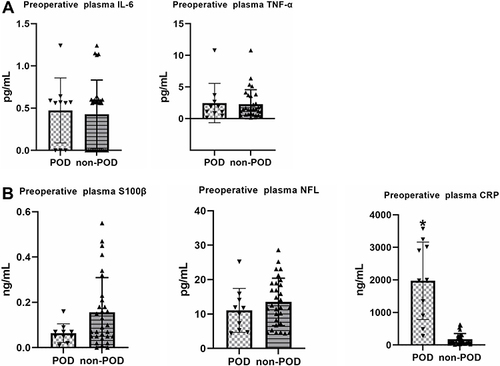
There was no difference between POD and Non-POD groups on the concentration of IL-6, TNF-α, S100β and NFL in preoperative plasma. However, the concentration of CRP was significantly higher in POD group than non-POD group ().
Figure 3 (A) There was no difference between POD and Non-POD groups on the concentration of IL-6 and TNF-α in preoperative plasma; (B) there was no difference between POD and Non-POD groups on the concentration of S100β and NFL in preoperative plasma. The concentration of CRP was significant higher in POD group than non-POD group in preoperative plasma. *p < 0.05 compared with non-POD group.
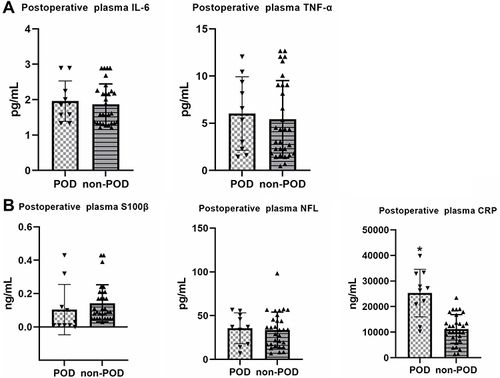
Comparison of Postoperative Plasma Levels of Cytokines Between POD and Non-POD Patients
There was no difference between POD and Non-POD groups on the concentration of IL-6, TNF-α, S100β and NFL in postoperative plasma. However, the concentration of CRP was significantly higher in POD group than non-POD group ().
Figure 4 (A) There was no difference between POD and Non-POD groups on the concentration of IL-6 and TNF-α in postoperative plasma; (B) there was no difference between POD and Non-POD groups on the concentration of S100β and NFL in postoperative plasma. The concentration of CRP was significant higher in POD group than non-POD group in postoperative plasma. *p < 0.05 compared with non-POD group.
Univariate and Multivariate Logistic Regressions
The logistic regressions were performed to identify the potential risk factors of POD. All the variables were screened using the univariate regression and the variables with P<0.05 were selected for multivariate logistic regression. Among the variables screened, the S100β concentrations in CSF might be an independent influence factor of POD ().
Table 2 Univariate and Multivariate Logistic Regressions
Diagnostic Analysis of Indicators for the Prediction of POD
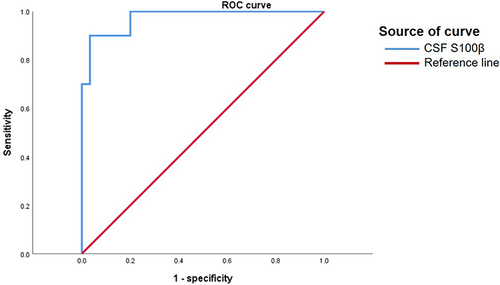
The total area under the curve AUC, cut-off, sensitivity, specificity, and Youden index of independent influence factors of POD are shown in .
Table 3 Diagnostic Analysis of Indicators for the Prediction of POD
The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off score for the diagnosis of POD. The optimal score was calculated according to the Youden index (maximum of [sensitivity + specificity-1]).Citation24
In the present study, 17.982 as the optimal cut-off score of S100β concentrations in CSF-associated with the POD. The sensitivity and specificity were 90.0% and 96.7%, respectively. The AUC was 0.973 (95% CI: 0.928–1.000, P < 0.05) (, ).
Discussion
PD is a common neurodegenerative disease in the elders, and the STN-DBS treatment is a standard option for advanced PD patients.Citation19 However, POD is common after DBS surgery and associated with worsened clinical outcomes, increased costs and mortality.Citation9
There was a link between inflammation markers and delirium.Citation25,Citation26 Elevated inflammatory biomarkers have been indicated as predictors of delirium in the general populationCitation27 including one of the most commonly examined markers of systemic inflammation CRP,Citation28,Citation29 TNF-αCitation30 and IL-6.Citation31,Citation32
Sarinnapha et al's study showed high preoperative and postoperative day 2 plasma CRP were independently associated with delirium incidence, duration, and feature severity. CRP might be useful to identify individuals who are at risk of developing delirium.Citation33 Slor, Chantal J also reported that delirium is associated with an increased systemic inflammatory response, and suggest that CRP plays a role in the underlying pathological pathway of POD.Citation34 Dimitrios Adamis reported that high levels of CRP are associated with delirium in acute orthopaedic and elective abdominal operations.Citation35 However, Mehmet Alper ÇINAR1and et al reported that there was no difference between POD and non-POD group on the blood level of CRP.Citation28 Therefore, CRP was also selected as a detection indicator in our study. We also noted that the concentration of CRP was significantly higher in POD group than non-POD group in CSF, preoperative plasma and postoperative plasma.
TNF-α and IL-6 were associated with memory and learning.Citation36,Citation37 High levels of IL-6 seem a consistent predictor for delirium in surgical samples.Citation35 Kazmierski et al reported an elevation of TNF-α levels in the postoperative period in coronary artery bypass graft (CABG) patients with delirium.Citation30 Ganna Androsova1 mentioned IL-6 and TNF-α could be biomarkers in predicting delirium.Citation32 Plaschke K reported that early POD after cardiac surgery is associated with increased IL-6.Citation31 However, in the present study, there was no difference between POD and Non-POD groups on the concentration of IL-6 and TNF-α in CSF, preoperative plasma and postoperative plasma.
NFL protein is an intermediate filament in the cytoplasm of neurons, which is mainly expressed in the dendrites and axons of neurons, and plays an important role in the construction of cytoskeleton to maintain the structural stability of neurons.Citation38,Citation39 At present, the increased level of NFL in CSF and blood has been detected in Alzheimer’s disease, amyotrophic lateral sclerosis (ALS) and brain injury, and it is believed that the increased concentration of NFL is correlated with the progression of the disease.Citation40,Citation41 In the study of Linda P, patients with higher level of CSF neurofilament light were accompanied with worse memory, attentional and executive functioning.Citation42 Saller mentioned in their study that NFL might be of benefit to identify high risk patients for delirium.Citation43 In the present study, the concentration of NFL was significantly higher in POD group than non-POD group.
S100β is a member of the calcium‑regulated protein S100 family and is characterized by two calcium‑binding sites with EF‑hand conformations.Citation44 It is mainly expressed by astrocytes and found both intra- and extra-cellularly in brain tissue.Citation45 Because of functional impairment of membrane integrity and/or increased blood-brain barrier permeability, it is usually elevated in blood and CSF after nervous system injury.Citation46 Therefore, it could have a clinical role in the assessment of brain injury and could be a marker of brain damage used in various diseases.Citation47
S100β has also attracted attention in the field of biomarkers of delirium.Citation48–51 Layth Al Tmimi et al reported that serum-levels of S100β measured on the first postoperative day in POD patients were significantly higher than non-POD patients.Citation52 S100β was proposed by Khan et al as a promising and specific marker for delirium.Citation53
In the present study, it was also showed that the S100β level in CSF was indicated to be independent influence factors of POD. There was no difference between POD and Non-POD groups on the concentration of S100β in preoperative plasma and postoperative plasma. However, the concentration of S100β was significant higher in POD group than non-POD group in CSF. And among the variables screened, S100β concentrations in CSF was displayed to be independent risk factors of POD.
One possible mechanism for the higher level of S100β after delirium is active stimulation of astrocytes or increased permeability of the blood-brain barrier.Citation54–56 The neurobehavioral and cognitive characteristic of delirium associated by the change in synaptic transmission, neural excitability, and cerebral blood flow resulted by high CSF S100β.Citation57 S100β has been considered as a putative biomarker of CNS damage, and increased CSF and serum levels of S100β are associated with the adverse CNS outcomes, especially delirium.Citation58–60 Another hypothesis could be activation of the nuclear factor κB signal pathways and lead to up-regulation of proinflammatory mediators.Citation61 S100β may play a role in BBB disruption and contribute to delirium.Citation54 Finally, CSF could accurately reflect biochemical changes in the central nervous system.Citation11 Compared with plasma, CSF S100β level was more significant in predicting the POD.Citation42
For PD patients undergoing DBS surgery, it is necessary to cut the dural during the surgery. Collecting CSF at this time is simple to do and without additional damage. So, the CSF S100β might be a marker for aiding early diagnosis of delirium after DBS surgery for PD patients.
We also did a number of measures to reduce the possible risk of POD just as following. Deep anesthesia might associate with delirium.Citation23 So, in the present study, BIS value was maintained within 40 to 60 in order to avoid episodes of deep anesthesia. POD is also associated with reduction in the intraoperative cerebral blood flow (CBF).Citation62 Therefore, the systemic blood pressure was kept higher than 90 mm Hg to ensure adequate CBF in the present study. Some studies showed severe pain may induce cognitive decline after surgery,Citation63 so, all the patients in this study received effective pain control.
Limitations
Although CSF S100β could be regarded as a potential biomarker of delirium in this study, limitations should be noticed. First, we aimed to recruit the maximum number of patients available and have not performed power calculations. Second, it was a monocentric study as only a few hospitals currently perform the DBS surgery. Third, there were only 10 POD patients and 30 non-POD patients in the biomarker analysis. Further analysis needs to be performed in future studies.
Conclusion
In this exploratory study of PD patients received DBS surgery, CSF S100β might be a marker for aiding detect high-risk patients of delirium. This requires further confirmation in clinical trials.
Data Sharing Statement
All the authors intend to share individual deidentified participant data including all the raw data. Immediately after the article is published, all the data could be shared by email to the corresponding author.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Georgiev D, Mencinger M, Rajnar R, et al. Long-term effect of bilateral STN-DBS on non-motor symptoms in Parkinson’s disease: a four-year observational, prospective study. Parkinsonism Relat Disord. 2021;89:13–16. doi:10.1016/j.parkreldis.2021.06.017
- Dafsari HS, Reddy P, Herchenbach C, et al. Beneficial effects of bilateral subthalamic stimulation on non-motor symptoms in parkinson’s disease. Brain Stimul. 2016;9(1):78–85. doi:10.1016/j.brs.2015.08.005
- Dafsari HS, Weiß L, Silverdale M, et al. Short-term quality of life after subthalamic stimulation depends on non-motor symptoms in Parkinson’s disease. Brain Stimul. 2018;11(4):867–874. doi:10.1016/j.brs.2018.02.015
- Wang XQ, Zhuang HX, Zhang LX, Chen X, Niu CS, Zhao M. Nomogram for predicting postoperative delirium after deep brain stimulation surgery for parkinson’s disease. World Neurosurg. 2019;130:e551–e557. doi:10.1016/j.wneu.2019.06.151
- Boussac M, Arbus C, Klinger H, et al. Personality related to quality-of-life improvement after deep brain stimulation in Parkinson’s disease (PSYCHO-STIM II). J Parkinsons Dis. 2021. doi:10.3233/JPD-212883
- Li H, Han S, Feng J. Delirium after deep brain stimulation in Parkinson’s disease. Parkinsons Dis. 2021;2021:8885386. doi:10.1155/2021/8885386
- Tanaka M, Tani N, Maruo T, et al. Risk factors for postoperative delirium after deep brain stimulation surgery for Parkinson disease. World Neurosurg. 2018;114:e518–e523. doi:10.1016/j.wneu.2018.03.021
- Zhan L, Wang XQ, Zhang LX. Nomogram model for predicting risk of postoperative delirium after deep brain stimulation surgery in patients older than 50 years with Parkinson disease. World Neurosurg. 2020;139:e127–e135. doi:10.1016/j.wneu.2020.03.160
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
- Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi:10.1016/j.bja.2020.06.063
- Han Y, Chen W, Song Y, et al. Proteomic analysis of preoperative CSF reveals risk biomarkers of postoperative delirium. Front Psychiatry. 2020;11:170. doi:10.3389/fpsyt.2020.00170
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852–857. doi:10.1001/jama.1996.03530350034031
- Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65(3):229–238. doi:10.1016/j.jpsychores.2008.05.019
- Katsumi Y, Racine AM, Torrado-Carvajal A, et al. The Role of Inflammation after Surgery for Elders (RISE) study: examination of [(11)C]PBR28 binding and exploration of its link to post-operative delirium. Neuroimage Clin. 2020;27:102346. doi:10.1016/j.nicl.2020.102346
- Lee SS, Lo Y, Verghese J. Physical activity and risk of postoperative delirium. J Am Geriatr Soc. 2019;67(11):2260–2266. doi:10.1111/jgs.16083
- Kealy J, Murray C, Griffin EW, et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020;40(29):5681–5696. doi:10.1523/JNEUROSCI.2876-19.2020
- Conti E, Andreoni S, Tomaselli D, et al. Serum DBI and biomarkers of neuroinflammation in Alzheimer’s disease and delirium. Neurol Sci. 2021;42(3):1003–1007. doi:10.1007/s10072-020-04608-x
- Sepulveda E, Adamis D, Franco JG, Meagher D, Aranda S, Vilella E. The complex interaction of genetics and delirium: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2021;271(5):929–939. doi:10.1007/s00406-021-01255-x
- Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266. doi:10.1002/mds.27372
- Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66(5):620–631. doi:10.1001/archneurol.2009.48
- Zhang H, Chen, L, Chen, S et al. Chinese deep brain stimulation therapy for Parkinson’s disease Expert Consensus (Second Edition). Chin Neurosurg J. 2020;36:325–337. doi:10.376/cma.j.cn112050-20200217-00062.
- Han Y, Zhang W, Liu J, et al. Metabolomic and lipidomic profiling of preoperative CSF in elderly hip fracture patients with postoperative delirium. Front Aging Neurosci. 2020;12:570210. doi:10.3389/fnagi.2020.570210
- Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. doi:10.1097/ANA.0b013e3182712fba
- Bantis LE, Nakas CT, Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70(1):212–223. doi:10.1111/biom.12107
- Beloosesky Y, Grinblat J, Pirotsky A, Weiss A, Hendel D. Different C-reactive protein kinetics in post-operative Hip-fractured geriatric patients with and without complications. Gerontology. 2004;50(4):216–222. doi:10.1159/000078350
- Vasunilashorn SM, Ngo L, Inouye SK, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol a Biol Sci Med Sci. 2015;70(10):1289–1295. doi:10.1093/gerona/glv083
- Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. doi:10.1111/j.1755-5949.2010.00173.x
- Çinar MA, Balikçi A, Sertoğlu E, Mehmet AK, Serdar MA, Özmenler KN. Role of CRP, TNF-a, and IGF-1 in Delirium Pathophysiology. Noro Psikiyatr Ars. 2014;51(4):376–382. doi:10.5152/npa.2014.6999
- Kotfis K, Ślozowska J, Safranow K, Szylińska A, Listewnik M. The practical use of white cell inflammatory biomarkers in prediction of postoperative delirium after cardiac surgery. Brain Sci. 2019;9:11. doi:10.3390/brainsci9110308
- Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-α concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr. 2014;26(5):845–855. doi:10.1017/S1041610213002378
- Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36(12):2081–2089. doi:10.1007/s00134-010-2004-4
- Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. doi:10.3389/fnagi.2015.00112
- Vasunilashorn SM, Dillon ST, Inouye SK, et al. High C-reactive protein predicts delirium incidence, duration, and feature severity after major noncardiac surgery. J Am Geriatr Soc. 2017;65(8):e109–e116. doi:10.1111/jgs.14913
- Slor CJ, Witlox J, Adamis D, et al. The trajectory of C-reactive protein serum levels in older Hip fracture patients with postoperative delirium. Int J Geriatr Psychiatry. 2019;34(10):1438–1446. doi:10.1002/gps.5139
- Adamis D, van Gool WA, Eikelenboom P. Consistent patterns in the inconsistent associations of Insulin-like growth factor 1 (IGF-1), C-Reactive Protein (C-RP) and Interleukin 6 (IL-6) levels with delirium in surgical populations. A systematic review and meta-analysis. Arch Gerontol Geriatr. 2021;97:104518. doi:10.1016/j.archger.2021.104518
- Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–452. doi:10.1001/archpsyc.58.5.445
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26(42):10709–10716. doi:10.1523/JNEUROSCI.3376-06.2006
- Laser-Azogui A, Kornreich M, Malka-Gibor E, Beck R. Neurofilament assembly and function during neuronal development. Curr Opin Cell Biol. 2015;32:92–101. doi:10.1016/j.ceb.2015.01.003
- Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035–1048. doi:10.1001/jamaneurol.2019.1534
- Martínez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler. 2015;21(5):550–561. doi:10.1177/1352458514549397
- Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–799. doi:10.1001/jamaneurol.2019.0765
- Oosterveld LP, Kuiper TI, Majbour NK, et al. CSF biomarkers reflecting protein pathology and axonal degeneration are associated with memory, attentional, and executive functioning in early-stage parkinson’s disease. Int J Mol Sci. 2020;21:22. doi:10.3390/ijms21228519
- Saller T, Petzold A, Zetterberg H, et al. A case series on the value of tau and neurofilament protein levels to predict and detect delirium in cardiac surgery patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019;163(3):241–246. doi:10.5507/bp.2019.043
- Gonçalves CA, Leite MC, Guerra MC. Adipocytes as an important source of serum S100B and possible roles of this protein in adipose tissue. Cardiovasc Psychiatry Neurol. 2010;(2010):790431. doi:10.1155/2010/790431
- Basile AM, Fusi C, Conti AA, et al. S-100 protein and neuron-specific enolase as markers of subclinical cerebral damage after cardiac surgery: preliminary observation of a 6-month follow-up study. Eur Neurol. 2001;45(3):151–159. doi:10.1159/000052114
- Grubb NR, Simpson C, Sherwood RA, Abraha HD, Cobbe SM. O', et al. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart. 2007;93(10):1268–1273. doi:10.1136/hrt.2006.091314
- Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008;41(10–11):755–763. doi:10.1016/j.clinbiochem.2008.04.003
- Beishuizen SJ, Scholtens RM, Vellekoop AE, et al. Timing is critical in determining the association between delirium and S100 calcium-binding protein B. J Am Geriatr Soc. 2015;63(10):2212–2214. doi:10.1111/jgs.13696
- Beishuizen SJ, Scholtens RM, van Munster BC, de Rooij SE. Unraveling the relationship between delirium, brain damage, and subsequent cognitive decline in a cohort of individuals undergoing surgery for hip fracture. J Am Geriatr Soc. 2017;65(1):130–136. doi:10.1111/jgs.14470
- Hov KR, Bolstad N, Idland AV, et al. Cerebrospinal fluid S100B and Alzheimer’s disease biomarkers in hip fracture patients with delirium. Dement Geriatr Cogn Dis Extra. 2017;7(3):374–385. doi:10.1159/000481853
- Jorge-Ripper C, Alemán MR, Ros R, et al. Prognostic value of acute delirium recovery in older adults. Geriatr Gerontol Int. 2017;17(8):1161–1167. doi:10.1111/ggi.12842
- Al Tmimi L, Van de Velde M, Meyns B, et al. Serum protein S100 as marker of postoperative delirium after off-pump coronary artery bypass surgery: secondary analysis of two prospective randomized controlled trials. Clin Chem Lab Med. 2016;54(10):1671–1680. doi:10.1515/cclm-2015-1012
- Khan BA, Zawahiri M, Campbell NL, Boustani MA. Biomarkers for delirium--a review. J Am Geriatr Soc. 2011;59 Suppl 2(0 2):S256–S261. doi:10.1111/j.1532-5415.2011.03702.x
- Kanner AA, Marchi N, Fazio V, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97(11):2806–2813. doi:10.1002/cncr.11409
- Kochanek PM, Berger RP, Bayir H, Wagner AK, Jenkins LW, Clark RS. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14(2):135–141. doi:10.1097/MCC.0b013e3282f57564
- Blyth BJ, Farhavar A, Gee C, et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. 2009;26(9):1497–1507. doi:10.1089/neu.2008.0738
- Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119(6):737–754. doi:10.1007/s00401-010-0674-1
- Bokesch PM, Izykenova GA, Justice JB, Easley KA, Dambinova SA. NMDA receptor antibodies predict adverse neurological outcome after cardiac surgery in high-risk patients. Stroke. 2006;37(6):1432–1436. doi:10.1161/01.STR.0000221295.14547.c8
- van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, de Rooij SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25(3):234–239. doi:10.1002/gps.2326
- Hall RJ, Ferguson KJ, Andrews M, et al. Delirium and cerebrospinal fluid S100B in Hip fracture patients: a preliminary study. Am J Geriatr Psychiatry. 2013;21(12):1239–1243. doi:10.1016/j.jagp.2012.12.024
- Li RL, Zhang ZZ, Peng M, et al. Postoperative impairment of cognitive function in old mice: a possible role for neuroinflammation mediated by HMGB1, S100B, and RAGE. J Surg Res. 2013;185(2):815–824. doi:10.1016/j.jss.2013.06.043
- Jones SC, Radinsky CR, Furlan AJ, et al. Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected]. Anesthesiology. 2002;97(2):488–496. doi:10.1097/00000542-200208000-00028
- Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102(4):1267–1273. doi:10.1213/01.ane.0000199156.59226.af