Abstract
This scoping review investigates the volume of evidence for home-based exercise and nutrition programs and their effect on muscle quality among senior adults to inform implementation and future research. It aims to answer the research question: What are the evidence, challenges, and needs for research regarding a home-based exercise and nutrition intervention program to improve muscle outcomes in senior adults? This scoping review was conducted following the PRISMA extension for Scoping Review. The following databases were searched: PubMed, Scopus, MEDLINE, CINAHL, EMBASE, and the Cochrane Library. Applied filters were used to help condense the research articles. A total of 13 studies met the inclusion criteria for this scoping review. Most exercise interventions were either resistance or multi-component exercise programs. The nature of the nutrition intervention varied between different supplements, foods, education, or counseling. Muscle outcomes included muscle mass in nine studies, muscle function in all the studies, muscle strength in ten studies, and biochemical analyses in two studies. Two studies found improvements in muscle mass; two studies revealed improvements in all their muscle function tests; and three studies revealed improvements in muscle strength. Muscle biopsy in a study revealed enhanced muscle fibers, but both studies did not reveal any biomarker improvements. The scoping review findings revealed mixed results on the effectiveness of a home-based exercise and nutrition program. However, the current evidence does have many gaps to address before recommending this form of intervention for senior adults as an effective way to prevent and manage sarcopenia. Since this review identified multiple knowledge gaps, strengths, and limitations in this growing field, it can be a starting point to help build future study designs and interventions in this population.
Introduction
In the 2019 United States 65 years and older population, 24.1 million men and 30 million women were reported as senior adults.Citation1 The world’s senior adult population is anticipated to double from 1 billion to 2.1 billion by 2050.Citation2 Aging is defined as the “irreversibly progressive decline of physiological function, eventually leading to age-related diseases, such as musculoskeletal disorders”.Citation3 Age-related musculoskeletal disorders can occur because of muscle protein synthesis (MPS) imbalance between anabolic and catabolic pathways.Citation4
Muscle mass and strength change throughout a person’s lifetime—increasing until young adulthood (up to 40 years old), being maintained in midlife (between 40 to 60 years old), and then decreasing with aging (age 65 years and up).Citation5 After 60 years, muscle mass can decrease 1.4–2.5% per year,Citation6 with women losing 0.64–0.7% per year and men losing 0.8–0.98% per year.Citation7 Muscle strength loss has been reported to be 1.5–5% per year beyond 50 years,Citation8 with men losing 3–4% per year and women losing 2.5–3% per year.Citation7
Fat mass increases as skeletal muscle mass decrease, negatively affecting a person’s physical function.Citation9 The continual worsening of a person’s physical function is one of the most critical age-related health issues.Citation9 Muscle function can decline 30–50% by 80 years, worsening the decline in inactive senior adults.Citation3 Consequences of poor physical function can lead to a high incidence of malnutrition, loss of independence, poor quality of life (QOL), morbidity, and mortality.Citation9 Therefore, senior adults with physical dysfunction can become an economic burden because of the increased need for healthcare resources.Citation9
Sarcopenia is the “progressive and generalized skeletal muscle disorder involving accelerated muscle mass, strength, and function loss”.Citation5 Sarcopenia can affect all muscles, including skeletal,Citation10 smooth,Citation11 and cardiac.Citation12 Therefore, sarcopenia is considered among the most common aging-related musculoskeletal disorders.Citation13 Sarcopenia can increase fall risk,Citation14 causes cognitive impairmentCitation15 and mobility disorders,Citation16 lowers QOL,Citation17 increase the need for long-term care placement,Citation18 and cause death.Citation19 In today’s research, the prevalence of severe cases of sarcopenia ranged from 0.2% to 45% in women and from 0.2 to 17.1% in men.Citation20
Early identification and intervention are essential to preventing or improving the outcomes in people with sarcopenia. There are three stages of sarcopenia diagnosis: 1) probable-low muscle strength, 2) confirmed-low muscle strength and mass, and 3) severe-low muscle strength, mass, and function.Citation5
Muscle strength is considered the most reliable measurement of muscle function. Therefore, the New European Working Group on Sarcopenia in Older People (EWGSOP) guidelines use muscle strength as the primary sarcopenia diagnosis stage.Citation5 When predicting adverse outcomes of sarcopenia, muscle strength is better than mass.Citation14 Examples of muscle strength measurement are grip strength,Citation21 isometric torque methods,Citation22 or chair stand and rise test.Citation23 Muscle mass can be measured by magnetic resonance imaging, computed tomography, dual-energy X-ray absorptiometry (DXA), or bioelectrical impedance analysis (BIA). Muscle function can be measured by Timed-Up and Go test (TUG), gait speed, and Short Physical Performance Battery (SPPB).Citation5
Blood biomarkers could be another way to diagnose sarcopenia and monitor their status. However, there is not a single biomarker that can identify sarcopenia in senior adults.Citation24 But some biomarkers may provide insight into sarcopenia pathophysiology, which may help identify susceptible individuals. These markers include an inflammatory response (eg, CRP,Citation25 IL-6,Citation25,Citation26 and TNF-αCitation25,Citation26), hormones (eg, DHEAS,Citation27 testosterone,Citation28 IGF-1,Citation29 and vitamin DCitation30), clinical parameters (eg, hemoglobin,Citation25 albumin,Citation31 and serum creatinine to cystatin C ratioCitation32), products of oxidative stress (eg, advanced glycation end products,Citation33 protein carbonyls,Citation34 and oxidized LDLCitation35), or antioxidants (eg, carotenoidsCitation36,Citation37 and alpha-tocopherolCitation37). Furthermore, comorbidities (eg, cancer, diabetes mellitus, and chronic kidney disease) must be considered when analyzing biomarker levels because they can affect them.Citation38
Although many factors contribute to sarcopenia in senior adults, the two crucial factors that can be controlled are inadequate nutrient intake and physical inactivity.Citation39,Citation40 The American College of Sports Medicine’s position states that daily physical activity can help promote healthy aging.Citation41 Additionally, physical activity interventions can increase muscle strength, endurance, and functional capacity to enhance cognitive status, independence, and QOL.Citation42,Citation43 Multi-component physical activity programs include strength (eg, resistance), endurance (eg, aerobic), and balance training because they effectively attenuate the adverse effects associated with aging, such as frailty, cognitive dysfunction, and decreased mobility.Citation9,Citation44
Resistance training (RT) has been regarded as one of the most successful interventions for sarcopenia to enhance muscle mass, strength, and function in senior adults.Citation39,Citation40,Citation45 RT is defined as “a high load [~80% one-repetition maximum] performed 2–3 days per week at moderate-to-vigorous intensity”.Citation45 However, high-load RT requires facilities with supervised observation and instructions in most cases.Citation45 Therefore, it could be difficult for senior adults to perform high-load RT as they may not have these accesses.Citation45
In comparison, home-based exercise programs have some benefits, including tailoring exercise to lifestyle preferences and autonomy, variability in timing, easier to maintain than group programs, low cost, and no need for travel.Citation9 In addition, for inactive senior adults, a home-based exercise program could allow them to adapt quickly and facilitates improvement in physical performance.Citation42 On the other hand, home-based programs have some limitations, including the lack of social aspect, the strong willpower needed to adhere to and keep consistent with the program, and the lack of equipment to ensure the exercise program, specifically resistance training, provides sufficient intensity.Citation9 Therefore, there needs to be a consensus on the type of programs that can further enhance senior adults’ muscle quality.
Nutrition has also been considered essential in countering sarcopenia.Citation45 A balanced diet including sufficient macro and micronutrients is required to prevent age-related sarcopenia.Citation46 Ingestion of dietary protein is required for MPS to prevent muscle breakdown, especially after exercising.Citation39 Senior adults must consume adequate protein to reduce muscle mass, strength, function loss, and slow sarcopenia.Citation39 The general recommendation for healthy senior adults is 1.0–1.2 grams per kilogram of body weight per day (g/kg BW/day).Citation47 However, the Society for Sarcopenia, Cachexia and Wasting DiseaseCitation48 recommends at least 1.0–1.5 g protein/kg BW/day combined with regular exercise to prevent and intervene in sarcopenia. Anabolic resistance from age-related impairment response to anabolic stimuli of the muscleCitation49 is the reason for senior adults’ higher protein needs.
Given the rise and prevalence of sarcopenia and the recent growing interest in the field, we conducted a scoping review with the overall aim of reviewing the current research in home-based exercise and nutrition intervention as a strategy to enhance muscle outcomes to help inform future research. This scoping review may provide a basis for planning future studies, identifying current research gaps, and advancing knowledge translation to improve participant outcomes.
Objectives
The growing amount of senior adults and the consistent rise in sarcopenia have called for research efforts to develop cost-saving, innovative, and effective countermeasures.Citation50 Furthermore, given the seriousness of sarcopenia, the need for a successful intervention has increased research attention.Citation51 However, there are limited research studies on interventions with home-based exercise and nutrition programs for senior adults to enhance muscle quality (muscle mass, strength, and function). To the authors’ knowledge, this scoping review is the first to explore this study area. Therefore, the following research question was formulated: What are the evidence, challenges, and needs for research regarding a home-based exercise and nutrition intervention program to improve muscle outcomes in senior adults?
The scope of this review will identify, describe, and summarize this question comprehensively. This review is tasked with summarizing the available evidence for 1) interventions that combine home-based exercise and nutrition intervention with senior adults to improve their muscle quality; 2) identifying what outcomes are being used to measure exercise, nutrition, and muscle quality; 3) identify research gaps; and 4) identify the needs for future design, application, and assessment providing the current evidence and their gaps.
Methods
This scoping review followed the components of the PRISMA extension for Scoping Review (PRISMA-ScR; )Citation52 and utilized the Critical Appraisal Skills Programme: Randomized Controlled Trials checklistCitation53 to critically appraise the included studies by assessing their relevance, trustworthiness, and results systematically. The checklist helped us assess any bias risk and evaluate the publications’ quality to see if it is appropriate for this study. A review protocol does not exist. The search was conducted on August 6, 2022. The following databases were searched from 2000–2022 to identify potentially relevant studies: PubMed, Scopus, MEDLINE, CINAHL, EMBASE, and the Cochrane Library.
Table 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) Checklist
Table 2 Summary of Included Articles
Table 3 Muscle Outcome Results
Table 4 Critical Appraisal Skills Programme: Randomized Controlled Trials Checklist
Keywords, other index terms, and the combination of these terms and appropriate synonyms were used to construct the search strategy. Applied filters were used to help condense the research articles, including academic journal source types, age 65+ years and 80 and over, English, and human trials. The detailed search strategy for databases included four concepts used to structure the search, including senior adults (example keywords: senior adults, older adults, elder, geriatric, elderly people, senior), home-based physical activity (example keywords: home physical activity program, home resistance training program, home exercise program), nutrition (example keywords: home nutrition program, nutrition, diet, supplement), and muscle outcomes (example keywords: muscle quality, muscle mass, muscle strength, muscle growth, blood biomarkers). The scoping review protocol recommends broad search terms to achieve high sensitivity.Citation58 The terms provided by the database and relevant to the study were used.
An example of an entire search strategy with exact terms used for MEDLINE: TX (elderly or aged or older or elder or geriatric or elderly people or old people or old people or senior) AND TX (home resistance training program OR TX home exercise program) AND TX (nutrition or diet or food or supplements) AND TX (muscle strength or muscle mass or muscle growth or muscle quality or blood biomarkers). Limiters: Aged 65+ years, 80 and over. Source Types: Academic Journals. Language: English
Studies were included if they reported 1) a home-based exercise program with a nutrition component added, 2) the senior adults as the subjects, and 3) muscle outcome, including muscle strength, muscle mass, muscle function, or muscle blood biomarkers. Studies were excluded if 1) the intervention was not home-based, 2) the intervention did not include both nutrition and exercise components, 3) nutrition was considered the control and not a part of the intervention, 4) the subject population was not senior adults, 5) muscle mass, strength, function, or blood biomarker tests were not an outcome, 6) animal study, 7) duplicate study during the initial screening, 8) not English translated, and 9) protocol, abstract, or review.
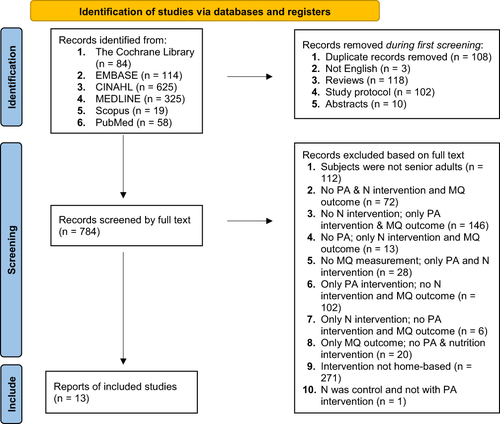
All articles’ citations retrieved by electronic searching were exported to EndNote 20 for organization. The screening process can be seen in . Two reviewers (ESG and SG) independently screened trials detailed for eligibility criteria. ESG screened the articles during the first pass. Duplicates, study protocols, abstracts, not English translated, and reviews were removed in first-pass screening. The full text was screened using inclusion and exclusion criteria during the second pass screening. SG confirmed articles met the inclusion criteria after the second pass. Other reviewers could resolve disagreements on study selection and data extraction if needed. Finally, the two prominent reviewers extracted the studies’ key data into a summary table () based on the review’s sub-objectives. The results were discussed and condensed accordingly.
Figure 1 Flow diagram of literature search. The process of systematically examining research evidence to assess its validity, results, and relevance before using it to inform a decision. This term is used for items 12 and 19 instead of “risk of bias” (which is more applicable to systematic reviews of interventions) to include and acknowledge the various sources of evidence that may be used in a scoping review (eg, quantitative and/or qualitative research, expert opinion, and policy document). From: Annals of Internal Medicine, Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. 2018;169:467–473. Copyright © 2018 American College of Physicians, Inc.Citation52
Only a summary of the articles’ study design and groups, country, subjects (number and types of participants involved), exercise intervention (type, duration, and items provided), nutrition intervention (type, duration, items provided, and ingredients), and outcome measurements (muscle and nutrition outcome used) are reported because many included studies were multi-component. The outcome measurement was extracted into four groups (muscle mass, muscle strength, muscle function, and biochemical analyses) seen in .
Results
Search Results
The initial search yielded 1125 articles from the database (). After removing the duplicates, reviews, study protocols, abstracts, and articles not translated into English, 784 remained. Of the 784 full-text articles evaluated for eligibility, 771 were excluded. A total of 13 studies met the inclusion criteria for the scoping review, as seen in .
Study Characteristics
The studies’ characteristics are seen in . All but one study of the included studies was reported as randomized controlled trials, while one was a prospective study.Citation65 All but one studyCitation46 was published within the past five years, demonstrating this area of study is in its novelty. The included studies came from high- and upper-middle-income countries: North America,Citation50 China,Citation62 France,Citation46 Canada,Citation66 Brazil,Citation40 Taiwan,Citation68 Korea,Citation60,Citation61 Japan,Citation59,Citation63,Citation65 and Austria.Citation64,Citation67 The studies ranged in size from 23 participantsCitation61 to 319 participants,Citation68 with the majority of subjects (9 of the 13 studies) being female (823 females vs 466 males; 64% vs 36%, respectively). The study population varied from healthy individuals,Citation59,Citation63,Citation66 sedentary individuals,Citation50,Citation61 those who underwent hip surgery,Citation60 those who have sarcopenia,Citation62 prefrail or frail individuals,Citation46,Citation64,Citation67,Citation68 those newly diagnosed with advanced pancreatic or non-small-cell lung cancer,Citation65 or dynapenic older adults with low protein intake.Citation40 The durations varied between 4 weeksCitation63 to 6 months.Citation59,Citation66,Citation68 One study did not have a comparison group,Citation65 six studies included two comparison groups,Citation46,Citation50,Citation60,Citation61,Citation64,Citation67 two studies included three comparison groups,Citation59,Citation63 and four studies included four comparison groups.Citation40,Citation62,Citation66,Citation68 A critical appraisal of the included studies was done using the Critical Appraisal Skills Programme: Randomized Controlled Trials checklist,Citation53 and the results can be seen in .
Exercise Intervention
The exact exercise programs performed, duration, and intensity widely varied among the studies (). Most studies were either RTCitation40,Citation50,Citation59,Citation61,Citation62,Citation65,Citation67 or multi-componentCitation46,Citation59,Citation60,Citation64,Citation66,Citation68 exercise programs, while one was strictly aerobics.Citation63 The RT was more potent towards muscle mass and strength, while the multi-component programs were more effective towards muscle function. Only five studiesCitation50,Citation59,Citation61,Citation63 included a warm-up and cool-down with their intervention, two studiesCitation64,Citation67 had only a warm-up, and the remaining studiesCitation40,Citation46,Citation60,Citation62,Citation65,Citation66,Citation68 did not report involving a warm-up or cool-down. Exercise programs providing warm-up or cool-down did not improve muscle outcomes within the studies. The majority of the studies (9 out of 13) provided participants with equipment for their exercise intervention, including an accelerometer,Citation50,Citation65 resistance bands,Citation40,Citation50,Citation59,Citation67 pole sticks for walking,Citation59 handouts,Citation46,Citation50,Citation60,Citation67 a DVD,Citation63 folding chairs,Citation61 exercise mats,Citation61 dumbbells,Citation40,Citation61 ankle weights,Citation40 or a personal computer (PC).Citation61 Studies that included resistance bands and handouts showed increased muscle mass and strength, while the other equipment revealed either increased, maintained, or did not affect muscle outcomes.
Participants were asked to exercise for a certain amount of time or perform a specific number of exercises per session. The amount of time ranged from 5Citation68 to 60 minutes.Citation40,Citation59,Citation60,Citation62,Citation68 The number of exercises per session varied greatly, with either a set number providedCitation40,Citation50,Citation59,Citation61,Citation63,Citation64,Citation66,Citation67 or the researchers individualized the session based on the participant’s current physical activity level.Citation46,Citation60,Citation62,Citation65,Citation68 The longer duration and individualized exercise interventions did show improvement in muscle outcomes compared to studies with shorter time and generalized exercise interventions. Each participant was asked to do their sessions a certain number of days per week, either every day,Citation46 two days/week,Citation59,Citation64,Citation67 three days/week,Citation40,Citation50,Citation60,Citation61,Citation63,Citation66 or individualized based on the participant’s physical activity level.Citation62,Citation65,Citation68 The individualized session improved muscle quality over a specific set number of days.
Nutrition Intervention
The nature of the nutrition intervention varied between different supplements, foods, or nutrition education/counseling (). Six studies provided only supplements or food in their intervention,Citation40,Citation46,Citation50,Citation59,Citation63,Citation66 three provided only nutrition education,Citation61,Citation64,Citation67 two provided only nutrition counseling,Citation60,Citation62 and two provided both supplement/food and nutritional counseling/education.Citation65,Citation68 Nutrition education indicated general information, while nutrition counseling provided individualized recommendations.
Regarding supplements and food, there was variation among studies concerning the type of supplement, dose, timing, and frequency of consumption. The supplement’s commonly used ingredients contained protein,Citation50,Citation66,Citation68 amino acids,Citation40,Citation59,Citation63,Citation65 or both.Citation46 Four studies provided a multi-nutrient supplement (nutritional powderCitation40,Citation50,Citation65 or drinkCitation46,Citation66) for daily consumption. One studyCitation40 provided a combination of food and supplements. The studies providing multi-nutrient supplements and a combination of food and supplement did show improvement in muscle outcomes rather than consuming protein or amino acid alone. Three studiesCitation40,Citation50,Citation61 reported that participants could maintain their regular dietary habits during the intervention, but it was unclear if the other studies reported the same. Four studiesCitation40,Citation50,Citation59,Citation63 provided specific instructions on when the supplement or food could be consumed; meanwhile, four studiesCitation46,Citation65,Citation66,Citation68 did not provide specific instructions but informed how many times per day to consume them. The studies providing specific instructions revealed improvements in muscle outcomes over those that did not. However, Tokuda and HoriCitation59 was the only study that provided the participants’ protein recommendations of 1.2 g/kg/day and showed improved muscle quality individually but not between groups.
Regarding studies with nutritional counseling or education, 8 of the 13 were provided nutrition education through customized dishware and colored meal pad,Citation68 a “buddy”,Citation64,Citation67 individualized counseling,Citation65 an app,Citation62 PC,Citation61 or phone call.Citation60 The app, customized dishware and colored meal pad, and “buddy” showed how participants being involved or having someone accountable for their intervention impacted their muscle outcomes. It should be noted that only seven studiesCitation46,Citation50,Citation59,Citation62,Citation63,Citation65,Citation68 had nutrition outcomes in addition to their muscle outcomes, while the rest had only muscle outcome measurements for their results. The nutrition outcomes data were collected by a type of food diary,Citation46,Citation50,Citation59,Citation63,Citation65,Citation68 Mini-Nutrition Assessment Short Form,Citation59 Mini-Nutrition Assessment,Citation46,Citation65 food frequency questionnaires,Citation62 body max index,Citation46,Citation65 body weight,Citation65 or nutrition impact symptoms.Citation65 The type of food diary used in the study varied, but it held the exact purpose of obtaining the participants’ food intake for a specific time. The studies involving a food diary and food frequency questionnaires revealed improved muscle outcomes.
Muscle Outcome
Muscle outcome in the 13 included studies included muscle mass in 9 studies,Citation40,Citation46,Citation50,Citation59,Citation61–63,Citation65,Citation67 muscle function in all the studies, muscle strength in 11 studies,Citation40,Citation50,Citation59–61,Citation63–68 and biochemical analyses in 2 studies.Citation40,Citation50 Each assessment method for these outcomes differed across the studies (). Most studies measured two muscle outcomes,Citation46,Citation60,Citation62,Citation64,Citation66,Citation68 while the remainder measured three outcomesCitation59,Citation61,Citation63,Citation65,Citation67 or four outcomes.Citation40,Citation50 The most popular pairing was muscle strength and function.Citation46,Citation60,Citation66,Citation68
Muscle Mass
The muscle mass was measured by either a DXA,Citation50,Citation61 BIA,Citation40,Citation59,Citation62,Citation63,Citation67 appendicular lean soft tissue-based formula,Citation61 fat-free mass,Citation46 or CT.Citation65 The results were mixed between the studies. In 6 studies, the home-based exercise and nutrition intervention did not improve the outcomes of skeletal muscle mass (SMM) index,Citation40,Citation59,Citation65 lean body mass,Citation67 appendicular skeletal muscle mass (ASM),Citation67 and skeletal muscle mass.Citation46,Citation63 These results could be due to the accuracy of the device used, insufficient protein intake, or the type of exercise intervention being done (aerobic, multi-component, low-intensity RT with an accelerometer, strength exercises, and progressive RT). However, two studies did show improvement in SMM,Citation50,Citation62 ASM,Citation50 or muscle-to-body fat ratioCitation50 compared to the control. The similarity of these studies was that RT for approximately three days/week can help enhance muscle mass. One studyCitation61 had mixed results where lower body muscle mass, ASM, and SMM were improved in the intervention group compared to the control, but not upper body muscle.
Muscle Function
There were multiple ways muscle function was measured which included SPPB,Citation50,Citation64,Citation67 gait speed,Citation40,Citation50,Citation59,Citation63,Citation65,Citation68 TUG,Citation40,Citation46,Citation50,Citation60,Citation61,Citation66 sit to stand,Citation40,Citation46,Citation50,Citation62,Citation63,Citation65 stair climb,Citation46,Citation50 balance test,Citation40,Citation59,Citation62 functional reach test,Citation60,Citation66 2-minute step,Citation61 back scratch,Citation61,Citation68 chair sit-and-reach,Citation61,Citation68 walking test,Citation46,Citation62,Citation65,Citation66 or heel lift.Citation63 Two studiesCitation64,Citation68 revealed improvement in all the intervention’s muscle function tests compared to the control. The only similarities between the studies were the strength component of the exercise intervention and nutrition education, indicating that these could be ideal interventions for enhancing muscle function. Many studiesCitation40,Citation50,Citation60,Citation61,Citation63,Citation65–67 showed mixed results of muscle function improvements in certain tests but not others. Three studiesCitation46,Citation59,Citation62 revealed no intervention effect in all their tests. The three studies had no similarities because they had different muscle function tests, exercise interventions, and nutrition interventions. Therefore, it is difficult to report what could be the reason why muscle function was not improved.
Muscle Strength
Muscle strength was measured by either grip strength,Citation40,Citation50,Citation59,Citation60,Citation63–65,Citation67,Citation68 leg press,Citation50 knee extension,Citation50,Citation59,Citation60 knee and hip flexor,Citation60 hip abductor,Citation60 arm curl,Citation61 chair stand,Citation61,Citation66 toe strength,Citation63 toe/heel raise,Citation63,Citation68 or angle of active flexion of the dominant shoulder.Citation63 Like muscle function, many studiesCitation50,Citation60,Citation61 showed mixed results of muscle strength improvement. Three studiesCitation40,Citation67,Citation68 revealed the intervention having strength improvements compared to the control. All three studies provided the participants with home items to help with their exercise or nutrition intervention, indicating that providing equipment at home for exercise or nutrition could be valuable for enhancing muscle strength. Kapan et alCitation64 revealed that individuals had improvement in grip strength but did not see a within-group change from control. Naito et alCitation65 saw improvement in interventions’ grip strength only at a one-time point but not with the other time points. Three studiesCitation59,Citation63,Citation66 showed no improvements with the intervention. The only similarity between the three studies was that supplementation (amino acid, tea catechins, or ensure high calcium) was part of their intervention, so it questions if an alternative nutrition intervention like food versus supplementation could be ideal.
Biochemical Analysis
Only one studyCitation50 performed muscle biopsies during pre-and post-intervention. It revealed muscle fibers improvement after performing whole-body elastic band resistance exercises with warm-up and cool-down for three days/week on non-consecutive days with daily consumption of a multi-nutrient supplement (M5). RT and exercising three days/week have been seen above as effective in enhancing muscle outcomes, further confirming their effectiveness. However, both studiesCitation40,Citation50 did not reveal any biomarkers improvements of liver function, inflammation, lipid profiles, or HOMA-IR with home-based exercise and nutrition intervention. Since recent studies are limited, further testing of common biomarkers to test for muscle mass is needed for home-based exercise and nutrition.
Discussion
In this scoping review, 13 studies were included with senior adults receiving home-based exercise and nutrition intervention to enhance their muscle quality. The studies were distinct in terms of participants included (nutrition, disease, or frailty status), duration (4 weeks to 6 months), groups (1 to 4 comparison groups), exercise intervention (type and duration), nutrition intervention (education, counseling, and supplementations), and outcomes (muscle, nutrition, and biomarkers). The main finding is that the data needs to be more consistent regarding whether muscle quality can be improved with a home-based exercise and nutrition intervention. In addition, examining the existing research revealed many gaps and further studies are needed to address these. These gaps included lack of participant blinding; lack of research done in low-to-middle-income countries; short study duration; population type and how it could have affected the intervention; and the type, dose, frequency, ingredients, and delivery of the nutrition intervention.
This area of research is novel, with most of the included studies published within the past five years suggesting growing interest in this topic. The growing interest could be because home-based interventions are generally inexpensive, flexible, time-saving, and promote independence.Citation50 For example, Hsieh et alCitation68 reported that the cost of their intervention for a home-based exercise and nutrition program was $81–100 for a three-month program. In addition, most of the studies were not blinded, leading to possible biased results. At the same time, it is difficult to blind participants to exercise intervention, but it can be done with nutritional supplements.
The included studies came from high- and upper-middle-income countries, which reveals an evidence gap in low- to middle-income countries to determine if home-based exercise and nutrition interventions are effective in these populations. The durations varied between 4 weeks to 6 months. The short length is likely due to difficulties recruiting and retaining senior adultsCitation69 or concerns about efficacy, protocol adherence, cost, and withdrawal/dropout rates.Citation50 Since the programs are short-term, it does question the long-term effect of this intervention on muscle quality is largely unknown, especially since there are no follow-up measurements taken long-term. In this review, the longer-duration interventions improved muscle quality, while the shorter-duration interventions did not. It should be noted that the 4-week study was the only aerobic study, and the participants received protein supplements. In contrast, the long-term studies were RT or multi-component exercise training receiving multi-nutrient supplementation, food, or education. Therefore, further studies are needed to address the effect of this intervention on muscle quality in low- to middle-income countries and long-term studies (1 year or more).
The populations utilized varied from healthy individuals to those with a medical condition. The medical condition could have other factors (eg, medication and standard of care) compromising the study’s intervention. For example, participants after hip surgeryCitation60 outcomes showed mixed muscle strength and function results. Participants with newly diagnosed advanced pancreatic or non-small cell lung cancerCitation65 outcomes revealed either maintenance (muscle mass and function) or improvement (muscle strength and function). Improved muscle quality could occur if the participants received personalized nutrition intervention through a professional nutrition educator (eg, a dietitian). The personalized intervention could help them meet their protein needs and adjust their diet for their conditions. Countries tend to have different medical environments and standards of care.Citation70 Therefore, the study’s result may not apply to different medical conditions in different countries. On the other hand, it could be ideal for the country’s standard of care to be included in the study, so the readers know potential outside factors affecting the outcome. The studies that revealed muscle outcome improvements were seen in sedentary, sarcopenic, dyspneic, and prefrail or frail participants. This fact indicates that this study area could have more of an impact on compromised than healthy people. Further studies should be conducted to confirm or deny this fact.
In the six studies that included exercise and only supplementation, the supplements differ in type, dose, frequency, ingredients, and distribution across the studies. Dietary protein requirements are higher in senior adults.Citation47 Unfortunately, most senior adults do not reach their daily requirements.Citation71 Therefore, before the study starts, researchers should review their participants to see if they are meeting their recommended protein intake and if their baseline protein intake is sufficient. In the included studies, whether the participants consumed sufficient or insufficient protein before the study is unknown. This data could strengthen the nutrition intervention by personalizing supplementation or food intake to the participant’s needs.
Additionally, the recommendations for sarcopenia prevention and intervention for senior adults vary from 1–1.5 g/kg/day combined with regular exercise.Citation48 Only one studyCitation59 tried to meet this recommendation by adjusting total protein intake to at least 1.2 g/kg/day by a nutritionist at the beginning of the study and increasing the participant’s protein intake throughout the intervention. This study showed improved muscle quality individually but not between groups. This study’s result could be due to the source or quality of protein being provided to or consumed by the participants. Protein-rich whole foods (eg, lean red meat) are starting to be used in research over protein supplements to promote MPS in senior adults.Citation72 Only one studyCitation68 provided combined food (skim milk powder and mixed nuts) with different supplementations (fish oil and Oxxynea FP) to reveal muscle strength and function improvement. Therefore, based on these findings, food combined with supplementation or food alone could positively affect muscle quality.
Lastly, the timing of protein intake could be another factor. The International Society of Sports Nutrition (ISSN) position stand recommends post-exercise high protein ingestion (immediately to 2 hours) to stimulate muscle growth.Citation73 Only two studiesCitation59,Citation63 recommended consuming their supplement after their exercise intervention. However, both studies did not improve muscle quality. The reason could be that either the participant did not consistently follow the instructions or the amount of protein consumed did not meet the participant’s needs. Additionally, consuming 20–40 g of high-quality protein doses based on 0.25–0.40 g/kg body is ideal per ISSN.Citation73 Those two studies provided either 8 g of proteinCitation63 or a protein intake of at least 1.2 g/kg/day.Citation59 The 8 g of protein did not provide adequate protein post-exercise intervention, while the other study did not report how much protein was provided post-exercise intervention. Therefore, further studies are needed to assess protein intake before the intervention and establish protein type, amount, and timing. Additional benefits for senior adults whose daily protein intakes are sufficient or insufficient based on their baseline intake should be investigated.
Three studies provided only nutrition education with their exercise intervention. The education was given sporadically. One studyCitation61 had education provided once every four weeks through PC, but what was explicitly discussed and who provided the education needed clarification. The other two studiesCitation64,Citation67 had “buddies” provide nutrition education during each session two days/week. All three studies had mixed results regarding muscle quality. They did not reveal if a professional expert (eg, a dietitian) was used in their intervention which could have compromised the education. The researchers employed trained non-professional volunteers as the “buddies” to assist the senior adults and implement the intervention. Such intervention efforts could have been compromised due to a lack of professional expertise. The “buddy” also felt that their role as the intervention’s supervisors was not always positively considered by the senior adult.Citation46 Therefore, further studies are needed to provide nutrition professional-led or approved education with appropriate training for those who provide the education for home-based exercise and nutrition intervention to see if both could positively affect muscle quality. Further research should also evaluate how an improved training program for the “buddy” impacts senior adults.
Two studies provided only nutrition counseling with their exercise intervention. One studyCitation60 provided nutrition counseling one day/week for 10 minutes and a leaflet for nutrition management. Who provided the counseling was not disclosed, but the researchers did state that their intervention was multi-professionally designed. This study reported mixed results regarding muscle strength and function. The other studyCitation62 delivered nutrition counseling on an app with recommendations based on the participant’s diet, and recipes were given. Muscle mass did improve, but not muscle function. Bias tends to be high when participants must report their diet for a food recall, especially in apps.Citation74 Additionally, the food frequency questionnaire was the nutrition outcome pre- and post-intervention, which can be affected by bias. Therefore, further studies are needed on effective individualized nutrition counseling techniques and interventions to change dietary patterns positively.
Two studies provided both supplement/food and nutritional counseling/education. One studyCitation65 provided individualized nutritional counseling and supplement (Inner Power), but who provided the counseling was not disclosed. The other studyCitation68 provided education through customized dishware and colored meal pad, in addition to having two nutrition subgroups receiving food and supplements. This study was the only one that improved all their outcomes (muscle strength and function) for the exercise and nutrition intervention group. The study revealed that a tailored exercise intervention based on participants’ capabilities, visualized and interactive education, and combining food and supplementation could show promising positive effects on home-based exercise and nutrition intervention toward muscle quality. However, further studies are needed to confirm this.
Conclusion
Thirteen intervention studies were evaluated using home-based exercise training and nutrition intervention to improve muscle quality in senior adults. This study area is in its novelty since all but one study was published within the past five years. The findings revealed mixed results in most studies but highlighted its potential as a strategy for preventing and managing sarcopenia. Resistance training enhanced muscle strength and mass more effectively, while multi-component programs were more effective towards muscle function. Providing equipment, like resistance bands and customized dishware, with handouts or a food diary to use at home amplified muscle outcomes. Longer duration and individualized programs with specific instructions were more effective than shorter, generalized exercise interventions. Providing nutrition education and a combination of food and supplement did show improvement in muscle outcomes instead to consuming protein or amino acid supplements alone. Further testing of biomarkers is needed since recent studies are limited.
However, the current evidence has many gaps (eg, blinding; low-to middle-income countries; short study duration; population type; and the type, dose, frequency, ingredients, and distribution of the nutrition intervention) to be addressed before confidently recommending this intervention to prevent and manage sarcopenia. Nevertheless, since this review addressed multiple knowledge gaps, strengths, and limitations in this growing field, it can be a starting point to help build future designs and interventions.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
No funding to declare.
References
- The Administration for Community Living. 2020 profile of older Americans. Available from: https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2020ProfileOlderAmericans.Final_.pdf. Accessed June 27, 2023.
- World Health Organization. Ageing and health; 2021, Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed June 27, 2023.
- Li Z, Zhang Z, Ren Y, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22(2):165–187. doi:10.1007/s10522-021-09910-5
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/s0140-6736(19)31138-9
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
- Zhu LY, Chan R, Kwok T, Cheng KC, Ha A, Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: a randomized controlled trial. Age Ageing. 2019;48(2):220–228. doi:10.1093/ageing/afy179
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi:10.3389/fphys.2012.00260
- Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013;3(4):346–350. doi:10.32098/mltj.04.2013.17
- Echeverria I, Amasene M, Urquiza M, et al. Multicomponent physical exercise in older adults after hospitalization: a randomized controlled trial comparing short- vs. long-term group-based interventions. Int J Environ Res Public Health. 2020;17(2):666. doi:10.3390/ijerph17020666
- Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997;83(5):1581–1587. doi:10.1152/jappl.1997.83.5.1581
- Kunieda T, Minamino T, Nishi JI, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114(9):953–960. doi:10.1161/CIRCULATIONAHA.106.626606
- Lin J, Lopez EF, Jin Y, et al. Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43(4):296–306. doi:10.1016/j.exger.2007.12.005
- Grote C, Reinhardt D, Zhang M, Wang J. Regulatory mechanisms and clinical manifestations of musculoskeletal aging. J Orthop Res. 2019;37(7):1475–1488. doi:10.1002/jor.24292
- Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol a Biol Sci Med Sci. 2018;73(9):1199–1204. doi:10.1093/gerona/glx245
- Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(12):1164.e7–1164.e15. doi:10.1016/j.jamda.2016.09.013
- Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. doi:10.1016/j.jamda.2011.04.014
- Beaudart C, Biver E, Reginster JY, et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8(2):238–244. doi:10.1002/jcsm.12149
- Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical Independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. doi:10.1002/jcsm.12160
- De Buyser SL, Petrovic M, Taes YE, et al. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing. 2016;45(5):602–608. doi:10.1093/ageing/afw071
- Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. doi:10.1002/jcsm.12783
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi:10.1016/s0140-6736(14)62000-6
- Francis P, Toomey C, Mc Cormack W, Lyons M, Jakeman P. Measurement of maximal isometric torque and muscle quality of the knee extensors and flexors in healthy 50- to 70-year-old women. Clin Physiol Funct Imaging. 2017;37(4):448–455. doi:10.1111/cpf.12332
- Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170. doi:10.1186/s12877-016-0349-4
- Tosato M, Marzetti E, Cesari M, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017;29(1):19–27. doi:10.1007/s40520-016-0717-0
- Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol a Biol Sci Med Sci. 2004;59(3):242–248. doi:10.1093/gerona/59.3.m242
- Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol a Biol Sci Med Sci. 2002;57(5):M326–32. doi:10.1093/gerona/57.5.m326
- Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–406. doi:10.1093/ageing/afp015
- Araujo AB, Travison TG, Bhasin S, et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56(11):2000–2008. doi:10.1111/j.1532-5415.2008.01965.x
- Onder G, Liperoti R, Russo A, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291(4):E829–34. doi:10.1152/ajpendo.00138.2006
- Mastaglia SR, Seijo M, Muzio D, Somoza J, Nuñez M, Oliveri B. Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J Nutr Health Aging. 2011;15(5):349–354. doi:10.1007/s12603-010-0287-3
- Visser M, Kritchevsky SB, Newman AB, et al. Lower serum albumin concentration and change in muscle mass: the health, aging and body composition study. Am J Clin Nutr. 2005;82(3):531–537. doi:10.1093/ajcn.82.3.531
- Sim M, Dalla Via J, Scott D, et al. Creatinine to cystatin C ratio, a biomarker of sarcopenia measures and falls risk in community-dwelling older women. J Gerontol. 2021;77(7):1389–1397. doi:10.1093/gerona/glab369
- Dalal M, Ferrucci L, Sun K, Beck J, Fried LP, Semba RD. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J Gerontol a Biol Sci Med Sci. 2009;64(1):132–137. doi:10.1093/gerona/gln018
- Semba RD, Ferrucci L, Sun K, et al. Oxidative stress and severe walking disability among older women. Am J Med. 2007;120(12):1084–1089. doi:10.1016/j.amjmed.2007.07.028
- Cesari M, Kritchevsky SB, Nicklas BJ, et al. Lipoprotein peroxidation and mobility limitation: results from the health, aging, and body composition study. Arch Intern Med. 2005;165(18):2148–2154. doi:10.1001/archinte.165.18.2148
- Alipanah N, Varadhan R, Sun K, Ferrucci L, Fried LP, Semba RD. Low serum carotenoids are associated with a decline in walking speed in older women. J Nutr Health Aging. 2009;13(3):170–175. doi:10.1007/s12603-009-0053-6
- Semba RD, Blaum C, Guralnik JM, Moncrief DT, Ricks MO, Fried LP. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15(6):482–487. doi:10.1007/bf03327377
- Calvani R, Marini F, Cesari M, et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle. 2015;6(4):278–286. doi:10.1002/jcsm.12051
- Naseeb MA, Volpe SL. Protein and exercise in the prevention of sarcopenia and aging. Nutr Res. 2017;40:1–20. doi:10.1016/j.nutres.2017.01.001
- de Carvalho Bastone A, Nobre LN, de Souza Moreira B, et al. Independent and combined effect of home-based progressive resistance training and nutritional supplementation on muscle strength, muscle mass and physical function in dynapenic older adults with low protein intake: a randomized controlled trial. Arch Gerontol Geriatr. 2020;89:104098. doi:10.1016/j.archger.2020.104098
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American college of sports medicine position stand. exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3181a0c95c
- Chang KV, Wu WT, Huang KC, Han DS. Effectiveness of early versus delayed exercise and nutritional intervention on segmental body composition of sarcopenic elders - A randomized controlled trial. Clin Nutr. 2021;40(3):1052–1059. doi:10.1016/j.clnu.2020.06.037
- George M, Azhar G, Pangle A, et al. Feasibility of conducting a 6-month long home-based exercise program with protein supplementation in elderly community-dwelling individuals with heart failure. J Physiother Phys Rehabil. 2017;2(2):1.
- de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. doi:10.1186/s12877-015-0155-4
- Watanabe Y, Yamada Y, Yoshida T, et al. Comprehensive geriatric intervention in community-dwelling older adults: a cluster-randomized controlled trial. J Cachexia Sarcopenia Muscle. 2020;11(1):26–37. doi:10.1002/jcsm.12504
- Bonnefoy M, Boutitie F, Mercier C, et al. Efficacy of a home-based intervention programme on the physical activity level and functional ability of older people using domestic services: a randomised study. J Nutr Health Aging. 2012;16(4):370–377. doi:10.1007/s12603-011-0352-6
- Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi:10.1016/j.clnu.2014.04.007
- Morley JE, Argiles JM, Evans WJ, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–396. doi:10.1016/j.jamda.2010.04.014
- Norman K, Haß U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. 2021;13(8):2764. doi:10.3390/nu13082764
- Nilsson MI, Mikhail A, Lan L, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. 2020;12(8):2391. doi:10.3390/nu12082391
- Li L, He Y, Jin N, Li H, Liu X. Effects of protein supplementation and exercise on delaying sarcopenia in healthy older individuals in Asian and non-Asian countries: a systematic review and meta-analysis. Food Chem X. 2022;13:100210. doi:10.1016/j.fochx.2022.100210
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
- CASP. CASP checklists. Available from: https://casp-uk.net/casp-tools-checklists/. Accessed June 27, 2023.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69.
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB Guidance for conducting systematic scoping reviews. Int J EvidBased Healthc. 2015;13:141–146.
- Peters MDJ, Godfrey C, McInerney P, Baldini Soares C, Khalil H, Parker D Scoping Reviews. In: Aromataris E, Munn Z, eds., and Joanna Briggs Institute Reviewer's Manual. Adelaide, Australia: Joanna Briggs Inst; 2017.
- Tonkin E, Brimblecombe J, Wycherley TP. Characteristics of smartphone applications for nutrition improvement in community settings: a scoping review. Adv Nutr. 2017;8(2):308–322. doi:10.3945/an.116.013748
- Tokuda Y, Mori H. Effect of ingestion of essential amino acids and tea catechins after resistance exercise on the muscle mass, physical performance, and quality of life of healthy older people: a randomized controlled trial. Asia Pac J Clin Nutr. 2021;30(2):213–233. doi:10.6133/apjcn.202106_30(2).0005
- Lee H, Lee SH. Effectiveness of multicomponent home-based rehabilitation in elderly patients after hip fracture surgery: a randomized controlled trial. J Pers Med. 2022;12(4). doi:10.3390/jpm12040649
- Hong J, Kim J, Kim SW, Kong HJ. Effects of home-based tele-exercise on sarcopenia among community-dwelling elderly adults: body composition and functional fitness. Exp Gerontol. 2017;87(Pt A):33–39. doi:10.1016/j.exger.2016.11.002
- Wang Z, Xu X, Gao S, et al. Effects of internet-based nutrition and exercise interventions on the prevention and treatment of sarcopenia in the elderly. Nutrients. 2022;14(12):1.
- Miyazaki A, Okuyama T, Mori H, Sato K, Kumamoto K, Hiyama A. Effects of two short-term aerobic exercises on cognitive function in healthy older adults during COVID-19 confinement in Japan: a pilot randomized controlled trial. Int J Environ Res Public Health. 2022;19(10):6202. doi:10.3390/ijerph19106202
- Kapan A, Luger E, Haider S, et al. Fear of falling reduced by a lay led home-based program in frail community-dwelling older adults: a randomised controlled trial. Arch Gerontol Geriatr. 2017;68:25–32. doi:10.1016/j.archger.2016.08.009
- Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle. 2019;10(1):73–83. doi:10.1002/jcsm.12351
- Johnson S, McLeod B, Gupta S, McLeod K. Impact of a home-based nutrition and exercise intervention in improving functional capacity associated with falls among rural seniors in Canada. Qual Ageing Old Adults. 2018;19:261–272. doi:10.1108/QAOA-11-2017-0044
- Haider S, Dorner TE, Luger E, et al. Impact of a home-based physical and nutritional intervention program conducted by lay-volunteers on handgrip strength in prefrail and frail older adults: a randomized control trial. PLoS One. 2017;12(1):e0169613. doi:10.1371/journal.pone.0169613
- Hsieh TJ, Su SC, Chen CW, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):119. doi:10.1186/s12966-019-0855-9
- Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56(12):2340–2348. doi:10.1111/j.1532-5415.2008.02015.x
- McPherson K. International differences in medical care practices. Health Care Financ Rev. 1989;Spec No:9–20.
- Fulgoni VL 3rd. Current protein intake in America: analysis of the national health and nutrition examination survey, 2003–2004. Am J Clin Nutr. 2008;87(5):1554s–1557s. doi:10.1093/ajcn/87.5.1554S
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562s–1566s. doi:10.1093/ajcn/87.5.1562S
- Kerksick CM, Arent S, Schoenfeld BJ, et al. International society of sports nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2017;14(1):33. doi:10.1186/s12970-017-0189-4
- Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi:10.2147/jmdh.S104807
