Abstract
Background
Women, and those older than 65 years of age, are particularly susceptible to dry eye disorders (DEDs). Inflammation is clearly involved in the pathogenesis of DEDs, and there is mounting evidence on the antioxidant and antiinflammatory properties of essential polyunsaturated fatty acids (EPUFAs).
Objective
To analyze whether a combined formulation of antioxidants and long-chain EPUFAs may improve the evolution of DEDs.
Methods
We used a prospective study to address the relationship between risk factors, clinical outcomes, and expression levels of inflammation and immune response (IIR) mediators in human reflex tear samples. Participants included: (1) patients diagnosed with nonsevere DEDs (DED group [DEDG]); and (2) healthy controls (control group [CG]). Participants were randomly assigned to homogeneous subgroups according to daily oral intake (+S) or not (−NS) of antioxidants and long-chain EPUFAs for 3 months. After an interview and a systematized ophthalmic examination, reflex tears were collected simultaneously from both eyes; samples were later subjected to a multiplexed particle-based flow cytometry assay. A specific set of IIR mediators was analyzed. All data were statistically processed through the SPSS 15.0 software program.
Results
Significantly higher expressions of interleukin (IL)-1β, IL6, and IL10 and significantly lower vascular endothelial growth factor expressions were found in the DEDG as compared to the CG. In the DEDG, significant negative correlations were detected between the Schirmer test and IL-1β, IL6, IL8, and vascular endothelial growth factor levels, and between the fluorescein breakup time with IL6 and IL8 levels. However, levels of IL-1β, IL6, and IL10 in tears were significantly lower in the DEDG+S versus the DEDG−NS and in the CG+S versus the CG−NS. Subjective symptoms of dry eye significantly improved in the DEDG+S versus the DEDG−NS.
Conclusion
IIR mediators showed different expression patterns in DED patients, and these patterns changed in response to a combined formulation of antioxidant and EPUFAs supplementation. Our findings may be considered for future protocols integrating clinical/biochemical data to help manage DED patients.
Introduction
Dry eye is a complex condition involving the lacrimal glands, eyelids, and tear film, as well as a variety of ocular surface tissues including epithelial, inflammatory, immune, and goblet cells.Citation1 From a pathogenic viewpoint, there are two major types of dry eye: the aqueous-deficient clinical form that is due to lacrimal gland dysfunction, and the evaporative dry eye that is mainly due to meibomian gland disorder.Citation2,Citation3 However, it is common to find dry eye patients (independently of the etiology) displaying a mixture of both aqueous-deficient and evaporative clinical manifestations. Dry eyes usually affect people aged ≥ 65 years;Citation3 moreover, dry eyes affect women selectively, as emphasized by the Women’s Eye Health Organization and other large studies,Citation4–Citation8 with an estimation of about 3.23 million American women suffering from dry eyes. The term “dry eye disorder” (DED) has recently been introduced to better define the ocular surface dysfunction that leads to tear film impairment and dry eye.Citation9
Objective tests for the clinical diagnosis of DEDs include the following: (1) the Schirmer test, with or without anesthesia, which determines tear production; (2) tear breakup time, with or without fluorescein, which reflects tear film stability; and (3) dye staining tests for evaluating the ocular surface tissue integrity, by using conjunctival lissamine green or corneal fluorescein staining.Citation1–Citation3,Citation9 Reflex tears are the functional response to an agent irritating to the eyes, including bright light, foreign particles, and irritant substances, as well as vomiting, coughing, and yawning maneuvres.Citation2,Citation3,Citation9 Reflex tears can be collected either by capillary tubes or by Schirmer strips without using local anesthetic. Basal tears can be collected by Schirmer strips with the use of local anesthetic. Two sampling methods have been used to assess biochemical traits of tear fluid composition in healthy and pathologic conditions: yawn collection and eye-flush collection.Citation10,Citation11 The glass capillary micropipettes extracting method has been proposed as the best means for obtaining reflex tear samples for laboratory assays.Citation11
Recent advances in biotechnology strengthen our understanding of DED pathophysiology. Among the newest diagnostic techniques are the Optical Quality Analysis System (Visiometrics®, Terrassa, Barcelona, Spain) with double-pass measurement of diffusion and light scattering, masks and goggles for managing meibomian gland dysfunctions, LipiView (TearScience®, Morrisville, NC, USA) for stimulating meibomian gland secretion, and the TearLab Osmolarity Test (Tear Lab Corporation, San Diego, CA, USA) for measuring tear osmolarity. In parallel to these advances, much laboratory research has been done to better manage DED patients.Citation12–Citation15 However, additional prospective studies are needed to elucidate other risk factors and molecular and cellular pathogenic mechanisms, discover new pharmacologic agents, improve eye health, and preserve better vision and quality of life.
Reactive oxygen species are chemically reactive molecules containing oxygen.Citation16 When reactive oxygen species formation becomes uncontrolled or the body’s antioxidant defense barrier fails, reactive oxygen species accumulate and trigger lipids, proteins, and nucleic acid damage, subsequently leading to cell disease and death. From a biochemical viewpoint, oxidative stress (OS) is the result of an imbalance between prooxidants and antioxidants. Strong evidence indicates that OS plays a significant role in a variety of ocular conditions. For example, a significant decrease in antioxidant defenses and a significant increase in prooxidants in aqueous humor, vitreous body, ocular tissues, and plasma have been reported in relation to primary open-angle glaucoma, cataracts, diabetic retinopathy, age-related macular degeneration, and DEDs.Citation17–Citation21 The initial steps of OS and whether this condition contributes to DED development and evolution remain unclear.
The eyes are exposed to external and internal damaging agents. Inflammation and immune response (IIR) mechanisms attempt to rescue the body from cell, tissue, and organ injuries and their downstream effects. The IIR mediators involve leukocytes and other innate immunity cells, as well as T, B, and natural killer lymphocytes (as adaptive immunity), which interact via cytokines, chemokines, and other molecules.Citation22 Tissue damage results from uncontrolled acute and chronic inflammation. Alteration of a wide spectrum of IIR mediators measured in blood, aqueous humor, vitreous body, or eye tissues supports an abnormal activity of the immune system in both anterior and posterior eye segment disorders.Citation21,Citation23–Citation27 New biotechnology strategies, such as multiplexed flow cytometry assays, metabolomics, and immunoproteomic profiling analyses, may improve our understanding of the pathogenesis and progression dynamics of eye diseases.Citation28–Citation31
The essential polyunsaturated fatty acids (EPUFAs) omega-3 (ω-3) and omega-6 (ω-6) play pivotal functions and display a wide spectrum of positive effects in the body, such as: helping to lower cholesterol and triglyceride levels; giving energy to the body; helping to reduce acute and chronic inflammation; reducing respiratory and asthma-like symptoms; enhancing appropriate pre- and postnatal development mainly of the central and peripheral nervous systems; helping in the regulation of blood pressure; reducing the odds of developing cancer, heart disease, and stroke; managing emotional distress and depression; and benefiting patients with neurodegenerative disorders, among others.Citation32–Citation38 The n-3 derived eicosanoids exert antiinflammatory actions, while the n-6 derived eicosanoids are proinflammatories.Citation32,Citation33 Recent studies have shed some light on the regulation of biochemical mechanisms of acute inflammation, which are performed in part by endogenous polyunsaturated fatty acid-derived autacoids, such as the series of specialized proresolving mediators, the lipoxins, resolvins, protectins, and maresins.Citation34 Based on these findings, EPUFAs are attractive molecules in eye and vision research,Citation35–Citation38 including DED treatment.Citation39
In the present study, we evaluated the relationship between oral supplementation of a combined formulation of antioxidants and EPUFAs on the improvement of the signs and symptoms of patients diagnosed with nonsevere DEDs, as compared to DED patients not taking these supplements and healthy controls.
Materials and methods
This prospective, open-label, randomized study was performed under the approval of the Institutional Review Boards of the University Hospital Dr. Peset (Valencia, Spain), as a nonsignificant risk investigational device study (Re: CEIC 59/10), and all tenets of the Declaration of Helsinki for the protection of human subjects in medical research were strictly observed.
Study design
A total of 66 subjects of both sexes, aged 23–80 years, were enrolled during ophthalmologic appointments at the study centers in Jerez de la Frontera, Cádiz, Spain and Valencia, Spain between March 2011 and June 2011 according to the main inclusion/exclusion criteria listed in .
Table 1 Inclusion and exclusion criteria
Prior to the baseline visit, subjects were required to discontinue use of nutritional supplements, systemic antihistamines, and dry eye (or meibomian gland disorder) related treatments such as antibiotics, nonsteroidal and antiinflammatory drugs, corticosteroids, as well as tears with vitamins, for at least 15 days, and participants were asked to strictly follow the recommendations of the ophthalmologists throughout the duration of the study. Ocular lubricants without nutritional agents were not restricted. Patients with obvious infection or significant eyelid inflammation were excluded from the present study. Patients diagnosed with mild-to-moderate DEDs (DED group [DEDG]) were enrolled. To classify our study participants, a systematized ophthalmologic examination and a questionnaire, with scores including objective/subjective criteria, were performed. Other signs and symptoms of DEDs were evaluated based on the personal viewpoint of the patients, as well as their comments and suggestions. According to this, and depending on their clinical form, patients were considered to be either aqueous deficient, evaporative (lipid layer insufficiency), or a combined form of both aqueous deficient/evaporative types. However, all of these clinical forms were enclosed in the DEDG, as a whole, and were compared to the group of healthy subjects. Further evaluation of the structure and function of the upper lid meibomian glands, as recently reported by Lane et al,Citation40 was not addressed in this study, but may be the subject of further research regarding the role of essential fatty acids in DED patients.
All participants (132 eyes) were randomized to the following groups: (1) patients diagnosed with DEDs (DEDG; n = 30); and (2) healthy individuals that constituted the control group ([CG]; n = 36). Two homogeneous subgroups were selected according to the oral intake of a supplement prescribed as two capsules a day (+S) or not receiving the oral supplement (−NS). The supplement formulation as reflected in (Brudysec 1.5 g; Brudy Laboratories, Barcelona, Spain) was based on ω-3 EPUFA, vitamins, glutathione, amino acids, and oligoelements, in a combined nutraceutical formulation with (per capsule): docosahexaenoic acid (350 mg), eicosapentaenoic acid (42.5 mg), docosapentaenoic acid (30 mg), vitamin A (133.3 μg), vitamin C (26.7 mg), vitamin E (4 mg), tyrosine (10.8 mg), cysteine (5.83 mg), glutathione (2 mg), zinc (1.6 mg), copper (0.16 mg), manganese (0.33 mg), and selenium (9.17 μg).
Table 2 Composition of Brudysec 1.5® (Brudy Laboratories, Barcelona, Spain) formula per capsule
Given that compliance with long-term self-administered medication therapy is approximately 50% for those who remain in care,Citation41 ophthalmologists were instructed to pay special attention to measure levels of compliance for participants in the personal interview, mainly in this case of prescribing self-administered doses of antioxidants and EPUFAs, in order to help patients to improve their compliance and increase the benefit they may receive from these supplements, as well as to ensure the validity of the final data of the study. Therefore, subjects were followed each month after the initial visit during the 3 months of follow-up.
Patient management
A personal interview was performed with all participants regarding their personal and familial backgrounds and characteristics of their disease, mainly symptoms of dry eyes and subjective sensations. The Ocular Surface Disease Index (OSDI) questionnaire assessed the subject’s frequency of dry eye symptoms and problems with their ocular surface.Citation42 The OSDI questionnaire sections are divided into the following questions:
Have you experienced photophobia, a gritty feeling, soreness, or blurred vision during the last week?
Have your eye problems limited you in reading, driving at night, computer work, or watching TV during the last week?
Have your eyes felt uncomfortable in windy or dry situations or because of air conditioning during the last week?Citation42
The questionnaire paid special attention to the comments of the interviewed participants regarding lifestyle and eye conditions.
A systematized ophthalmologic examination was carried out on all participants as follows: best corrected visual acuity (BCVA) in each eye, value of the eyelid Schirmer test, slit lamp examination for the eye adnexa and anterior eye segment, tear breakup time with fluorescein, and corneal surface details with fluorescein. All ophthalmologists completed a full sheath to enclose all data, and were advised to strictly follow the study protocol. The primary outcomes of the ophthalmologic measures for effectiveness of the oral nutraceutical formulation were the Schirmer test and fluorescein tear breakup time; the secondary measure was dry eye symptoms.Citation43
Tear sampling procedures
The expression of a set of cytokines/chemokines in reflex tear samples obtained by the gentle rubbing method was assayed by the Multiplex System (Luminex® R-200; Luminex Corporation, Austin, TX, USA). Polystyrene beads coupled covalently to specifically directed antibodies (human cytokine/chemokine panel) were allowed to react with 30–40 μL of each tear sample containing an unknown amount of cytokine, or with a standard solution containing a known amount of cytokine, at room temperature for 1 hour, following the manufacturer’s instructions. The cytokines/chemokines that were analyzed using this detection method were the interleukins (IL)-1β, IL2, IL4, IL5, IL6, IL7, IL8, IL10, and IL12; tumor necrosis factor-alpha (TNF-α); vascular endothelial growth factor (VEGF); granulocyte-macrophage-colony stimulating factor (GM-CSF); and interferon-gamma (IF-γ).
Briefly, a series of washes were carried out to remove unbound proteins. Then, a biotinylated detection antibody specific for a different epitope on the cytokine was added to the beads and incubated at room temperature for 30 minutes. Streptavidin-phycoerythrin (which binds to the biotinylated detection antibodies), was used to detect the reaction mixture. The flow-based Bio-Plex® (Bio-Rad Laboratories, Hercules, CA, USA) suspension array system was used to identify and quantify each antigen–antibody reaction. Identification of the assayed molecules was based on bead color and fluorescence, using fluorescently labeled reporter molecules associated with each target protein. Unknown cytokine/chemokine concentrations were calculated automatically by the Bio-Plex® Manager software (Bio-Rad Laboratories) using a standard curve derived from a recombinant cytokine standard. Cytokine/chemokine levels were corrected for the initial total protein concentration of each human tear sample during analysis.
Data are presented as the mean ± standard deviation for two or three determinations and expressed in picograms per milliliter per milligram.
Statistical analysis
Demographic, clinical, and biochemical data were recorded into a previously designed Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). A nonparametric Mann–Whitney U-test was selected for comparing two independent sample groups by means of Statistical Package for the Social Sciences (SPSS) software (v15.0; IBM Corporation, Armonk, NY, USA). A value of P < 0.05 was considered to indicate a statistically significant difference between groups.
Results
The median age of the participants was 52 ± 15 years (DEDG) versus 50 ± 12 years (CG); 59% of the DEDG and 58% of the CG were older than 45 years. Regarding gender, men and women accounted for 28% and 72% of the DEDG versus 32% and 68% of the CG, respectively.
Our sample of mild-to-moderate DED patients had a history of dry eyes for more than 1 year. All of them reported one or more of the following symptoms: ocular irritation, soreness, burning, foreign body sensation, dryness, photophobia, and/or blurred vision. The DED patients had known dry eyes before starting our study, and most of them (89%) used eye drops regularly for treating their tear film alterations. None of the subjects of the DEDG suffered severe dryness or Sjögren’s syndrome. By using the OSDI for measuring dry eye symptoms and severity in our study participants, and by taking into consideration all comments and suggestions from all of them, we found that scores significantly worsened when comparing healthy controls with dry eye patients (P = 0.0015).
All participants were examined under a slit lamp in relation to their anterior eye segment and media, and the DEDG displayed ocular surface disorder morphological alterations such as marginal blepharitis and stinging of the cornea. The interviews with the healthy subjects, the OSDI data, and the biomicroscopy examination of the anterior eye segment did not reveal any DED signs or symptoms in the CG.
The Schirmer test scores (wetting of the paper after 5 minutes) were significantly lower in the DEDG (4.26 ± 0.59 mm) than in the CG (13.25 ± 2.46 mm; P = 0.0002), reflecting the altered tear secretion in this mild-to-moderate group of DED patients. Regarding the age of the DEDG of our study participants, those younger than 45 years normally moistened 8–11 mm of each paper strip, while those older than 45 years usually wet about 5–8 mm in 5 minutes.
The fluorescein tear film breakup time was much shorter in the DEDG patients (4.35 ± 1.23 seconds) than that in the healthy group (14.24 ± 3.22 seconds), which reflects the statistically significant altered tear film stability in mild-to-moderate DED individuals as compared to the controls (P = 0.0001).
An overall amelioration in the signs and symptoms was observed in the DEDG+S as compared to the DEDG−NS groups at 3 months. Surprisingly, the CG+S group also reported an improvement of 50% or greater in ocular signs such as visual fatigue, heaviness, and improved eyelashes, nails, hair, and skin.
Neither the DEDG nor the CG showed significant differences in BCVA and intraocular pressure from baseline to 3 months, and no changes in these parameters were noted in the oral supplementation subgroups from pre- to postsupplementation. The mean changes in BCVA and intraocular pressure were less than 0.1 and 1 mmHg, respectively.
The gentle collection method of reflex human tears by means of the capillary tube was a noninvasive, useful, and a relatively easy procedure to achieve the main objectives of the present study. The responses were calculated by subtracting background cytokine concentrations from the cytokine concentrations in the tears. The standard curves for both the kit assay and the extraction buffers were similar for the 12 analyzed molecules. The amount of tear samples obtained from the participants in the study permitted detection in up to 90% of the sampling procedures. The set of assayed molecules showed a wide variety of expression levels in tear samples, with acceptable precision, as measured by the Luminex multianalyte profiling bioassay system (Luminex Corporation).
When comparing the main groups, significantly higher expressions of IL-1β (P = 0.015), IL6 (P = 0.0001), and IL10 (P = 0.050), and significantly lower VEGF expression (P < 0.050) were found in the DEDG as compared to the CG. In the DEDG, significant negative correlations were detected between the Schirmer test score and IL-1β (P < 0.01), IL6 (P < 0.001), and IL8 (P < 0.05) levels, and between the fluorescein breakup time and IL6 (P < 0.001) and IL8 (P < 0.5) levels. However, tear levels of IL-1β, IL6, and IL10 were significantly lower in DEDG+S versus DEDG−NS (P = 0.05), and in CG+S and CG−NS (P = 0.001; P = 0.01, respectively).
Subjective symptoms of DED significantly improved in the DEDG+S versus DEDG-NS group, as reflected in . A significantly positive correlation was detected between cytokine/chemokine tear expression levels and aging when the participants were subclassified by age. In particular, the expression levels of IL4, IL10, IL6, IL12, IFγ, and GM-CSF were correlated with age in the DEDG patients 45 years of age or older (). In this context, the inflammation/immune response mediators involved in tears from DED patients in relation to aging is shown in .
Table 3 Cytokine/chemokine expression in tears
Figure 1 Remaining subjective symptoms of DEDs in DED−S or DED+S patients.
Note: **P< 0.001.
Abbreviations: DED, dry eye disorder; DED−S, dry eye disorder without antioxidants/omega-3 fatty acid oral supplements; DED+S, dry eye disorder with antioxidants/omega-3 fatty acid oral supplements.
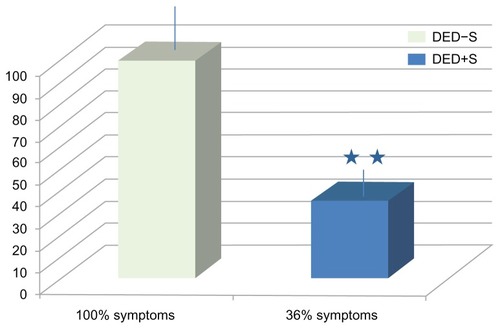
Figure 2 Subjective DED symptoms after supplementation with a combined formulation mainly containing antioxidants and docosahexaenoic acid, and its effects on human tear IL expression levels.
Abbreviations: DED, dry eye disorder(s); IL, interleukin.
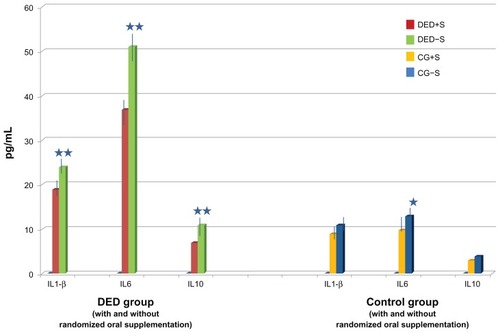
We found no significant correlation between cytokine/chemokine levels and gender in our study participants.
Discussion
DEDs are among the most common conditions observed in ophthalmic appointments, and are the ocular status with the highest prevalence rates worldwide. Among the hallmarks of DEDs are ocular discomfort and loss of vision, and there are derived complications for reading, cooking, working with computers, driving, and using makeup and eye cosmetics among the affected individuals.Citation1–Citation3 The main goal of this work was to analyze possible changes in the expression of IIR mediators in tears of nonsevere DED patients (those pertaining to the three clinical types: the aqueous deficient, evaporative [lipid layer insufficiency], or combined form of both aqueous deficient/evaporative [lipid layer anomalies]) as compared to healthy controls, by using a new tool that is able to simultaneously measure various molecules in a single microplate well (the Luminex multianalyte profiling assay system).
A wide variety of cytokines and chemokines are present in human tears for maintaining the morphology and function of the ocular surface.Citation44–Citation46 In the present study, tear samples from the DEDG (constituted by the aqueous deficient clinical form), evaporative (lipid layer insufficiency), or a combined type of both aqueous deficient/abnormal lipid layer clinical DED forms displayed significantly increased levels of IL-1β, IL6, and IL10 and decreased VEGF with respect to the CG. When comparing our results on the cytokine/chemokine expression in tears with other studies that consider different subtypes of dry eye, such as the one by Boehm et al,Citation47 several concordances exist, such as the significantly higher expression of IL-1β in the DEDG as compared to the CG. Brignole-Baudouin et alCitation48 reported that the destructive cell-signaling chemicals IL6, TNF-α, and VEGF were higher in DED patients. The proinflammatory cytokines, IL6 and TNF-α, have also been detected in tears and in the conjunctival epithelium of individuals with DEDs.Citation43–Citation48 Enríquez-de-Salamanca et alCitation45 reported that IL6 was detected in only 65% of the tear samples from patients with mild-to-moderate DEDs, as collected by Schirmer strips, and that those concentrations did not differ from controls. In the same study, the authors emphasized that IL-1β and TNF-α were detected in 30% and 2% of their study patients, respectively.Citation45 With the gentle rubbing collection method used here, we detected IL-1β in 81.89% of the DEDG, IL6 in 73.77%, IL10 in 59.01%, and VEGF in 93.44%.
In our study sample, patients with mild-to-moderate DEDs showed positive results in the clinical tests and symptoms. It is possible that an underlying cytokine/chemokine-mediated inflammatory disorder is common to all ocular surface alterations, although the etiology of any DED in our patients is highly variable. The most dangerous proinflammatory cytokines and chemokines are TNF-α, IL-1β, IL6, and IL8, and among these IL-1β and IL6 levels were increased in the DEDG of our study participants, reflecting an inflammatory profile in the ocular surface of these patients.
Interesting data observed included the significantly lower VEGF expression (P < 0.050) found in our DEDG as compared to the CG. The expression of VEGF is tightly regulated. In fact, a wide range of signals also stimulate VEGF transcription, such as (inflammatory) cytokines and oncoproteins, including IL-1β, IL6, insulin-like growth factor (ILGF)-1, TGF-β1, c-Src, v-Raf, and Ras, among others. VEGF is the archetypical example of the cross-talk between nerves and vessels. Its role in conjunctival biopsies and tears in ocular chronic inflammatory diseases, such as vernal keratoconjunctivitis or atopic keratoconjunctivitis, has been recently revisited.Citation49 Moreover, it has been suggested that, in addition to its angiogenic actions, VEGF can also act as a direct proinflammatory mediator during the pathogenesis of rheumatoid arthritis.Citation50 Enríquez-de-Salamanca et alCitation45 stated that VEGF levels were increased in tear samples from mild-to-moderate DED patients. VEGF is the prototypic example of the crosstalk between nerves and vessels. The observation that reduced levels of VEGF are causally involved in neurodegeneration might have implications in anterior and posterior eye segment disorders.Citation51 Eye pain and soreness is a common symptom in dry eyes. It is likely that our patients suffered from a mild neurotrophic epitheliopathy that caused a reduced expression of the VEGF, as reflected in the tear analyses. This finding requires further research to elucidate the role of VEGF in dry eye syndrome.
The EPUFAs (ω-3 and ω-6) have to be consumed daily in order to cover all of their functions in an organism. Recent evidence has demonstrated that the intake of ω-3 fatty acids and the ratio of consumption with ω-6 fatty acids influences the expression of global inflammatory markers in humans.Citation52 These findings suggest a presumptive protective function of ω-3 supplementation in managing DED patients.Citation53–Citation55 Therefore, we analyzed whether a combined formulation of antioxidants and long-chain EPUFAs may influence the evolution of DEDs. We found that oral supplementation with a combined formulation of antioxidants and EPUFAs significantly changed the expression pattern of cytokines/chemokines in tears from DED patients when compared with DED patients not taking the supplements. These results are in agreement with those of Roncone et alCitation53 and Brignole-Baudouin et al.Citation48 Furthermore, Cortina and BazanCitation54 proposed that the eicosapentaenoic acid and docosahexaenoic acid derivatives, particularly resolvin E1 (RvE1) and neuroprotectin D1, appear to be responsible for the antiinflammatory effects of both EPUFAs. This was supported by a study from the same research group,Citation55 in which topical RvE1 resulted in decreased inflammation in a mouse dry eye model; RvE1 promoted tear production, corneal epithelial integrity, and decreased inflammatory inducible COX-2, suggesting that RvE1 and similar resolvin analogs have therapeutic potential in the treatment of DEDs.
Currently, the diagnosis of DEDs is still a challenging task due to the difficulties of identifying risk factors that may contribute to obscuring the diagnosis; in fact, this point requires further research. Moreover, the few available standardized clinical protocols for DED diagnosis, such as the Schirmer test, the fluorescein tear film breakup time, and the ocular surface staining probes, together with insufficient knowledge about the pathologic mechanisms of DEDs and the coexistence of other local and systemic conditions, contribute to the present situation and the inability to cure and eliminate the disease.
Differential expression of IIR mediators has also been detected in human tears in relation to aging. In this study, we confirmed a positive correlation of cytokine/chemokine tear expression with the aging process. Particularly, the expression levels of IL4, IL6, IL10, IL12, IFγ, and GM-CSF were correlated with age in members of the DEDG who were 45 years or older, as shown in . This relationship should be considered when managing patients in this age range.Citation56 In spite of the fact that the main purpose of the present study was to compare the expression patterns of specific immune mediator molecules in the tears of DED patients, we detected significant changes in our older DED patients in respect to the healthy controls based on age, thus confirming previous findings from our group and others that age is the most common risk factor for dry eyes.Citation57–Citation59 Taking this into consideration, dry eye has to be considered a normal part of aging. When specifically focusing on the topic of sex disparities in DEDs, we found no significant correlation between cytokine/chemokine levels and gender among our study participants.
It is interesting to consider that cytokines such as IL-1β and IL6, which act by inducing an inflammatory response (while others act on specific cell types in the immune response), were significantly augmented in tears from our sample of DED patients. IL6 has been described as one of the key molecules in DEDs.Citation44,Citation60 This molecule was identified in the tears and conjunctival epithelium of DED patients, and its presence was correlated with some clinical parameters in patients with severe DED forms. Furthermore, the clinical and biochemical parameters related to DEDs, as well as the symptoms and subjective sensations of the patients were significantly reduced in members of the DEDG+S subgroup. Thus, our findings established that lower IIR mediators in tear levels were correlated with increased quality of life, this being an interesting point to appraise. Together, our findings suggest that oral supplementation with a combination of antioxidants and EPUFAs fully benefit DED patients.
Our findings also suggest that IL-1β and IL6 expression in tears can be used as presumptive biomarkers of DEDs and dry eye related-quality of life. Topical IL-1β and IL6 modulators may be a new therapeutic approach to DEDs. This topic offers ophthalmologists and researchers a great opportunity for future investigations. Larger trials with a greater number of participants may help to implement specific guidelines for the use of EPUFAs in DED patients.
Acknowledgments
Part of this work was presented to the Association for Research in Vision and Ophthalmology Meeting, Fort Lauderdale, Florida (Galbis Estrada et al, 4243/A120 ARVO Posters, 2012). For more information about this study, please visit http://www.unidadinvestigacionoftalmologica.es.
The present work was partially financed by a research grant from the Junta de Andalucia, Servicio Andaluz de Salud, Sevilla, Spain (IP: Javier Benítez-del-Castillo; 2011–2012). Carmen Galbis-Estrada received a research fellowship from Brudy Laboratories, Barcelona, Spain (2011–2012).
Disclosure
The authors report no conflicts of interest in this work.
References
- PerryHDDry eye disease: pathophysiology, classification, and diagnosisAm J Manag Care200814Suppl 3S79S8718452371
- de TavaresFPFernandesRSBernardesTFBonfioliAASoaresEJDry eye diseaseSemin Ophthalmol2010253849320590418
- LabbéABrignole-BaudouinFBaudouinCOcular surface investigations in dry eyeJ Fr Ophtalmol20073017697 French17287676
- ScheinODMuñozBTielschJMBandeen-RocheKWestSPrevalence of dry eye among the elderlyAm J Ophthalmol199712467237289402817
- MuñozBWestSKRubinGSCauses of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation StudyArch Ophthalmol2000118681982510865321
- MossSEKleinRKleinBELong-term incidence of dry eye in an older populationOptom Vis Sci200885866867418677233
- SchaumbergDASullivanDABuringJEDanaMRPrevalence of dry eye syndrome among US womenAm J Ophthalmol2003136231832612888056
- GipsonIKSpurr-MichaudSArgüesoPTisdaleANgTFRussoCLMucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell linesInvest Ophthalmol Vis Sci20034462496250612766048
- BaudouinCA new approach for better comprehension of diseases of the ocular surfaceJ Fr Ophtalmol2007303239246 French17417148
- VanDerMeidKRSuSPKrenzerKLWardKWZhangJZA method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using LuminexMol Vis2011171056106321552500
- Enríquez-de-SalamancaACastellanosESternMETear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye diseaseMol Vis20101686287320508732
- BaudouinCBrignoleFBecquetFPisellaPJGoguelAFlow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammationInvest Ophthalmol Vis Sci1997387145814649191610
- VignaliDAMultiplexed particle-based flow cytometric assaysJ Immunol Methods20002431–224325510986418
- MassingaleMLLiXVallabhajosyulaMChenDWeiYAsbellPAAnalysis of inflammatory cytokines in the tears of dry eye patientsCornea20092891023102719724208
- HalliwellBFree radicals and antioxidants: updating a personal viewNutr Rev201270525726522537212
- Zanon-MorenoVMarco-VenturaPLleo-PerezAVOxidative stress in primary open-angle glaucomaJ Glaucoma200817426326818552610
- BerthoudVMBeyerECOxidative stress, lens gap junctions, and cataractsAntioxid Redox Signal200911233935318831679
- Garcia-MedinaJJPinazo-DuranMDGarcia-MedinaMZanon-MorenoVPons-VazquezSA 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathyEur J Ophthalmol201121563764321218388
- SuzukiMTsujikawaMItabeHChronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degenerationJ Cell Sci2012125Pt 102407241522357958
- VenkataSJNarayanasamyASrinivasanVTear ascorbic acid levels and the total antioxidant status in contact lens wearers: a pilot studyIndian J Ophthalmol200957428929219574697
- Pinazo-DuránMDZanón-MorenoVGarcía-MedinaJJGallego-PinazoREvaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucomaCurr Opin Pharmacol20121319810723142105
- MedzhitovRJanewayCJrInnate immunityN Engl J Med2000343433834410922424
- ZoukhriDEffect of inflammation on lacrimal gland functionExp Eye Res200682588589816309672
- KrabbeKSPedersenMBruunsgaardHInflammatory mediators in the elderlyExp Gerontol200439568769915130663
- StevensonWChauhanSKDanaRAn immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- YoshidaNIkedaYNotomiSLaboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosaOphthalmology Epub September 15, 2012
- Gallego-PinazoRMarsigliaMMrejenSYannuzziLAOuter retinal tubulations in chronic central serous chorioretinopathyGraefes Arch Clin Exp Ophthalmol Epub September 7, 2012
- GrusFHPodustVNBrunsKSELDI-TOF-MS ProteinChip array profiling of tears from patients with dry eyeInvest Ophthalmol Vis Sci200546386387615728542
- HiguchiAKawakitaTTsubotaKIL-6 induction in desiccated corneal epithelium in vitro and in vivoMol Vis2011172400240621976951
- ChoyCKChoPChungWYBenzieIFWater-soluble antioxidants in human tears: effect of the collection methodInvest Ophthalmol Vis Sci200142133130313411726613
- StevensonWChauhanSKDanaRDry eye disease: an immune-mediated ocular surface disorderArch Ophthalmol201213019010022232476
- FredmanGSerhanCNSpecialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolutionBiochem J2011437218519721711247
- SwenorBKBresslerSCaulfieldLWestSKThe impact of fish and shellfish consumption on age-related macular degenerationOphthalmology2010117122395240120630597
- ChongEWKreisAJWongJYSimpsonJAGuymerRHDietary-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysisArch Ophthalmol2008126682683318541848
- LicinioJWongMLThe role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotectionMol Psychiatry19994431732710483047
- RenHMagulikeNGhebremeskelKCrawfordMPrimary open-angle glaucoma patients have reduced levels of blood docosahexaenoic and eicosapentaenoic acidsProstaglandins Leukot Essent Fatty Acids200674315716316410047
- Pinazo-DuranMDBoscá-GomarLAnti-inflammatory properties of polyunsaturated fatty acid omega 3 Indications in ophthalmologyArch Soc Esp Oftalmol2012877203205 Spanish22732118
- SapiehaPStahlAChenJ5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acidsSci Transl Med201136969ra12
- RosenbergESAsbellPAEssential fatty acids in the treatment of dry eyeOcular Surf2010811828
- LaneSSDuBinerHBEpsteinRJA new system, the LipiFlow, for the treatment of meibomian gland dysfunctionCornea201231439640422222996
- StewartMThe validity of an interview to assess a patient’s drug takingAm J Prev Med198732951003330658
- SchiffmanRMChristiansonMDJacobsenGHirschJDReisBLReliability and validity of the Ocular Surface Disease IndexArch Ophthalmol2000118561562110815152
- SaviniGPrabhawasatPKojimaTGrueterichMEspanaEGotoEThe challenge of dry eye diagnosisClin Ophthalmol200821315519668387
- BrignoleFPisellaPJGoldschildMDe Saint JeanMGoguelABaudouinCFlow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyesInvest Ophthalmol Vis Sci20004161356136310798650
- HiguchiAKawakitaTTsubotaKIL-6 induction in desiccated corneal epithelium in vitro and in vivoMol Vis2011172400240621976951
- Enríquez-de-SalamancaACastellanosESternMETear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye diseaseMol Vis20101686287320508732
- TuominenISTervoTMTeppoAMValleTUGrönhagen-RiskaCVesaluomaMHHuman tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRKExp Eye Res200172663164111384151
- BoehmNRiechardtAIWiegandMPfeifferNGrusFHProinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarraysInvest Ophthalmol Vis Sci201152107725773021775656
- Brignole-BaudouinFBaudouinCAragonaPA multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patientsActa Ophthalmol2011897e591e59721834921
- Abu El-AsrarAMAl-MansouriSTabbaraKFMissottenLGeboesKImmunopathogenesis of conjunctival remodelling in vernal keratoconjunctivitisEye (Lond)2006201717915746957
- YooSAKwokSKKimWUProinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic interventionMediators Inflamm2008200812987319223981
- WangYMaoXOXieLVascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis miceJ Neurosci200727230430717215390
- RandALAsbellPANutritional supplements for dry eye syndromeCurr Opin Ophthalmol201122427928221597374
- RonconeMBartlettHEperjesiFEssential fatty acids for dry eye: A reviewCont Lens Anterior Eye2010332495420031476
- CortinaMSBazanHEDocosahexaenoic acid, protectins and dry eyeCurr Opin Clin Nutr Metab Care201114213213721157308
- LiNHeJSchwartzCEGjorstrupPBazanHEResolvin E1 improves tear production and decreases inflammation in a dry eye mouse modelJ Ocul Pharmacol Ther201026543143920874497
- MossSEKleinRKleinBEPrevalence of and risk factors for dry eye syndromeArch Ophthalmol200011891264126810980773
- LeeAJLeeJSawSMPrevalence and risk factors associated with dry eye symptoms: a population based study in IndonesiaBr J Ophthalmol2002861347135112446361
- ChiaEMMitchellPRochtchinaELeeAJMarounRWangJJPrevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye StudyClin Experiment Ophthalmol200331322923212786773
- VisoERodriguez-AresMTGudeFPrevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study)Ophthalmic Epidemiol2009161152119191177
- LuoLLiDQDoshiAFarleyWCorralesRMPflugfelderSCExperimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surfaceInvest Ophthalmol Vis Sci200445124293430115557435