Abstract
Background
Parkinson’s disease is a neurodegenerative disorder caused by loss of dopaminergic neurons in the substantia nigra. The dopamine precursor, levodopa, remains the most effective and common treatment for this disorder. However, long-term administration of levodopa is known to induce characteristic dyskinesia, and molecular mechanisms underlying dyskinesia are poorly understood.
Methods
In this study, we investigated the effect of 6-hydroxydopamine lesions in dopaminergic neurons and chronic treatment with levodopa on expression of G protein-coupled receptor kinase 6 and â-arrestin-1, two key regulators of G protein-coupled receptors, in the rat striatum.
Results
We found that a unilateral 6-hydroxydopamine lesion reduced expression of G protein-coupled receptor kinase 6 and â-arrestin-1 protein in the lesioned striatum. Reduction of these two proteins persisted in 6-hydroxydopamine-lesioned rats on chronic levodopa treatment for 23 days. In addition, coadministration of the N-methyl-D-aspartate receptor antagonist, MK-801, and levodopa reversed the reduction of G protein-coupled receptor kinase 6 and â-arrestin-1 in the striatum. MK-801 also attenuated levodopa-induced dyskinetic behavior.
Conclusion
These data indicate that G protein-coupled receptor kinase 6 and â-arrestin-1 in striatal neurons are sensitive to dopamine depletion and are downregulated in rats with Parkinson’s disease and in levodopa-treated rats with the disease. This downregulation seems to require activation of N-methyl-D-aspartate glutamate receptors.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by static tremor, rigidity, bradykinesia, and an irregular gait.Citation1 The disease is caused by degeneration of dopaminergic neurons in the substantia nigra and consequent depletion of dopamine content in the striatum.Citation2 As a result, levodopa, a dopamine precursor that increases availability of dopamine, is still the most effective treatment for this disease of dopamine depletion.Citation3 While levodopa alleviates the main motor symptoms of PD, long-term treatment with the drug causes abnormal involuntary movements, ie, levodopa-induced dyskinesias, in a subset of patients.Citation4 To date, little is known about the pathogenesis of levodopa-induced dyskinesias, which remain a challenge to the therapeutic management of PD.
The striatum is a key structure in the basal ganglia and is enriched with glutamatergic innervations from multiple forebrain sites. Ionotropic glutamate receptors, such as N-methyl-D-aspartate (NMDA) receptors, are densely expressed in striatal neurons.Citation5 Accumulating evidence supports NMDA receptor activity being linked to levodopa-induced dyskinesias.Citation6 For instance, glutamate release in the basal ganglia was enhanced in parkinsonian rats receiving chronic treatment with levodopa.Citation7 NMDA receptors were altered in their expression and phosphorylation levels in the striatum of rats with levodopa-induced dyskinesias.Citation6,Citation8 Moreover, levodopa-induced dyskinesias were consistently ameliorated by NMDA receptor antagonists in parkinsonian patients and experimental animals.Citation9–Citation15 Thus, the NMDA receptor seems to serve as an important regulator mediating the development and expression of levodopa-induced dyskinesias.
Dopamine receptors are G protein-coupled receptors, mainly the D1 and D2 subtypes, and highly expressed in striatal neurons, becoming hyperactive in rats with PD because of depletion of dopamine ligand.Citation2 Like other G protein-coupled receptors, dopamine receptors are subject to regulation by G protein-coupled receptor kinases (GPK). These kinases, once bound to G protein-coupled receptors, phosphorylate G protein-coupled receptors and trigger binding of arrestin to induce receptor endocytosis, leading to inhibition of G protein-coupled receptor signaling.Citation16 Five G protein-coupled receptor kinase isoforms are distributed in the brain, of which GRK6 is expressed at the highest level in the striatum.Citation17,Citation18 Similar to the high level of GRK6, two arrestin subtypes (â-arrestin-1 and â-arrestin-2) are abundant in the striatum, with â-arrestin-1 at a level 20-fold higher than that of â-arrestin-2.Citation19–Citation21 Enriched expression of GRK6 and â-arrestin-1 in striatal neurons implies their roles in regulating dopamine receptors and possible links with PD and levodopa-induced dyskinesias. Indeed, GRK6 knockout enhanced the sensitivity of dopamine receptors.Citation22 In rats with levodopa-induced dyskinesias, overexpression of GRK6 promoted dopamine D1 receptor internalization and attenuated dyskinesia, while GRK6 knockdown exacerbated dyskinesia.Citation23,Citation24
In this study, we investigated changes in expression of GRK6 and â-arrestin-1 protein in the striatum in a PD model of rats which received 6-hydroxydopamine (6-OHDA) dopamine lesions. We further detected changes in expression of these two proteins in dopamine-lesioned rats with or without chronic levodopa treatment. We also investigated the roles of NMDA receptors in chronic levodopa-induced changes in GRK6 and â-arrestin-1 expression and dyskinesias.
Materials and methods
Animals
Adult Sprague-Dawley rats (200 ± 20 g) were housed in the Institute of Experimental Animal Science, Shanghai JiaoTong University School of Medicine, at a constant temperature of 23°C with free access to food and water. All animal use and procedures were approved by the institutional review board of Xinhua Hospital, and were performed according to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs and antibodies
6-OHDA, apomorphine, levodopa methyl ester, benserazide, ascorbic acid and MK-801 were purchased from Sigma Chemical Company (St Louis, MO, USA). Rabbit polyclonal antibodies against GRK6 and â-arrestin-1 were obtained from Abcam Inc (Cambridge, UK). Mouse anti-â-actin antibody, and horseradish peroxidase-conjugated goat antirabbit and antimouse IgG secondary antibodies were obtained from Beyotime Institute of Biotechnology (Shanghai, People’s Republic of China).
6-OHDA lesions and rotation screening
Rats were anesthetized using an intraperitoneal injection of sodium pentobarbital 50 mg/kg. The rat head was mounted onto a stereotaxic apparatus (Narishige, Japan). Nigrostriatal lesions were produced by injecting 6-OHDA (8 μg/4 μL) into the right medial forebrain bundle at bregma 4.5 mm, lateral 0.9 mm, and dura 7.5 mm.Citation25 The injection was administered over 5 minutes and the injector was kept in position for 10 minutes to prevent backflow of the injected drugs. Control rats received a sham lesion procedure in which only a vehicle solution was injected. Rotation tests were performed 3 weeks after surgery to confirm the 6-OHDA lesion. To this end the 6-OHDA-lesioned rats were injected intraperitoneally with apomorphine 0.25 mg/kg and placed in a stainless steel bowl. The number of rotations was counted visually for each rat. Rats exhibiting a vigorous rotational response (> 100 turns) to apomorphine were deemed to be successfully lesioned animals and selected for further studies.
Drug treatment and experimental groups
Four groups of rats (n = 10 per group) were used in the present study, ie, sham rats, PD rats (unilateral 6-OHDA-lesioned), PD rats on chronic levodopa administration, and PD rats on chronic levodopa administration with the noncompetitive NMDA receptor antagonist, MK-801, administered on the final day. The sham-lesioned rats underwent sham surgery and were then treated with intraperitoneal saline twice daily for 23 days. 6-OHDA-lesioned rats were treated with intraperitoneal saline twice daily for 23 days following their dopamine lesion. The rats with 6-OHDA lesions were treated with levodopa methyl ester (25 mg/kg with 6.25 mg/kg of intraperitoneal benserazide twice daily for 22 days). On day 23, the animals were divided into two groups. The first group was cotreated with intraperitoneal MK-801 0.1 mg/kg and levodopa twice daily while the second group was cotreated with vehicle and levodopa twice daily.
Behavioral observations
The rats were monitored for abnormal involuntary movements using a procedure similar to one described previously.Citation26 Briefly on test days, the rats were individually placed in plastic trays 5 minutes before drug treatment. After injection, each rat was assessed for orolingual, limb, axial, and locomotor movements. At 20-minute intervals, abnormal involuntary movements were rated for 60 seconds over a period of 3 hours, during which a severity score of 0–4 was assigned to each category of abnormal involuntary movement. These scores were then summed at each time point.
Western blotting
The rats were anesthetized with intraperitoneal sodium pentobarbital 50 mg/kg 30 minutes after the last drug administration. Their brains were removed and bilateral striatal structures were dissected. Striatal tissue was lysed in lysis buffer containing phenylmethanesulfonyl fluoride. The supernatant was collected after centrifugation at 14,000 g for 30 minutes. Protein concentrations were measured using a bicinchoninic acid assay kit (Pierce, Rockford IL, USA). Protein samples were mixed with 2× sample buffer (4% sodium dodecyl sulfate, 20% glycerol, 10% mercaptoethanol, and bromophenol blue dye in 125 mM Tris-HCl, pH 6.8) and heated to 95°C for 5 minutes. The samples were then loaded onto sodium dodecyl sulfate polyacrylamide gel (15%), and the proteins were transferred onto a 0.2 mm polyvinylidene difluoride membrane (Bio-Rad Hercules, CA, USA). The membrane was blocked in Tris-buffered saline with 0.1% Tween 20 and 5% milk, and then incubated with a primary antibody against GRK6 (1:500), â-arrestin-1 (1:500), or â-actin (1:1000) at 4°C overnight. Omission of the primary antibody served as a negative control. The membrane was then washed and incubated with a horseradish peroxidase-conjugated secondary antibody (1:1000, 2 hours). Immunoblot signals were visualized in chemiluminescent solution using a Bio-Rad gel imager.
Statistical analysis
Experimental data were presented as the mean ± standard error of the mean. Analysis of variance followed by least significant difference post hoc comparison tests were used to analyze Western blot data for the different groups. Nonparametric tests were performed to analyze abnormal involuntary movement scores from the behavioral experiments. P < 0.05 was considered to be a statistically significant difference.
Results
Effects of MK-801 on abnormal involuntary movements
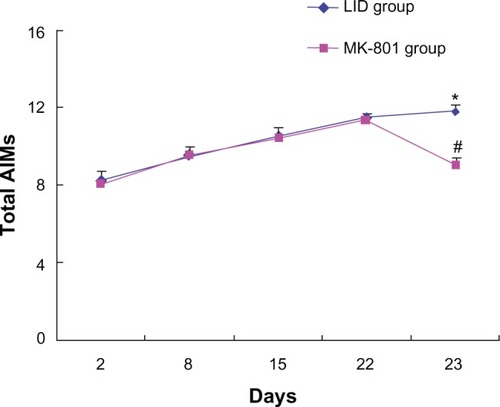
Chronic administration of levodopa is known to produce abnormal involuntary orolingual, limb, and axial movements.Citation4 In this study, total abnormal involuntary movement scores progressively increased following repeated levodopa administration (twice daily for 23 days with vehicle + levodopa coadministered on the final day, ). In a separate group of rats, MK-801 was coadministered with levodopa on the final day (day 23) following daily treatment with levodopa for 22 days. We found that total abnormal involuntary movement scores were significantly reduced in this group compared with the control group (). Thus, pharmacological blockade of NMDA receptors appeared to alleviate levodopa-induced dyskinesias resulting from chronic levodopa administration.
Figure 1 Effects of MK-801 on abnormal involuntary movements in rats with Parkinson’s disease.
Abbreviations: 6-OHDA, 6-hydroxydopamine; LID, levodopa-induced dyskinesia; AIM, abnormal involuntary movement.
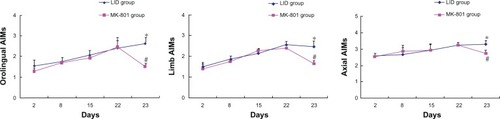
We also analyzed abnormal involuntary orolingual, limb, and axial movements. Similar to the total abnormal involuntary movements described in , abnormal involuntary orolingual, limb, and axial movements showed progressive increases over the period of repeated levodopa injections in control rats (levodopa-induced dyskinesia group, ). Coadministration of MK-801 on day 23 significantly reversed these abnormal involuntary movements (). These data support the role of NMDA receptors in mediating levodopa-stimulated abnormal involuntary movements.
Figure 2 Effects of MK-801 on individual abnormal involuntary movements in rats with Parkinson’s disease.
Abbreviations: 6-OHDA, 6-hydroxydopamine; LID, levodopa-induced dyskinesia; AIM, abnormal involuntary movement.
Effects of MK-801 on GRK6 expression in the striatum
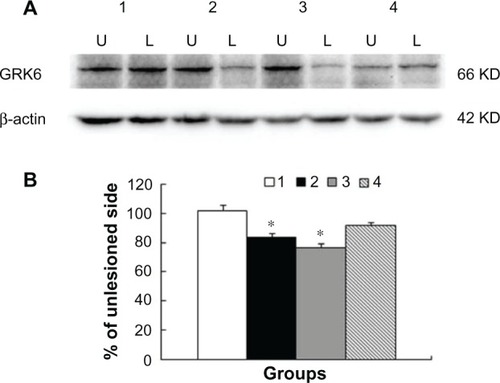
We next examined possible changes in GRK6 protein expression in the striatum for all groups of animals. To this end, we removed striatal tissue from both the lesioned and nonlesioned sides. We then compared GRK6 protein levels on both sides with Western blots. Comparable GRK6 expression was seen in the lesioned and nonlesioned striatum of sham-treated control rats (). The 6-OHDA lesion resulted in a significant decrease in GRK6 expression in the lesioned striatum relative to the nonlesioned striatum (83.7% ± 2.1% of nonlesioned side, P < 0.05). This decrease persisted in the 6-OHDA-lesioned rats undergoing chronic levodopa administration. As shown in , following 23 daily treatments with levodopa, GRK6 protein levels in the lesioned striatum remained lower than those in the nonlesioned striatum (76.8% ± 2.2% of nonlesioned side, P < 0.05). Interestingly, in 6-OHDA-lesioned rats cotreated with MK-801 and levodopa on day 23, we did not see a significant decrease in striatal GRK6 expression on the lesioned side compared with the nonlesioned side (91.1% ± 2.7%, P > 0.05; ). This indicates that the NMDA receptor antagonist normalized altered GRK6 expression in striatal neurons.
Figure 3 Changes in GRK6 protein expression in rat striatum. Rats were subject to sham surgery (group 1), 6-OHDA lesions (group 2), 6-OHDA lesions followed by 23 daily treatments with levodopa (group 3), and coadministration of MK-801 and levodopa on the last day (group 4). GRK6 protein levels in the striatum on both the lesioned (L) and nonlesioned (U) sides were assayed by Western blotting. Representative immunoblots (A) are shown above the quantification of data (B).
Abbreviation: 6-OHDA, 6-hydroxydopamine; KD, 1000 Dalton.
Effects of MK-801 on â-arrestin-1 expression in the striatum
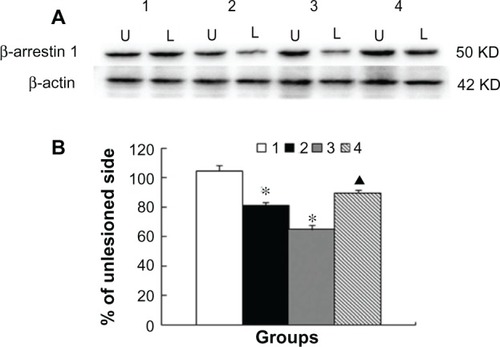
To determine changes in â-arrestin-1 expression in the striatum, we performed Western blots on striatal protein samples from the same animals. The basal level of â-arrestin-1 remained stable on both sides of the striatum in sham-treated animals (). However, protein levels in 6-OHDA-lesioned rats were significantly lower in lesioned than in nonlesioned striatum (81.0% ± 2.2% of nonlesioned side, P < 0.05). Similarly, â-arrestin-1 levels in the levodopa-treated rats remained lower (64.9% ± 3.1% of nonlesioned side, P < 0.05). Coadministration of MK-801 significantly reversed the reduction of â-arrestin-1 expression in the lesioned striatum (P < 0.05 versus vehicle + levodopa). These data indicate that â-arrestin-1, like GRK6, is sensitive to dopamine signals and subject to MK-801-sensitive down-regulation in response to administration of levodopa.
Figure 4 Changes in â-arrestin-1 protein expression in rat striatum. Rats were subject to sham surgery (group 1), 6-OHDA lesion (group 2), 6-OHDA lesion followed by 23 daily treatments with levodopa (group 3), or coadministration of MK-801 and levodopa on the last day (group 4). Protein levels of â-arrestin-1 in the striatum on the lesioned (L) and nonlesioned (U) sides were assayed by Western blotting. Representative immunoblots (A) are shown above the quantification of data (B).
Abbreviation: 6-OHDA, 6-hydroxydopamine; KD, 1000 Dalton.
Discussion
This study assessed changes in GRK6 and â-arrestin-1 expression in the striatum for four groups of rats, ie, sham-treated rats, PD rats (with a unilateral 6-OHDA lesion), PD rats on chronic administration of levodopa, and PD rats on chronic levodopa administration with MK-801 administered at the final day. We found that expression of the two proteins was lower in the lesioned striatum of PD rats and PD rats on chronic treatment with levodopa. Moreover, pharmacological blockade of NMDA receptors with the antagonist MK-801 prevented reduction of GRK6 and â-arrestin-1 in the lesioned striatum. These data indicate that GRK6 and â-arrestin-1 in striatal neurons are sensitive to dopamine depletion. Downregulation of expression of the two proteins may contribute to the development of levodopa-induced dyskinesias.
G protein-coupled receptors are the largest and most diverse family of cell signaling proteins.Citation27 Generally, ligand binding activates G protein-coupled receptors, which leads to activation of specific downstream signaling pathways. Active G protein-coupled receptors are also subject to phosphorylation modification by GRKs. GRK-mediated phosphorylation of receptors promotes association of arrestin molecules with the phosphorylated receptors.Citation28,Citation29 In many cases, this arrestin-receptor association triggers translocation (internalization) of the receptor, leading to feedback inhibition of receptor signaling. In this study, we found that a 6-OHDA lesion resulted in a decrease in GRK6 and â-arrestin-1 expression in the striatum. Given that both proteins are involved in inhibition of G protein-coupled receptors, reduced expression of GRK6/â-arrestin-1 may contribute to the hyperactivity of dopamine receptors (one family of G protein-coupled receptors) in 6-OHDA-lesioned rats. This is in accordance with a mutagenesis study in which GRK6 knockout in mice led to supersensitivity of dopaminergic signaling.Citation22
Striatal GRK6 and â-arrestin-1 expression remained reduced in PD rats after chronic treatment with levodopa. Interestingly, the NMDA receptor antagonist, MK-801, could normalize this reduction, given that no significant reduction in GRK6 and â-arrestin-1 expression was seen in the lesioned striatum compared with the nonlesioned striatum in rats treated with MK-801. It has been shown that increased availability of GRK6 ameliorates dyskinesia, while reduced GRK6 levels aggravate it.Citation23 Thus, the reduced GRK6 and â-arrestin-1 expression seen in this study may contribute to the pathogenesis of levodopa-induced dyskinesias. Blocking reduction of GRK6 and â-arrestin-1 expression with MK-801 may result in suppression of levodopa-induced dyskinetic behavior, as seen in this study and others.Citation9–Citation15
In our present study, we found that GRK6 and â-arrestin-1 expression in the striatum was reduced in PD rats with 6-OHDA lesions. This is consistent with an early observation that 6-OHDA lesioning reduced GRK6 expression in the caudal portion of the caudate putamen, although GRK6 expression in the rostral caudate putamen and nucleus accumbens was upregulated.Citation17 However, while chronic treatment with levodopa normalized PD, it did not reverse the reduction of GRK6 and â-arrestin-1 expression in this or another study.Citation17 We assume that different mechanisms may contribute to the reduction of GRK6 and â-arrestin-1 in PD rats and in PD rats treated with levodopa. Reduction of the two proteins contributes, at least in part, to the development of levodopa-induced dyskinesias, particularly in levodopa-treated PD rats.
Amantadine, an NMDA receptor antagonist, is one of the most effective drugs for the clinical treatment of dyskinesias. In our study, we found that MK801 might be a good adjunct in the treatment of levodopa-induced dyskinesias. GRK6 and â-arrestin-1 are effective targets for treatment of dyskinesia. Further, the concise mechanisms involved in regulation of GRK6 and â-arrestin-1 expression by MK801 need more study in the future.
Acknowledgments
This study was supported by grants from the National Science Foundation of China (81071025, 81171203, 81171204), the Shanghai Committee of Science and Technology (11nm0503300, 11410708900, 12XD1403800), and the Key Science Development Program of Shanghai Municipal Education Commission (10ZZ72).
Disclosure
The authors report no conflicts of interest in this work.
References
- CenciMAL-DOPA-induced dyskinesia: cellular mechanisms and approaches to treatmentParkinsonism Relat Disord200713Suppl 3263267
- ObesoJAOlanouCWNuttJGLevodopa motor complications in Parkinson’s diseaseTrends Neurosci200023Suppl 10S2S711052214
- CalabresiPPicconiBTozziADi FilippoMDopamine-mediated regulation of corticostriatal synaptic plasticityTrends Neurosci200730521121917367873
- ThanviBLoNRobinsonTLevodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatmentPostgrad Med J20078398038438817551069
- StandaertDGTestaCMYoungABPenneyJBJrOrganization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the ratJ Comp Neurol199434311168027428
- KongMBaMSongLLiuZComparative effects of acute or chronic administration of levodopa to 6-OHDA-lesioned rats on the expression and phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in the striatumNeurochem Res20093481513152119283475
- RobeletSMelonCGuilletBSalinPKerkerian-Le GoffLChronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson’s diseaseEur J Neurosci20042051255126615341597
- DunahAWWangYYasudaRPAlterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s diseaseMol Pharmacol200057234235210648644
- BaMKongMYangHChanges in subcellular distribution and phosphorylation of GluR1 in lesioned striatum of 6- hydroxydopamine-lesioned and L-dopa-treated ratsNeurochem Res200631111337134717053970
- BibbianiFOhJDKielaiteACollinsMASmithCChaseTNCombined blockade of AMPA and NMDA glutamate receptors reduces levodopa-induced motor complications in animal models of PDExp Neurol2005196242242916203001
- Hadj TaharAGregoireLDarreABelangerNMeltzerLBedardPJEffect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naïve parkinsonian monkeysNeurobiol Dis200415217117615006686
- LugingerEWenningGKBoschSPoeweWBeneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson’s diseaseMov Disord200015587387811009193
- LundbladMAnderssonMWinklerCKirkDWierupNCenciMAPharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s diseaseEur J Neurosci200215112013211860512
- PapaSMChaseTNLevodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeysAnn Neurol19963955745788619541
- Verhagen MetmanLDel DottoPvan den MunchhofPFangJMouradianMMChaseTNAmantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s diseaseNeurology1998505132313269595981
- PremontRTGainetdinovRRPhysiological roles of G protein-coupled receptor kinases and arrestinsAnnu Rev Physiol20076951153417305472
- AhmedMRBychkovEGurevichVVBenovicJLGurevichEVAltered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by L-dopa treatmentJ Neurochem200810461622163617996024
- Erdtmann-VourliotisMMayerPAmmonSRiechertUHölltVDistribution of G protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brainMol Brain Res2001951–212913711687284
- AhmedMRGurevichVVDalbyKNBenovicJLGurevichEVHaloperidol and clozapine differentially affect the expression of arrestins, receptor kinases, and extracellular signal-regulated kinase activationJ Pharmacol Exp Ther2008325127628318178904
- DeWireSMAhnSLefkowitzRJShenoySKÂ arrestins and cell signalingAnnu Rev Physiol20076948351017305471
- GurevichEVBenovicJLGurevichVVArrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal developmentNeuroscience2002109342143611823056
- GainetdinovRRBohnLMSotnikovaTDDopaminergic super-sensitivity in G protein-coupled receptor kinase 6-deficient miceNeuron200338229130312718862
- AhmedMRBerthetABychkovELentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson’s diseaseSci Transl Med201022828ra28
- ManagoFEspinozaSSalahpourAThe role of GRK6 in animal models of Parkinson’s disease and L-dopa treatmentSci Rep2012230122393477
- PaxinosGWatsonCThe Rat Brain in Stereotaxic Coordinates4th edNew York, NYAcademic Press1998
- DeumensRBloklandAPrickaertsJModeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathwayExp Neurol2002175230331712061862
- GurevichVVGurevichEVThe structural basis of arrestin mediated regulation of G protein coupled receptorsPharmacol Ther2006110346550216460808
- LuttrellLMLefkowitzRJThe role of â-arrestins in the termination and transduction of G-protein-coupled receptor signalsCell Sci2002115Pt 3455465
- PerrySJLefkowitzRJArresting developments in heptahelical receptor signaling and regulationTrends Cell Biol200212313013811859025