Abstract
Purpose
Osteoarthritis (OA) is a degenerative disease characterized by a progressive loss of articular cartilage extracellular matrix and is due to functional impairments occurring in chondrocytes. In previous works, we highlighted that Regenerative Tissue Optimization (TO-RGN) treatment with radioelectric asymmetric conveyer (REAC) technology influenced the gene expression profiles controlling stem cell differentiation and the pluripotency of human skin-derived fibroblasts in vitro. Since interleukin-1 beta signaling has been implicated in the induction and progression of this disease (through metalloproteinase-3 synthesis and nitric oxide production), we investigated whether REAC TO-RGN might influence the biochemical and morphological changes induced by interleukin-1 beta in normal and OA chondrocytes.
Methods
The induction of metalloproteinase-3 and proteoglycan synthesis was evaluated by a solid-phase enzyme-amplified sensitivity immunoassay, and nitric oxide production was evaluated with the Griess method. Ultrastructural features were observed by transmission electron microscopy.
Results
REAC TO-RGN treatment decreased nitric oxide and metalloproteinase-3 production in normal and OA chondrocytes, while inducing an increase in proteoglycan synthesis. OA chondrocytes were more affected by REAC TO-RGN treatment than were normal chondrocytes. Ultrastructural changes confirmed that REAC TO-RGN may counteract the negative effects of interleukin-1 beta incubation.
Conclusion
The results of this in vitro study suggest that REAC TO-RGN treatment may represent a new, promising approach for the management of OA.
Introduction
Osteoarthritis (OA) is the most common articular disorder of hyaline cartilage and subchondral bone. The disease is responsible for substantial direct and indirect socioeconomic costs.Citation1,Citation2 Chondrocytes are responsible for the synthesis and maintenance of extracellular matrix within articular cartilage, as they regulate the equilibrium between synthetic and degradative processes. Loss of homeostasis in favor of catabolic activities leads to the destruction of articular cartilage, and chondrocyte death has been postulated to be a crucial event in the pathogenesis of OA.Citation3,Citation4 Interleukin-1 beta (IL-1β) is a cytokine involved in the processes of cartilage degradation, also acting as a potent inhibitor of extracellular matrix synthesis. In response to IL-1β, chondrocytes secrete proinflammatory cytokines, chemokines, neutral metalloproteinase (MMP), and nitric oxide (NO).Citation5 The latter promotes numerous effects on chondrocytes, including inhibition of proteoglycan (PG) and collagen synthesis, activation of MMPs, as well as induction of apoptosis, and can give rise to free radicals, which are implicated in tissue injury in a variety of degenerative diseases. Furthermore, inhibitors of NO synthesis have been shown to delay the development of clinical and histological signs in various experimental models of OA.Citation6
Currently, the optimal management of OA requires a combination of pharmacological and nonpharmacological treatment modalities.Citation7 Pharmacologic treatment of OA has been largely confined to analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs), which are only symptom-modifying agents. These drugs control pain but lack known disease-modifying effects. Furthermore, treatment with NSAIDs is limited by their negative secondary effects on the gastrointestinal tract and on cartilage metabolism.Citation8 Identification of a tool capable of counteracting the cartilage degeneration occurring during OA without side effects could represent an interesting approach in OA therapy. In this regard, we have recently observed that Regenerative Tissue Optimization (TO-RGN) treatment with radioelectric asymmetric conveyer (REAC) technologyCitation9,Citation10 significantly influenced the differentiation capability of mouse embryonic stem cellsCitation11 as well as the pluripotency and plasticity of human skin-derived fibroblasts.Citation12 These findings have suggested the purpose of this study – to clarify whether the positive effect of REAC TO-RGN might be related to a capability to counteract the IL-1β-induced signaling pathway, a primary cause of OA. REAC TO-RGN treatment was applied to cultures of normal and OA human articular chondrocytes, in the presence and absence of IL-1β. The release of PG, MMP-3, and NO was evaluated, and morphological assessment of chondrocytes, using transmission electron microscopy (TEM), was carried out.
Materials and methods
Cell culture
Normal human articular cartilage was obtained from the femoral heads of five subjects with displaced femoral neck fractures (three males and two females), and OA human articular cartilage was obtained from the femoral heads of five patients with OA, defined by the clinical and radiological criteria of the American College of Radiology,Citation13 undergoing surgery for total hip prostheses. The mean age of the group was 33 years (range: 20–41) for the normal subjects and 70.6 years (range: 67–75) for the OA patients. Written consent was signed by each participant in this study, along with the guidelines of the Ethical Committee that had approved the study. These samples were sufficient to provide a large number of cell cultures to perform the study protocol. Normal chondrocytes were obtained from the middle layer of cartilage in the femoral heads, whereas the OA chondrocytes originated from the area adjacent to the OA lesion. OA cartilage was characterized by macroscopic focal fibrillation of the articular surface. Normal cartilage was characterized by a glossy, white, completely smooth surface and a healthy appearance without irregularities. Following surgery, the cartilage was dissected aseptically and minced into small pieces. The fragments were washed in Dulbecco’s modified Eagle’s medium (DMEM) without phenol red, containing 2% penicillin/streptomycin solution and 0.2% amphotericin B (Life Technologies, Carlsbad, CA, USA). The chondrocytes were isolated from the articular cartilage by sequential enzymatic digestion: 30 minutes with 0.1% hyaluronidase, 1 hour with 0.5% pronase, and 1 hour with 0.2% collagenase (Sigma-Aldrich, St Louis, MO, USA), at 37°C in the wash solution (DMEM + penicillin/streptomycin solution + amphotericin B). The resulting cell suspension was filtered twice using 70 μm nylon mesh, then washed and centrifuged at 700 g for 10 minutes. As shown by the trypan blue viability test, 90%–95% of the recovered cells were alive. The primary cultures of chondrocytes so obtained were maintained in a humidified atmosphere of 5% CO2 at 37°C, for 2 weeks.
REAC technology for therapeutic use
The REAC is an innovative patented technologyCitation9,Citation10 for biostimulation and/or bioenhancement that induces weak radioelectric currents in tissue, to induce cell-reprogramming activity.Citation12 The model used in this study (BENE Bio Enhancer – Neuro Enhancer®; ASMED, Florence, Italy) is specifically designed for regenerative treatments. Recently, using a TO-RGN treatment protocol, the REAC technology was able to drive stem cell pluripotency and differentiationCitation11 and cell-reprogramming activity.Citation12
REAC TO-RGN treatment protocol
The REAC device was placed into a CO2 incubator, set with the TO-RGN protocol,Citation11,Citation12 and its conveyer electrodes were immersed into separate culture media of human normal and OA chondrocytes, in the presence and absence of IL-1β, during 48 hours of culture.
The distance between the emitter (at 2.4 GHz) and the culture medium was approximately 35 cm. The electromagnetic parameters of a single radiofrequency burst of 200 ms duration were the following: radiated power was ~2 mW, electric field = 0.4 V/m, magnetic field was 1 mA/m, specific absorption rate (SAR) was 0.128 μW/g; the density of radioelectric current flowing in the culture medium during the REAC single radiofrequency burst was J = 30 μA/cm2.
Exposure of chondrocytes to IL-1β and REAC TO-RGN
Normal and OA human articular chondrocytes were cultured in 8-well microplates at a density of 4 × 104 cells/well, with 1 mL of medium without phenol red, containing 10% fetal bovine serum (FBS), 200 U/mL penicillin, 200 mg/mL streptomycin, 2 mM glutamine (Life Technologies), until they became preconfluent. Chondrocytes were divided into eight groups: normal and OA chondrocytes at basal conditions; normal and OA chondrocytes at basal conditions treated with REAC TO-RGN; normal and OA chondrocytes treated with 5 ng/mL IL-1β (Sigma-Aldrich); and normal and OA chondrocytes treated with REAC TO-RGN and IL-1β. The evaluation of cell viability was carried out in each group at every experimental step, using the Countess Automated Cell Counter (Life Technologies) and trypan blue. After 48 hours of treatment, the media were removed and stored at −80°C for the subsequent detection of PGs and MMP-3. Once terminated the REAC treatment, the cells were fixed for TEM. The supernatants were frozen in part to determine subsequently the MMP-3 and PG. Release of NO was immediately detected before freezing using Griess’s assay.
PG assay
The quantity of PGs was measured in the culture medium by a solid-phase enzyme-amplified sensitivity immunoassay (EASIA) (DIAsource ImmunoAssay SA, Nivelles, Belgium), performed on microtiter plates (Heinegard and SaxneCitation13). This technique uses two monoclonal antibodies that are directed against keratan sulfate proteoglycans and another monoclonal antibody that is directed against the binding site of hyaluronic acid proteoglycans. Standards or samples containing the PG were reacted with captured monoclonal antibodies (MAbs) coated on the microtiter well and with a monoclonal antibody labeled with horseradish peroxidase (MAb-HRP). After an incubation period allowing the formation of a sandwich (coated MAbs1/PG/MAbs2-HRP), the microtiter plate was washed to remove unbound enzyme-labeled antibodies. Bound enzyme-labeled antibodies were measured through a chromogenic reaction. Then, chromogenic solution (tetramethylbenzidine + H2O2) was added and incubated. The reaction was stopped with the addition of stop solution (HCl), and the microtiter plate was then read at the appropriate wavelength (450 nm). The amount of substrate turnover was determined colorimetrically by measuring the absorbance, which was proportional to the PG concentration. The assay sensitivity was 0.9 ng/mL. The results obtained for the different culture supernatants were normalized with number the cells. The amount of PG was expressed as ng/106 cells.
MMP-3 assay
MMP-3 was assayed by a solid-phase EASIA (Bender-Medsystems, Vienna, Austria).Citation15 An anti-human MMP-3 coating antibody was absorbed on microwells; any human MMP-3 present in the sample was then bound to the antibodies adsorbed to the microwells. The substrate turnover was determined colorimetrically by measuring the absorbance, which was proportional to the MMP-3 concentration. The sensitivity of the method was estimated to be <0.1 ng/mL. The results obtained for the different culture supernatants were normalized to the number of cells. The amount of MMP-3 was expressed as ng/106 cells.
Nitrite assay
The quantity of nitrites in the culture medium was measured by the Griess method (1% sulfanilamide, 0.1% (napthyl)ethylenediamine dihydrochloride, and 2.5% phosphoric acid).Citation16 Equal volumes (100 μL) of supernatant and the Griess reagent were incubated on microplates at room temperature for 15 minutes. The absorbance was measured with a spectrophotometer at 550 nm. The concentration of nitrites was calculated using a standard curve made by successive dilutions with a solution of sodium nitrite in water. The results obtained for the different culture supernatants were normalized to the number of cells. The amount of nitrite was expressed as ng/106 cells.
TEM
Cultures of normal and OA human chondrocytes were fixed in cold Karnovsky fixative and maintained at 4°C for 2 hours, postfixed in 1% buffered osmium tetroxide, then dehydrated in a graded ethanol series, and embedded in epon araldite medium. Ultrathin sections, stained with uranyl acetate and lead citrate, were observed and photographed with a TEM (EM 208; Royal Philips Electronics, Amsterdam, The Netherlands). At least 100 cells from each group were examined.
Morphometric and statistical analysis
For the morphometric studies, we analyzed sections of three different blocks from each group. For standardization and comparison of the different groups, only medially sectioned chondrocytes were investigated; 100 chondrocytes were selected using the nucleus/cytoplasm ratio as the selection criterion. Our analysis was based on an established methodCitation17,Citation18 for ultrastructural quantitative evaluation of changes in chondrocyte function. Mitochondria and Golgi bodies were counted and recorded. Moreover, the presence of cytoplasmic vacuolization was reported. This cytoplasmic characteristic is known to be predominant in the presence of negative stimulus, represented by IL-1β. To evaluate the presence of vacuolization in medially sectioned chondrocytes, we considered five vacuoles as the point of reference.
Data were expressed as the mean ± standard deviation (SD) of triplicate values for each experiment. For each group (of normal and OA chondrocyte), the comparisons between the basal condition, REAC TO-RGN alone, IL-1β alone, and REAC TO-RGN + IL-1β treated were analyzed using the Friedman test, followed by Dunn’s post hoc test. Normal and OA groups were compared using the Mann–Whitney test, considering the differences, for each group, between the basal condition and REAC TO-RGN, between basal and IL-1β, and between basal and REAC TO-RGN + IL-1β. A P value < 0.05 (two-tailed) was considered statistically significant. All the data were analyzed using GraphPad Prism software for Windows (v 5.00; GraphPad Software Inc, La Jolla, CA, USA).
Results
Viability evaluation
Analysis of cell viability, detected by trypan blue staining before starting, revealed that more than 95% of normal chondrocytes and 87% of OA chondrocytes were viable; after 48 hours, cell viability was 86.67% in normal and 77.83% in OA chondrocytes. The treatment with IL-1β significantly affected (P < 0.05) chondrocyte viability in both groups of cells (normal, 64.83%; OA, 61%). REAC TO-RGN exposure was applied at basal conditions, and cell viability resulted to be increased, particularly in OA chondrocytes (normal, 88.83%; OA, 86.33%). When REAC TO-RGN treatment was performed in the presence of IL-1β, both normal and OA chondrocytes maintained a remarkable viability (73% and 72.69%, respectively). It was interesting to note that normal and OA chondrocytes showed a significantly different response to IL-1β and REAC TO-RGN treatment. In particular, OA chondrocytes significantly maintained their viability after REAC TO-RGN treatment, both in the presence or absence of IL-1β, as compared with normal chondrocytes in the same experimental conditions (P < 0.01).
PGs, MMP-3, and NO evaluation
The amount of PGs (), MMP-3 (), and NO () in the culture medium of normal and OA chondrocytes incubated in the different experimental conditions are shown.
Figure 1 Effects of incubation of human normal and OA chondrocytes, at basal conditions; with REAC TO-RGN treatment alone; with IL-1β (5 ng/mL) alone; or coincubation with IL-1β and REAC TO-RGN treatment for 48 h in the culture medium. (A) The quantity of PG (ng/106); (B) the MMP-3 level (ng/106 cells); and (C) the NO production (ng/106 cells).
Abbreviations: OA, osteoarthritis; REAC, radioelectric asymmetric conveyer technology; TO-RGN, Regenerative Tissue Optimization; IL, interleukin; PG, proteoglycan; MMP, metalloproteinase; NO, nitric oxide.
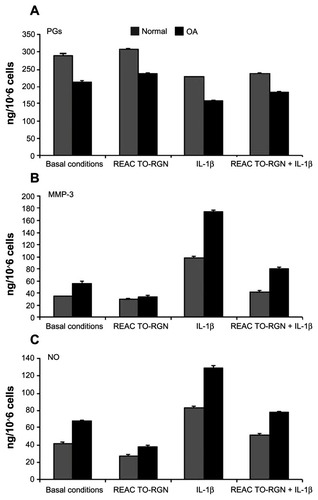
The total PG concentration in the culture medium increased in the presence of REAC TO-RGN, both at basal conditions and in IL-1β-treated normal and OA chondrocytes (). Noteworthy, OA chondrocytes significantly increased (P < 0.01) the levels of PGs in the presence of REAC TO-RGN and IL-1β as compared with normal chondrocytes.
The REAC TO-RGN stimulus that was applied to normal or OA chondrocytes reduced MMP-3 and NO levels, both in the absence or presence of IL-1β ( and C). Interestingly, the effect of REAC TO-RGN treatment on the decrease in MMP-3 levels was significantly higher in OA chondrocytes, as compared with normal cells, even after the addition of IL-1β (P < 0.01). The level of NO in the OA chondrocyte culture significantly decreased with respect to that of normal chondrocytes after REAC TO-RGN at basal conditions (P < 0.01).
TEM evaluation
Normal chondrocytes showed a euchromatic nucleus and a cytoplasm containing abundant rough endoplasmic reticulum, Golgi bodies, and mitochondria (Figure not shown) (). Cultured OA chondrocytes showed a reduction in cytoplasmic components, such as Golgi bodies and mitochondria, and the presence of vacuoles ( and ). The REAC TO-RGN treatment, applied at basal conditions, improved the morphological status of the cultured normal and OA chondrocytes; in particular, in the OA cells (), a significantly increased number of mitochondria (P < 0.05) and Golgi bodies (P < 0.05) was displayed ().
Table 1 Number (n) of mitochondria, Golgi bodies and presence of vacuolization (percentage, %) in normal and OA chondrocytes, under basal conditions and after treatment with the REAC TO-RGN, in absence or presence of IL-1b (5 ng/mL)
Figure 2 TEM micrographs of human OA chondrocytes. (A) Basal conditions: The cell shows an euchromatic nucleus (N), a reduction in cytoplasmic components, such as rough endoplasmic reticulum (RER) and mitochondria (M). The plasma membrane presents cytoplasmic processes. (B) Incubation with REAC TO-RNG: The nucleus (N) is euchromatic; the cytoplasm shows a significant increase of the presence of rough endoplasmic reticulum and mitochondria (M). (C) Incubation with IL-1b: The cytoplasm shows a diffuse vacuolization (V) and it contains a reduced quantity of typical organelles, such as Golgi bodies, rough endoplasmic reticulum and mitochondria (M). (D) Incubation with REAC TO-RNG + IL-1β: The cell partially restores its morphology. The nucleus (N) is euchromatic, the cytoplasm shows a restored organization: a much reduced number of vacuoles (V) is present, rough endoplasmic reticulum is abundant and mitochondria (M) are well shaped. The plasma membrane presents many cytoplasmic processes. Bar = 1μm.
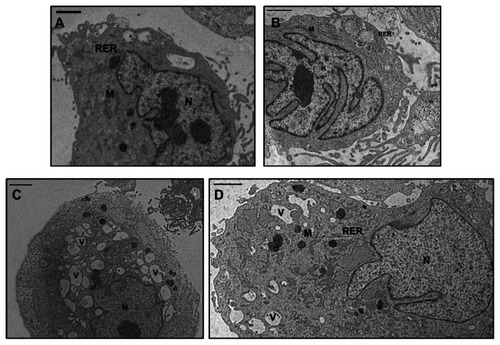
In the normal and OA chondrocytes incubated with IL-1β, a peculiar presence of vacuolization was observed ( and ); for this reason, the number of chondrocytes with an evident vacuolization (more than five vacuoles) was evaluated in each group; this number was significantly increased (P < 0.05) in both normal and OA chondrocytes incubated with IL-1β as compared with untreated cells. Moreover, the effect of IL-1β treatment on the number of chondrocytes with vacuolization was significantly higher in OA chondrocytes as compared to normal cells (P < 0.01). In the presence of IL-1β REAC, TO-RGN stimulus partially restored the cytoplasmic ultrastructure () in both normal and OA chondrocytes, as shown by the decreased number of chondrocytes with vacuolization observed in both groups of cells. Moreover, in OA chondrocytes cultured in the presence of IL-1β, the number of mitochondria and Golgi bodies was significantly increased (P < 0.05) after REAC TO-RGN treatment.
On the whole, significant differences emerged from the comparative analysis of the effects of REAC TO-RGN therapy on the number of mitochondria, Golgi bodies, and the number of vacuolized cells between normal and OA chondrocytes, confirming a higher receptivity of OA chondrocytes to REAC TO-RGN, both in basal conditions and in the presence of IL-1β (P < 0.05, mitochondria at basal conditions; P < 0.01, mitochondria in the presence of IL-1β), as compared with normal ones.
Discussion
Previous work has already demonstrated the effectiveness of a pulsed electromagnetic field (PEMF) to counteract IL-1β activityCitation19–Citation21 and to regulate NO production,Citation22 and also its effect on gene expression in human mesenchymal stem cells and chondrocytes.Citation23
The present study shows that in both normal and OA chondrocytes, REAC TO-RGN exposure determined a reduction in the catabolic effect of IL-1β, downregulating MMP-3 and NO production and increasing PG synthesis. We also observed that, in OA chondrocytes, the REAC TO-RGN-induced PG synthesis was higher as compared with normal cells. This effect may play an important role in the management of OA. It is well known that OA is an inflammatory disease that presents clinical features amenable to the limited regenerative capability of cartilage tissue and which is characterized by several structural changes in the cartilage tissue, including the degradation of cartilage matrix (due to the inability of OA chondrocytes to synthesize sufficient matrix to repair damaged tissues).Citation24–Citation26 Considering the adverse effect of anti-inflammatory drugs commonly used for OA handling, REAC treatments, having no side effect, can play an important role in the management of a disease that affects an increasing number of patients.
In a previous work, our group demonstrated a positive role of a physical approach, the pulsed signed therapy, in counteracting the matrix degeneration occurring in IL-1β OA chondrocytes in vitro.Citation27 Moreover, studies by different authors have shown that under in vivo conditions, PEMF therapy could reduce pain and improve functional performance in patients with OA;Citation28 and that under in vitro conditions, PEMF therapy may exert chondroprotective effects on human articular cartilage, particularly in the early stages of OA.Citation29 However, PEMF treatments are different from REAC treatments for various reasons. First of all, PEMF therapy needs much longer-term exposure to treatment; secondly, at present there are no standardized treatment protocols (with specified characteristics of the applied PEMF) that have been tested in pilot studies and supported by compelling evidence. Finally, some clinical trials that have described positive results in OA applied electromagnetic fields over the levels recommended by the International Commission on Non-Ionizing Radiation Protection (http://www.who.int/peh-emf/about/WhatisEMF/en/index4.html),Citation30,Citation31 an approach that may result in adverse effects on human health. In the present work, we analyzed the effect of REAC TO-RGN on the biochemical and morphological changes occurring in cultures of normal and OA chondrocytes, mimicking also the in vivo inflammatory milieu by the addition of IL-1b. In fact, the proinflammatory cytokine IL-1b has chemical characteristics that influence protein catabolism and seems to be involved in the initiation and progression of OA, and also in NO production.Citation32 Furthermore, increasing evidence suggests that chondrocyte death may contribute to the progression of OA. In particular, Blanco et alCitation3 described apoptotic processes induced by NO in OA chondrocytes, which contribute to the decrease in the number of these extracellular matrix-producing cells.Citation33 Within this context, in this study, we showed a decrease in NO production after REAC TO-RGN stimulation, both in IL-1b-treated and IL-1b-untreated OA and normal chondrocytes. Interestingly, the effect of REAC TO-RGN was significantly more pronounced in the OA chondrocytes than in normal cells. Furthermore, morphometric analysis of the normal and OA chondrocytes treated with IL-1β showed a statistically significant reduction in the number of mitochondria and Golgi bodies. A significant improvement in morphometric analysis and in the number of mithocondria and Golgi bodies was highlighted in cells treated with REAC TO-RGN treatment and with IL-1β. The number of normal and OA chondrocytes with a diffuse vacuolization was also significantly reduced in the presence of REAC TO-RGN stimulus, determining a recovery of cellular features and confirming a shift toward anabolic activity. At the moment, we have not identified the exact sequence of events activated by REAC TO-RGN in chondrocytes. Although future investigations are needed in order to understand the molecular mechanisms involved in the positive effect observed in REAC TO-RGN-exposed chondrocytes, the present work clearly discloses the possibility that this instrument may represent a viable alternative to traditional OA therapy.
Author contributions
Salvatore Rinaldi and Vania Fontani developed the experimental design and contributed to the manuscript. Giulia Collodel and Nicola Antonio Pascarelli, conceived and executed most of the experimental plan, performed the transmission electron microscopy, and contributed to the manuscript. Antonella Fioravanti and Margherita Maioli conceived and executed most of the experimental plan and contributed to the manuscript. Antonello Lamboglia, Elena Moretti, Francesca Iacoponi, Sara Santaniello, Gianfranco Pigliaru, and Alessandro Castagna performed the experiments. Carlo Ventura designed/supervised the project, and contributed to the manuscript.
Disclosure
Salvatore Rinaldi and Vania Fontani are the inventors of the radioelectric asymmetric conveyer. The authors report no other conflicts of interest in this work.
References
- SrikanthVKFryerJLZhaiGWinzenbergTMHosmerDJonesGA meta-analysis of sex differences prevalence, incidence and severity of osteoarthritisOsteoarthritis Cartilage200513976978115978850
- ZhangYJordanJMEpidemiology of osteoarthritisClin Geriatr Med201026335536920699159
- BlancoFJOchsRLSchwarzHLotzMChondrocyte apoptosis induced by nitric oxideAm J Pathol1995146175857856740
- HashimotoHNagasawaTAbeTShibataSMishimaYComparative studies of clinical findings and prognosis of polyarteritis nodosa, Wegener’s granulomatosis, allergic granulomatous angiitis and malignant rheumatoid arthritisRyumachi1988283145155 Japanese2907185
- GoldringSRGoldringMBThe role of cytokines in cartilage matrix degeneration in osteoarthritisClin Orthop Relat Res2004427 SupplS27S3615480070
- AbramsonSBOsteoarthritis and nitric oxideOsteoarthritis Cartilage200816Suppl 2S15S2018794013
- JordanKMArdenNKDohertyMStanding Committee for International Clinical Studies Including Therapeutic Trials ESCISITEULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)Ann Rheum Dis200362121145115514644851
- SavarinoLFioravantiALeoGAloisiRMianMAnthraquinone-2,6-disulfonic acid as a disease-modifying osteoarthritis drug: an in vitro and in vivo studyClin Orthop Relat Res200746123123717806152
- RinaldiSFontaniVRadioelectric asymmetric conveyer for therapeutic use EP1301241 (B1)10112006
- RinaldiSFontaniVRadioelectric asymmetric conveyer for therapeutic use United States patent US 7333859 2 192008
- MaioliMRinaldiSSantanielloSRadiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regenerationCell Transplant20122161225123521975035
- MaioliMRinaldiSSantanielloSRadio electric conveyed fields directly reprogram human dermal-skin fibroblasts towards cardiac-, neuronal-, and skeletal muscle-like lineagesCell Transplant Epub October 2, 2012
- HeinegardDSaxneTConnective tissue macromolecules as markers for tissue processes in joint diseaseEur J Rheumatol Inflamm1991119199
- AltmanRAlarcónGAppelrouthDThe American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hipArthritis Rheum19913455055142025304
- LazzeriniPECapecchiPLNerucciFSimvastatin reduces MMP-3 level in interleukin 1beta stimulated human chondrocyte cultureAnn Rheum Dis200463786786915194586
- GhasemiAZahediaslSPreanalytical and analytical considerations for measuring nitric oxide metabolites in serum or plasma using the Griess methodClin Lab2012587–861562422997962
- AnnefeldMA new test method for the standardized evaluation of changes in the ultrastructure of chondrocytesInt J Tissue React1985742732894066202
- FioravantiACollodelGPetragliaANerucciFMorettiEGaleazziMEffect of hydrostatic pressure of various magnitudes on osteoarthritic chondrocytes exposed to IL-1betaIndian J Med Res201013220921720716822
- OngaroAPellatiASettiSElectromagnetic fields counteract IL-1β activity during chondrogenesis of bovine mesenchymal stem cellsJ Tissue Eng Regen Med Epub December 17, 2012
- RohdeCChiangAAdipojuOCasperDPillaAAEffects of pulsed electromagnetic fields on interleukin-1 beta and postoperative pain: a double-blind, placebo-controlled, pilot study in breast reduction patientsPlast Reconstr Surg201012561620162920527063
- HammondDCDiscussion. Effects of pulsed electromagnetic fields on interleukin-1 beta and postoperative pain: a double-blind, placebo-controlled, pilot study in breast reduction patientsPlast Reconstr Surg201012561630163120517085
- FitzsimmonsRJGordonSLKronbergJGaneyTPillaAAA pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signalingJ Orthop Res200826685485918240331
- WaltherMMayerFKafkaWSchützeNEffects of weak, low-frequency pulsed electromagnetic fields (BEMER type) on gene expression of human mesenchymal stem cells and chondrocytes: an in vitro studyElectromagn Biol Med200726317919017886005
- BuckwalterJAMankinHJArticular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantationInstr Course Lect1998474875049571450
- HowellDSAltmanRDCartilage repair and conservation in osteoarthritis. A brief review of some experimental approaches to chondroprotectionRheum Dis Clin North Am19931937137248210583
- MoskowitzRWAltmanRDHochbergMCBuckwalterJAGoldbergVMOsteoarthritis: Diagnosis and Medical/Surgical Management4th edPhiladelphiaLippincott Williams & Wilkins2007
- FioravantiANerucciFCollodelGMarkollRMarcolongoRBiochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapyAnn Rheum Dis200261111032103312379533
- Ryang WeSKoogYHJeongKIWiHEffects of pulsed electromagnetic field on knee osteoarthritis: a systematic reviewRheumatology (Oxford) Epub April 13, 2012
- OngaroAPellatiAMasieriFFChondroprotective effects of pulsed electromagnetic fields on human cartilage explantsBioelectromagnetics201132754355121412809
- TrockDHBolletAJMarkollRThe effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trialsJ Rheumatol19942110190319117837158
- ThamsborgGFlorescuAOturaiPFallentinETritsarisKDissingSTreatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled studyOsteoarthritis Cartilage200513757558115979009
- TetlowLCAdlamDJWoolleyDEMatrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changesArthritis Rheum200144358559411263773
- ClancyRRediskeJKoehneCActivation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependenceOsteoarthritis Cartilage20019429429911399092